Evaluation of novel Prosopis juliflora water soluble leaf ethanolic extract as preservation coating material of cucumber
Abstract
Cucumber (Cucumis sativus) is an economically important vegetable worldwide. Cucumbers have short shelf life with sensitivity to chilling injuries and fungal and bacterial postharvest diseases. Prosopis juliflora water-soluble leaf ethanolic (PJ-WS-LE) extract has a strong antimicrobial activity against many spoiling agents with time-stability and no phytotoxicity on fruits. The present study explores PJ-WS-LE extract efficacy as a coating material to preserve cucumber. 8 mg/ml of PJ-WS-LE extract increased cucumber shelf life at 22°C by 77%. At week 2, cucumber coated with PJ-WS-LE extract alone showed less than 10 CFU/g of mold as well as 76.9% and 85.8% reduction of total yeast and aerobic bacterial counts, respectively. PJ-WS-LE extract treated samples maintained the slightest change in total soluble solids and 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity. Embedding PJ-WS-LE extract in 0.5% chitosan did not improve the quality maintaining results. In vivo overall results highlight the potentials of PJ-WS-LE extract as a possible coating replacement of chemical fungicides.
Novelty impact statement
- Prosopis juliflora water-soluable leaf ethanolic (PJ-WS-LE) extract extended cucumbers shelf life at 22°C by 77%.
- PJ-WS-LE extract served as an antimicrobial agent that lowers surrounding bacterial and fungal counts of coated cucumber samples.
- 8 mg/ml of Prosopis juliflora water-soluble leaf ethanolic maintained cucumbers storage quality-parameters up to three weeks at 8°C.
1 INTRODUCTION
The increase in human population on earth has put stress on food demand (Fróna et al., 2019). Postharvest diseases are among the main causative agents of fresh produces’ loss in the world (Opara et al., 2021). Extension of fruits and vegetables shelf life is a hot research topic nowadays (Patel & Panigrahi, 2019). Delaying senescence symptoms is one of the main objectives of postharvest technologies developers (Beta & Ndolo, 2019). Many of the commonly used spoilage control methods are either costly or polluting (Garnier et al., 2017). Chemical antifungal and antibacterial agents used in agriculture increase fresh produces’ shelf life; however, they may damage the environment and pose a potential threat to human health (Mahajan et al., 2014). As a result, many countries have set maximum residue limit (MRL) of chemicals on the skin of their exported fruits and vegetables (Mostafidi et al., 2020). The resistance of spoilage microorganisms to commonly used antimicrobial agents is an additional problem that needs to be addressed (Basavegowda et al., 2020; Salehet al., 2020). For all these reasons, scientists are exploring possible chemicals’ replacements within natural products for both fields and postharvest levels usages (Alirezalu et al., 2017; Bill et al., 2014).
Among the economically important crops, cucumber (Cucumis sativus) is an internationally important vegetable (Che & Zhang, 2019). This member of the Cucurbitaceae family has a short shelf life of below 14 days which is usually terminated by chilling injuries, loss of firmness, color, and water content which could occur with or without fungal and bacterial rot (İbrahim & Serhat, 2019). During storage, cucumbers’ physiological changes could enable microorganisms development which makes them more susceptible to postharvest diseases (Wenneker & Thomma, 2020). Cucumbers are eaten fresh or as pickles, they are low in calories, around 95% of their weight is water with some carbohydrates, protein, magnesium, vitamin C, vitamin K, and antioxidant compounds (Patel & Panigrahi, 2019). Cucumbers benefits are not only nutritional but they are also used in cosmetology due to their radian effects on skin (Mukherjee et al., 2013).
A list of studies has evaluated various natural products for their bio-controller effectiveness. Many, including essential oils have shown good results (Kazemi et al., 2020; Raveau et al., 2020). However, a number of limitations were encountered, this includes the insolubility of the active natural product in water, which makes them hard to apply (Dhifi et al., 2016). In addition, many of the active phytochemicals are volatile and not stable when exposed to heat, light and/or oxygen, which also lower their value for commercialization (Turek & Stintzing, 2013). To stabilize natural antimicrobial compounds, encapsulation was widely used (Becerril et al., 2020). Besides being environmentally friendly, edible coatings should give the fruits and the vegetables a better look in terms of color and texture, and should allow gases (oxygen and carbon dioxide) and moisture exchanges, lessen water loss, lower microbial growth, and extend fresh produces storage shelf life (Esmaeili et al., 2021; Raghav et al., 2016). A coating material with antimicrobial effectiveness and no phytotoxicity would be of great interest (Hernalsteens, 2020). Among the widely studied encapsulation material, chitosan, a generally recognized as safe (GRAS) compound, has shown good biodegradability, antimicrobial activity, and capacity of loading and delivering a wide range of active biological compounds (Detsi et al., 2020).
