Efficacy and safety of bexagliflozin compared with dapagliflozin as an adjunct to metformin in Chinese patients with type 2 diabetes mellitus: A 24-week, randomized, double-blind, active-controlled, phase 3 trial
Abstract
Background
Bexagliflozin and dapagliflozin are sodium-glucose cotransporter-2 (SGLT2) inhibitors. No direct comparison of SGLT2 inhibitors in a randomized controlled trial has been reported to date.
Methods
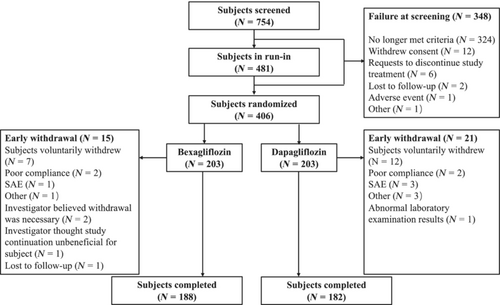
This was a multicenter, randomized, double-blind, active-controlled trial comparing bexagliflozin to dapagliflozin for the treatment of type 2 diabetes mellitus in adults with disease inadequately controlled by metformin. Subjects (n = 406) were randomized to receive bexagliflozin (20 mg) or dapagliflozin (10 mg) plus metformin. The primary endpoint was noninferiority of bexagliflozin to dapagliflozin for the change in glycated hemoglobin (HbA1c) from baseline to week 24. Secondary endpoints included intergroup differences in fasting plasma glucose (FPG), 2-h-postprandial glucose (PPG), body weight, and systolic blood pressure (SBP) from baseline to week 24. The trial also evaluated the safety profiles.
Results
The model-adjusted mean change from baseline to week 24 HbA1c was −1.08% for bexagliflozin and −1.10% for dapagliflozin. The intergroup difference of 0.03% (95% confidence interval [CI] −0.14% to 0.19%) was below the prespecified margin of 0.4%, confirming the noninferiority of bexagliflozin. The changes from baseline in FPG, PPG, body weight, and SBP were −1.95 mmol/L, −3.24 mmol/L, −2.52 kg, and −6.4 mm Hg in the bexagliflozin arm and −1.87 mmol/L, −3.07 mmol/L, −2.22 kg, and −6.3 mm Hg in the dapagliflozin arm. Adverse events were experienced in 62.6% and 65.0% and serious adverse events affected 4.4% and 3.5% of subjects in the bexagliflozin and dapagliflozin arm, respectively.
Conclusions
Bexagliflozin showed nearly identical effects and a similar safety profile to dapagliflozin when used in Chinese patients on metformin.
CONFLICT OF INTEREST STATEMENT
Wei Zhang, Jinyu Chen, and Qian Zhu were employees of Newsoara Biopharma Co., Ltd. at the time of study conduct. The remaining authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its Supplementary Materials.