In cucumber, some researchers have been working on developing edible coatings with some promising results (Nair et al., 2020). However, our study is the first describing a stable and water-soluble extract of an available, easy to grow plant. Prosopis juliflora is an invasive species in the state of Qatar and in many countries around the world (Hussain et al., 2020). Chemical and mechanical invasion control are expensive and not recommended because of their possible adverse effects on the environment, which highlights the importance of control through utilization (Wei et al., 2018). A recent study of our team showed the effectiveness of P. juliflora water-soluble leaves ethanolic (PJ-WS-LE) extracts against a list of fungi and bacteria (Saleh & Abu-Dieyeh, 2021). This study aims to evaluate the value of PJ-WS-LE extract, individually and in combination with chitosan, as a protective coating material for cucumber samples. PJ-WS-LE extract has been proven as an effective, stable natural compound against many postharvest spoiling agents. Having a feasible extraction method and solvent-free final composition, make PJ-WS-LE extract a promising replacement of chemical fungicides.
2 MATERIALS AND METHODS
2.1 Effect of PJ-WS-LE extract on cucumbers shelf life at room temperature
2.1.1 Fruits
Sixty samples of fresh good looking, locally grown (Qatari) cucumbers (C. sativus) were purchased from the market upon arrival from farms. Only mature and undamaged fruit were chosen for the experiment. Samples were not washed or sterilized to mimic the market’s conditions.
2.1.2 Preparation of PJ-WS-LE extract
Fresh leaves of P. juliflora were collected and processed as previously describes by our team. A stock solution of 100 mg/ml of PJ-WS-LE extract was prepared as previously described (Saleh & Abu-Dieyeh, 2021). A stock solution was diluted for further usages.
2.1.3 Cucumbers’ shelf-life evaluation
Cucumber samples were divided into a control group and an experimental group. The control group samples were not treated and kept in sterile well-ventilated boxes at room temperature set at 22°C. Experimental group samples were first sprayed thoroughly with PJ-WS-LE extract solution, then left to dry in a sterile drainer to be moved to sterile well-ventilated boxes at room temperature set at 22°C. The concentration of PJ-WS-LE extract used was set to 8 mg/ml which is double the highest in vitro minimum inhibitory concentration (MIC) of the extract as demonstrated by our team in an earlier study (Saleh & Abu-Dieyeh, 2021).
Samples were monitored every 24 hr to determine shelf life and to subculture fungal spoiling agents. Each sample that showed fungal growth was removed from the box and the fungal contaminant was transferred with a sterile needle to a clean PDA plate before discarding the sample. PDA plates were incubated at 25°C for 5 days then fungi were microscopically identified (Saleh & Al-Thani, 2019). Samples that showed physiological characteristics of deterioration with no fungal growth were also discarded. Average shelf life per hour of control and experimental groups were calculated. The percentage of edible samples at 6 days was also determined.
2.2 In vivo long-term preservative activity of PJ-WS-LE extract individually and when embedded in chitosan coating material
2.2.1 Preparation of coating solutions
Chitosan solution was prepared at 0.5% by stirring chitosan powder (CAS 9012-76-4, Himedia, India) in 1% glacial acetic acid solution (IsoLab, Germany) overnight. NAOH (Sigma-Aldrich, Germany) at 0.5 M was used to adjust the pH of the solution to 5.6. PJ-WS-LE extract stock solution was sterilized by filtration using 0.2 µm syringe filter (Sigma-Aldrich, Germany) and diluted and embedded in chitosan coating solution to achieve a final extract concentration of 8 mg/ml PJ-WS-LE extract in 0.5% chitosan (Chien et al., 2007). Chitosan concentration in the study (0.5%) was chosen as the highest chitosan concentration suitable for our cucumber samples. A pilot study evaluating various chitosan concentration (0.25%, 0.5%, 1%, and 2%) on locally grown cucumber samples showed stress-like symptoms at 1% chitosan and above.
2.2.2 Sample preparation
- Batch A: untreated samples.
- Batch B: samples were thoroughly sprayed with 8 mg/ml PJ-WS-LE extract and left to air-dry.
- Batch C: samples were thoroughly sprayed with 0.5% chitosan and left to air-dry.
- Batch D: samples were thoroughly sprayed with 8 mg/ml PJ-WS-LE extract embedded in 0.5% chitosan and left to air-dry.
Every five samples of cucumber made a single replicate and were stored together in one sterile box, each treatment was performed in seven replications and the entire experiment was repeated twice (Mohammadi et al., 2016).
2.2.3 Evaluation of sensory quality
The sensory quality of the samples was evaluated using a 5-point scale for smell, color change, and overall appearance. The weekly withdrawn samples were used to evaluate the three attributes. Scores were given using the following scale: 5 points for “extremely liked,” 4 points for “liked,” 3 points for “acceptable,” 2 points for “disliked,” and 1 point for “extremely disliked.” Evaluating committee was made of ten panelists of age between 25 and 50 years old (5 females and 5 males). The average score per batch per week was then calculated (Patel & Panigrahi, 2019).
2.2.4 Estimation of weight loss
All cucumber samples used in the trial had their weight taken at day zero. Weights of the remaining samples were measured weekly. Total weight loss is the variation between the primary and weekly weights of the sample during storage time. The average percent weight loss per batch was calculated (Patel & Panigrahi, 2019).
2.2.5 Estimation of total aerobic bacterial count
Cucumber samples withdrawn weekly were washed from the outside with 10 ml sterile distilled water (SDW) each. Washing water was serially diluted (10−1 to10−3) and spread in duplicates on nutrient agar (NA) (Scharlau, Spain) plates for the total aerobic bacterial count. All plates were incubated at 37°C for 24 hr. Colony-forming unit (CFU) per sample was calculated and the weekly average total aerobic bacterial counts CFU per treatment batch were also calculated. The number of bacterial strains in each sample was evaluated relying on colonies’ morphologies. The average number of strains per treatment batch was calculated (Moradi et al., 2019).
2.2.6 Estimation of total fungal count
The wash water previously prepared was also plated on duplicates of Potato Dextrose Agar (PDA) (Difco-USA) plates for total mold and yeast count. PDA plates were incubated at 25°C for 5 days. Yeast CFU and mold CFU per sample were calculated separately. Weekly average mold CFU and yeast CFU per treatment batch were calculated. The mold that shows growth was aseptically isolated on clean PDA plates and microscopically identified upon growth (Moradi et al., 2019).
2.2.7 Evaluation of respiration rate
Cucumber samples of each treatment batch were placed in closed containers and the level of carbon dioxide produced was measured using a carbon dioxide meter (OMEGA AMQ-102, UK). A reading of the CO2 in ppm was taken every two seconds for around 50 min. Respiration rate (ppm/s) was calculated using the slope of the trend line passing by the collected data, and weekly results were compared (Mohammadi et al., 2016).
2.2.8 Determination of samples firmness
Weekly evaluated cucumber samples had their firmness measured using a penetrometer (Agriculture Solutions, USA). The probe of the penetrometer was inserted in two different locations in the middle of each fruit, and the average reading in Newton (N) was recorded. The average samples firmness (N) in each treatment batch was calculated weekly (Emamifar et al., 2019).
2.2.9 pH measurement
Cucumber samples withdrawn at every measurement interval were individually mixed in a blender and then filtered using cheesecloth. Juice pH was measured using a digital pH meter (Jenway, UK). Average samples pH per treatment batch was calculated on a weekly basis. A pH 7 buffer solution was used to calibrate the pH meter before measurements (Naeem et al., 2019).
2.2.10 TSS measurement
Prepared cucumber juice samples had their total soluble solids (TSS) in brix measured. Two drops of juice were poured on a refractometer (ANTAHI, New Zealand) and measurements were taken for each sample. Average samples TSS (%Brix) per treatment batch was calculated weekly. Distilled water was used to calibrate the refractometer before measurements (Moradi et al., 2019).
2.2.11 DPPH radical scavenging assay
2.3 Cucumbers water content
Thirty samples of fresh good looking cucumbers were purchased from the market upon arrival from Qatari farms. Samples were weighted and each cucumber was cut into two halves and kept in a paper bag inside an oven at 65°C. When completely dried, samples’ final weights were taken and percent water content was calculated (Shahari et al., 2014).
2.4 Statistical analysis
Completely Randomized Design (CRD) was used as an experimental design. T-test was used to evaluate the significance of the shelf-life differences. The weekly percent weight change data were evaluated using one-way ANOVA followed by Tukey post hoc test at p ≤ .05 to separate the means among treatment levels. One-way ANOVA test was also used to evaluate the significance of firmness, pH, and TSS changes among different treatment groups at p ≤ .05. Correlation matrix among percent change in weight, bacteria CFU, mold CFU, and yeast CFU levels throughout the experiment was constructed using Pearson correlation test. Data were presented as average ± standard error of the Means (SEM). Statistical analyses were performed using SPSS (Version 27, IBM, NY, USA, 2020).
3 RESULTS
3.1 Effect of PJ-WS-LE extract on cucumbers shelf life at room temperature
The treated fruits showed longer shelf life than control samples which means that the active phytochemicals in the crude extract were capable of preventing fungal growth on cucumber samples. The calculated average shelf life of treated cucumbers at room temperature was 221 hr (9.2 days) while none treated fruits showed an average shelf life of 125 hr (5.2 days) with a 77% increase in shelf life. T-test validated variances equality assumption and showed statistically significant difference between control and experimental samples shelf life (p ≤ .01). Percentage of the remaining edible samples after 6 days of incubation was also calculated, 66.7% of the treated samples were still good for consumption while only 26.7% of the non-treated samples were still edible at day 6 (Figure 1). Cucumbers are more prone to yeast and bacterial spoilage this is why many samples showed deterioration in their physiological characteristics and were taken out of the experiment without showing hyphal growth. Pure cultures were taken from cucumbers that showed fungal growth, microscopic identification showed Cladosporium growth in 26.7% of the evaluated samples.

3.2 Sensory quality of cucumber samples at long-term storage experiment
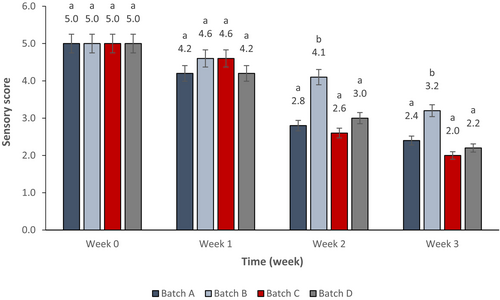
The change in sensory characteristics of cucumbers stored at 8°C was evaluated on a weekly basis. Figure 2 represents the change in the score of fruits belonging to different treatment batches. As the storage period passed, the overall sensory score of all cucumber batches declined. There was no significant difference between the end quality of cucumbers belonging to batch D and those of the control samples according to the post hoc test of one-way ANOVA (p ≥ .05). Best end quality was seen in batch B which samples maintained acceptable quality during the 3 weeks of the experiment, Quality of batch B samples is significantly better than other batches during weeks 2 and 3 (p ≤ .05). At week 2, cucumbers sprayed with the PJ-WS-LE extract alone showed an overall quality score of 4.1, which demonstrates one more time the capability of the extract to extend cucumbers shelf life.
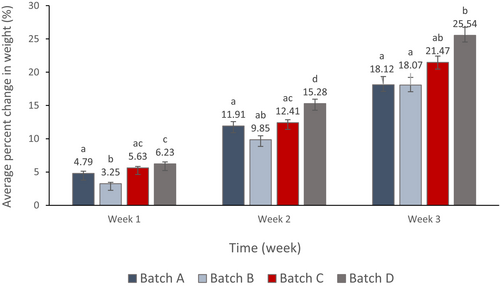
3.3 Cucumbers’ weight loss
Data of percent change in weight of each treatment batch every week is presented in Figure 3. Cucumber samples coated with 0.5% chitosan alone or with 8 mg/ml PJ-WS-LE extracts embedded in 0.5% chitosan showed unexpectedly higher weight loss that samples that are not coated. Tukey test following one-way ANOVA for each week’s data showed no significant difference between weight loss of batches C and D during weeks 1 and 3 (p = .397 and p = .064, respectively), which indicates that chitosan treatment did not suit the cucumber skin and increased weight loss. Cucumber batch with the lowest change in weight was batch B treated with 8 mg/ml PJ-WS-LE extract, in week 1 the extract showed a significant effectiveness in protecting cucumber samples against weight loss when compared with the control samples (batch A) (p = .001 <.05). The weight loss of samples of batch B increased with time to remain the lowest among other batches.
3.4 Respiration rates of cucumber samples belonging to different treatment batches during storage
The respiration rate of different cucumber batches was calculated using the slope of the trend line passing by the CO2 data collected. A comparison between the carbon dioxide levels of the four treatment categories during the storage period is shown in Figure 4.
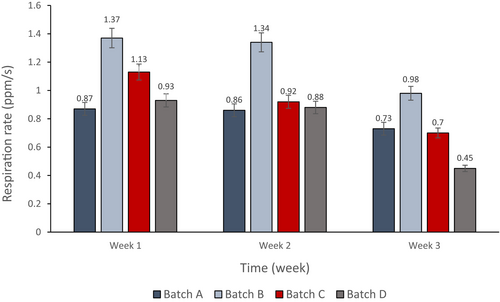
Figure 4 shows that the respiration rate of cucumber samples of all treatments, represented by the carbon dioxide level, decreased with time during the 3 weeks of the experiment. Week 1 results indicate that samples treated with PJ-WS-LE (batch B) extract have the highest rate of respiration while the negative control group (batch A) have the lowest. Plants of batch B coated with the extract alone showed the highest rate of respiration throughout the three weeks, which indicates the effectiveness of PJ-WS-LE extract in protecting cucumber samples and keeping their normal respiration rate. Chitosan-coated samples showed the lowest rates of respiration at the third week.
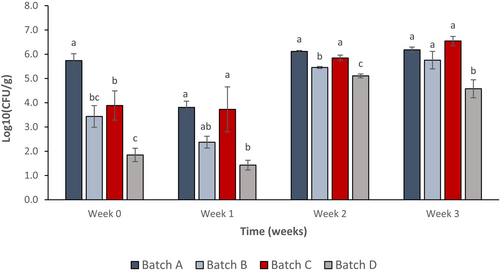
3.5 Total aerobic bacterial count
PJ-WS-LE extract in the cucumber coating material served as an antimicrobial barrier that kills surrounding bacteria. From reading zero, samples coated for few hours showed lower bacterial count than negative control samples and samples coated with chitosan alone. Both batches treated with PJ-WS-LE extract alone and PJ-WS-LE extract embedded in chitosan showed aerobic bacterial count CFU significantly lower than the control batch according to one-way ANOVA post hoc Tukey test (p ≤ .05). Effect is stronger in samples of batch D coated with the extract embedded in 0.5% chitosan (Figure 5).
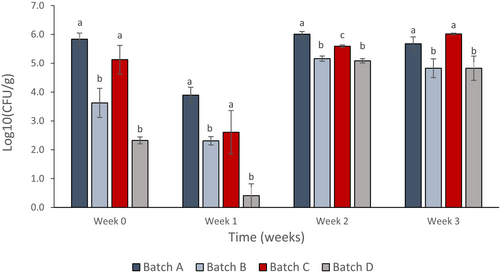
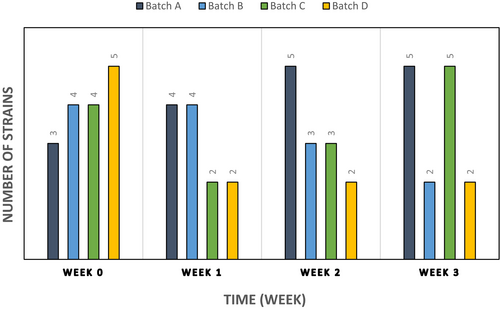
Figure 5 shows that samples coated with 8 mg/ml PJ-WS-LE extract alone (batch B) and extract embedded in 0.5% chitosan (batch D) have their total aerobic bacterial count dropped drastically compared to the control batches in the first week. However, the CFU of aerobic bacteria surrounding the cucumber samples of batches B and D increased in weeks 2 and 3, yet the final CFU counts were still lower than the control batch samples (batch A) in which aerobic bacterial CFU is 5.5× more than batch B CFU and 2.8× more than batch D CFU. Among the factors evaluated was the number of strains per treatment batch. Results show a decrease in the number of strains per sample in the two batches treated with PJ-WS-LE extract (batches B and D) which show an average of only two bacterial strains per sample compared to an average of five strains per sample in control batches (batches A and C; Figure 6).
3.6 Total fungal count
Cucumber rotting involves more bacteria than mold, spread plate method of wash water used to wash the outside of cucumber samples of different batches proved this idea and showed a low number of mold spores. However, samples coated with PJ-WS-LE extract showed a total mold count of less than 1 log CFU/g for batches B and D throughout the weeks of the experiment, compared to 2.9 log CFU/g for the control samples at reading zero, which proves the effectiveness of the extract against spores germination on cucumbers.
For yeast count, the variation of average CFU showed a pattern similar to that of bacteria (Figure 7), with very low yeast CFU count of cucumbers of batch D during reading 0 and 1, the number increases in weeks 2 and 3 to remain lower than the yeast CFU of the control batch A.
Mold samples showing growth upon spread plate method were subjected to microscopic identification, the number of samples showing a certain fungi species was recorded regardless of the number of colonies per samples. Results show that the dominant strain on cucumber samples stored at 8°C was Penicillium found in 14 samples of different treatment batches throughout the storage period, followed by Clostridium, Aspergillus, and Alternaria shown on 3, 3, and 2 samples, respectively.
3.7 Effectiveness of PJ-WS-LE extract in maintaining strawberry samples weight
A correlation between the samples’ weight loss pattern and the change in microbial counts throughout the study would support our hypothesis stating that PJ-WS-LE extract acts as an antimicrobial barrier around the fruits and maintain their storage quality during the experiment including the lessening of weight loss. Pearson test results showed a significant correlation between bacterial and yeast counts patterns. Most interestingly, the results show a significant correlation between the percent weight change data and the total CFU counts of bacteria and yeast note that mold CFU count is not correlated with the percent change in weight in the tested samples (Table 1).
| Correlation matrix | ||||||
|---|---|---|---|---|---|---|
| Mean | SD | % Weight change | Mold CFU | Yeast CFU | Bacteria CFU | |
| % Weight change | 11.78 | 7.07 | 1 | |||
| Mold CFU | 138.89 | 1069.19 | −0.162 | 1 | ||
| Yeast CFU | 933,703.31 | 2,089,316.20 | 0.384** | −0.059 | 1 | |
| Bacteria CFU | 338,484.26 | 479,181.52 | 0.433** | −0.092 | 0.529** | 1 |
- ** p-value < .01
3.8 Changes in physical and chemical properties during storage time (Firmness, pH, TTS, antioxidant ability, and ascorbic acid level)
Weekly cucumber samples monitoring included physical parameters that were measured for each treatment groups. The results of averages firmness, pH, and TSS of samples stored at 8°C throughout the 3 weeks are shown in Table 2.
| Treatment batch | Storage week | Firmness (N) | pH | TSS (Bx) |
|---|---|---|---|---|
| Batch A negative control samples | 1 | 40.8 ± 7.63 | 6.07 ± 0.056 | 30.8 ± 0.86 |
| 2 | 40.75 ± 3.85 | 6.71 ± 0.225 | 30.6 ± 1.63 | |
| 3 | 37.38 ± 7.20 | 6.78 ± 0.073 | 35 ± 2.14 | |
| Batch B coated with 8 mg/ml PJ-WS-LE extract | 1 | 38.05 ± 5.36 | 6.38 ± 0.184 | 31.2 ± 1.77 |
| 2 | 38.72 ± 3.12 | 6.76 ± 0.075 | 30.8 ± 2.76 | |
| 3 | 38.70 ± 2.29 | 6.83 ± 0.033 | 32.4 ± 3.01 | |
| Batch C coated with 0.5% chitosan | 1 | 42.26 ± 8.45 | 6.15 ± 0.042 | 32.8 ± 0.37 |
| 2 | 41.96 ± 6.04 | 6.89 ± 0.071 | 33.2 ± 1.58 | |
| 3 | 42.57 ± 6.38 | 6.91 ± 0.026 | 34.2 ± 3.42 | |
| Batch D coated with 8 mg/ml PJ-WS-LE extract in 0.5% chitosan | 1 | 42.66 ± 6.72 | 6.92 ± 0.056 | 31.0 ± 1.67 |
| 2 | 39.65 ± 5.25 | 6.87 ± 0.109 | 35.25 ± 2.05 | |
| 3 | 43.29 ± 5.20 | 6.88 ± 0.098 | 39.2 ± 2.26 |
Negative control batch of cucumber showed 8% loss of their firmness throughout the storage period. However, all other batches showed stability in their firmness. When results of the overall storage period of each treatment batch were compared using one-way ANOVA followed by Tukey test, no significant variation in the firmness averages was seen (p = .857). However, batches A and B treated with PJ-WS-LE extract showed average firmness of 40.2N and 40.6N, respectively, compared to average firmness of 43.4N and 43.5N in batches C and D that was not treated with PJ-WS-LE extract.
pH results of cucumber juices of different batches show increase in numbers throughout the weeks except of batch D which samples showed a slight decrease in their pH. However, when each batch results (all weeks results combined) were tested using one-way ANOVA followed by Tukey test, the overall results did not show any significant difference in pH of cucumbers of different batches (p = .716). The highest pHs were recoded with batch D samples (average pH = 6.51).
Total soluble solids results showed an increase in TSS level of the control (batch A) samples by 12% and of the experimental batch D samples by 21% during the 3 weeks of storage. Cucumber samples of batches B and C showed a slight change in their TSS levels with the slightest change in samples treated with 8 mg/ml PJ-WS-LE extract (batch B). One-way ANOVA did not show any significant difference in the TSS levels of different batches. DPPH radical scavenging activity of cucumber samples of the four treatment batches showed an increase with time (unpublished data). There was no significant difference in the overall percentage of DPPH free radical scavenging activity among the four batches upon running one-way ANOVA followed by the Tukey test (p = .774). Yet the lowest rate of antioxidant increase was seen in samples of batch B.
3.9 Cucumbers water content
Results showed that the average water content of the evaluated samples is 96.25 ± 0.074% of the cucumber samples’ net weight, which is above the literature average of 90% to 95%.
4 DISCUSSION
Cucumber samples treated with 8 mg/ml PJ-WS-LE extract and stored at room temperature showed that control fruits started rotting within 48 hr and all of them have shown fungal growth at day six with an average shelf life of 5 days. Treatment has extended the shelf life of the samples to an average of 9 days with 76.8% average shelf-life extension. At day 5, 66.7% of the treated samples were still marketable. Results can be compared to those of a study conducted on treating cucumber samples with chitosan nanoparticles loaded with Zataria multiflora essential oil. An experiment was conducted at 10°C which makes shelf life higher than those of our study, yet the cost of the storage is also higher. During Mohammadi et al. (2016) experiment the uncoated cucumber samples showed signs of fungal decay (31.5% of the samples) after 9 days while coated samples were all still good for consumption. Another study conducted on cucumber tested the efficacy of chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil, at the end of the study, only 21.6% of the treated samples stored at 10°C showed decay after 21 days compared to 100% decay of the control sample (Mohammadi et al., 2015). A recent study showed that starch-glucose edible coating (1.5 µM starch–2.5 µM D-glucose) of cucumber has slowed down the ripening process and increased shelf life of the fruits during the long storage period at fridge temperature up to 30 days (Patel & Panigrahi, 2019). Despite the difference in storage temperature, samples treated with PJ-WS-LE extract showed the highest shelf-life increase (76.8%) among other studies conducted on cucumber when comparing treated samples to control samples stored at the same temperature. It is also worth noting that the extract preparation method and the coating procedure performed are simple and economically feasible, which give PJ-WS-LE extract an advantage among other coatings. Overall results show that 8 mg/ml of PJ-WS-LE extract had a protective effect on cucumbers against natural spoiling.
Long-term fruits preservation experiment was conducted on cucumber samples stored at 8°C, treatments were divided into four batches, batch A are the untreated control samples, batch B were treated with 8 mg/ml PJ-WS-LE extract, batch C were coated with 0.5% chitosan, and batch D were coated with 8 mg/ml PJ-WS-LE extract with 0.5% chitosan. The best sensory scores were seen with samples coated with 8 mg/ml PJ-WS-LE extract alone (Batch B) with liked quality conserved. Low quality of samples coated with 8 mg/ml PJ-WS-LE extract embedded in 0.5% chitosan indicates that chitosan edible coat is stressful on cucumber skin.
An effective edible coatings should act as a barrier against water loss. Different edible coatings are formulated using different compositions including proteins, lipids, and polysaccharides (Raghav et al., 2016). Every composition has its advantages and disadvantages, lipids are hydrophobic compounds so they can limit water loss, although, their non-polymeric nature limits their ability to form films with good mechanical integrity (Yousuf et al., 2021). On the other hand, polysaccharides have efficient gas barrier properties but are highly hydrophilic which can show high water vapor permeability (Kocira et al., 2021). Among polysaccharides, chitosan, obtained from alkaline deacetylation of chitin has been widely used because of its antimicrobial properties (Elieh-Ali-Komi & Hamblin, 2016). However, Qatari cucumber are grown in greenhouses where they are continuously watered. Water content results showed higher water content in Qatari samples. This can explain why chitosan coating caused fast water loss and therefore shrinking of the fruits which limits its usefulness as a local coating material (Gutiérrez-Pacheco et al., 2020).
Samples coated with 8 mg/ml PJ-WS-LE extract (batch B) showed also lower weight loss when compared to the other three batches during the first 2 weeks. During the first week, batch B weight loss was significantly lower than the control batch A. Lower weight loss in coated fruits might be caused by partial pores blockage which lowers in turn water loss (Patel & Panigrahi, 2019). At the end of the trial batches coated with chitosan showed the highest weight loss, which proves one more time the incompatibility of this edible coating with locally grown cucumbers. Weight loss is usually related to multiple reactions in the fruits including browning, vitamin degradation, and enzymatic activities that enhance the rate of microorganisms growth including fungal decay (Mohammadi et al., 2016). The coating of cucumber samples with chitosan nanoparticles loaded with C. zeylanicum (CEO-CSN) essential oil showed the lowest weight loss in coated samples compared to the untreated control samples (Mohammadi et al., 2015). Another study conducted by Mohammadi team showed a weight loss in cucumber samples treated with chitosan nanoparticles loaded with Z. multiflora essential oil (ZEO@CSNPs) of less than 10% compared to weight loss of 12.5% in uncoated samples within 15 days (Mohammadi et al., 2016). Similarly, batch B samples treated with PJ-WS-LE extract showed weight loss lower than 10% compared to 11.9% weight loss in control samples within the first 15 days.
Respiration rate is an indicator of plants’ normal activity. The respiration rate monitoring throughout the experiment showed the best rate with batch B. It is worth noting that the PJ-WS-LE extract embedded in 0.5% chitosan had a respiration rate of 54% lower than that of batch B samples toward week 3. Similarly, cucumber samples coated with ZEO@CSNPs showed a lowering in their respiration rate (Mohammadi et al., 2016).
Fruits and vegetables surrounding microorganisms increase in numbers during fruits ripening and play a role in fruits spoilage. Cucumbers are usually more prone to bacterial spoilage than fungal spoilage. Total aerobic bacterial CFU monitoring between week 0 (time of treatment) and week 3 showed that PJ-WS-LE extract has effective antimicrobial qualities which lowered the final CFU count by 5.5× in batch B and 2.8× in batch D compared to the control samples (batch A). The capacity of PJ-WS-LE extract to lower the surrounding microorganisms count plays a role in increasing plants’ shelf life. In addition, the average number of bacterial types per treatment batch indicated lower bacterial diversity on batches B and D samples (two types) when compared with control batches A and C samples (5 types), which indicates the total intolerance of some bacterial types to PJ-WS-LE extract. As for fungi, batches coated with PJ-WS-LE extract did not show fungal growth, which indicates the effectiveness of the extract against spore germination. Similar results were seen with yeast count which showed a final CFU count lower in batch D samples that the control samples. It is worth noting that the total bacterial and yeast count dropped sharply in week 1 to rise again in weeks 2 and 3, which is not the case with mold count that remained low. Those results could be an indicator of the mode of action of the PJ-WS-LE extract against the different microorganisms, which could be a microbicidal effect against mold and a microbiostatic effect against some of the mold and aerobic bacteria. On the other hand, the microbial count increase in week 2 could be related to the dose of PJ-WS-LE extract used, although previous study on strawberry showed total effectiveness with this dose (Saleh & Abu-Dieyeh, 2022). However, the variation in the types of spoiling agents between the two fruits can require higher doses for better protection of cucumber samples. Overall results showed a high antimicrobial effect of PJ-WS-LE extract. In accordance with our results, CEO-CSN and ZEO@CSNPs showed antimicrobial activities of the coating with lower CFU of treated samples compared to control (Mohammadi et al., 2015, 2016). Noting that PJ-WS-LE extract showed the lowest bacterial and mold CFU compared to other treatments.
Our teams’ previous in vitro analysis showed a strong effectiveness of PJ-WS-LE extract against a variety of fungi, bacteria, and even yeast with MICs ranging from 0.5 to 4 mg/ml (Saleh & Abu-Dieyeh, 2021). This explains the correlation between yeast and bacterial CFU changes. Being a valuable source of the phenolic compound gives P. juliflora extracts the capacity of initiating multiple antioxidative mechanisms, which can act as antimicrobial agents. The antimicrobial activity of P. juliflora has been previously described by many older studies (de Brito Damasceno et al., 2018). Previous results, together with the significant correlation proven by the Pearson test imply that the strong antimicrobial activity of the extract protects cucumber samples from yeast and bacterial rot and maintain their weights among other quality parameters. The insignificant results in the case of mold are due to the low number of spores encountered around cucumber samples from days 1 which although reached zero after treatment but it did not change widely in correlation with percent change in weight.
Firmness is among the important sensory attributes in fruits and vegetables. Firmness is affected by pectin depolymerization leading to texture deterioration during fruit ripening (Paniagua et al., 2014). There was no significant variation in the firmness averages throughout the 3 weeks of the experiment, however, batches A and B samples showed slightly lower firmness averages compared to batches C and D, which imply that chitosan coating played a role in hardening the cell wall of the cucumber samples. In agreement with our results, the older studies conducted on CEO-CSN and ZEO@CSNPs coatings showed also firmness stability in chitosan-coated samples (Mohammadi et al., 2015, 2016). Furthermore, there was no significant change in pH during the experiment. Yet it was noted that samples of batches A, B, and C showed pH increase with time while samples of batch D showed pH decrease, which indicates that the combination of the plant extract and the chitosan has induced a different set of reactions in cucumbers compared to other treatments. The highest increase in TSS during the experiment was seen with batch D (21%) followed by batch A (12%). As an increase in soluble sugars could be due to the increase in water loss and therefore weight loss, the TSS results show consistency with the average weight loss of cucumber samples discussed earlier in which samples of batch D showed the highest average weight loss by the end of the incubation period. The lowest TSS increase was seen in cucumbers coated with 8 mg/ml PJ-WS-LE (bacth B) extract, which showed the best sensory qualities during the experiment. Overall, the increase in total soluble sugars during cucumber ripening has been described, as the time passes, macromolecules produce soluble micromolecules which increases TSS (Patel & Panigrahi, 2019).
The total antioxidant change was not significant with time, however, batch B showed the lowest DPPH radical scavenging activity increase, which reflects their slower ripening process compared to other samples. Usually, DPPH-radical scavenging activity increases as an indicator of antioxidant compounds development during postharvest storage (Mohammadi et al., 2016). It is worth noting that previous studies have demonstrated that chitosan could induce an increase in antioxidant activity in fruits such as tomato, orange, and sweet cherry (Dang et al., 2010; Liu et al., 2007; Meng et al., 2008).
5 CONCLUSION
The strong antimicrobial activity of PJ-WS-LE extract, previously demonstrated, against a wide range of spoiling agents and its stability with time, make it a strong candidate for in vivo applications. Cucumber was chosen as a model for its economical value and importance in human diet in the region. Spraying cucumber samples with 8 mg/ml of PJ-WS-LE extract extended cucumbers shelf life at 22°C by 77% and maintained samples’ acceptable quality for 3 weeks of storage at 8°C. Samples sprayed with the extract and stored 8°C showed 32% less weight loss and 57% higher respiration rate that the un-treated control samples within the first week of storage. PJ-WS-LE extract in cucumber coating material served as an antimicrobial barrier that kills surrounding aerobic bacteria, mold spores, and yeast. Embedding PJ-WS-LE extract in a polysaccharide edible coating material to improve efficacy did not work, as chitosan coating increased water loss which deteriorates samples’ physiological characteristics. Nevertheless, the simple application of the water-soluble extract alone was highly effective in increasing cucumber shelf life and maintaining samples quality characteristics. Those results supported our research hypothesis about PJ-WS-LE extract. Future studies on PJ-WS-LE extract will involve more fruits and vegetable, and more pathogenic spoiling agents for a better understanding of all possible applications of this natural bio-controller in agriculture. Fractionation of PJ-WS-LE extract and identification of active phytochemical(s) are also among the future objectives of the team.
ACKNOWLEDGMENTS
The publication of this article was funded by Qatar National Library.
CONFLICTS OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
Iman Saleh: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing. Mohammed Abu-Dieyeh: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors




