Cardiovascular disease and risk of incident diabetes mellitus: Findings from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL)
心血管疾病与糖尿病发病风险:拉美裔社区健康研究/拉丁裔研究(HCHS/SOL)的研究结果
Abstract
enBackground
Studies have reported an association between prevalent cardiovascular disease (CVD) and risk of diabetes mellitus (DM). However, factors that may explain the association remain unclear. We examined the association of prevalent CVD with incident DM and assessed whether weight gain and medication use may explain the association.
Methods
Data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Visit 1 (2008-2011) and Visit 2 (2014-2017) were used to compare incidence of DM among individuals with and without self-reported CVD at Visit 1. A total of 1899 individuals with self-reported CVD were matched to controls free of self-reported CVD at Visit 1 using 1:1 propensity score matching. Covariates included in the propensity model were sociodemographic characteristics, lifestyle factors, comorbid conditions, and study site. The effect of self-reported CVD on incident DM was examined using a generalized estimating equation. The mediating effects of weight gain and use of cardiovascular medications were evaluated.
Results
Covariate distributions were similar among individuals with and without self-reported CVD. The incidence of DM among persons with self-reported CVD was 15.3% vs 12.7% among those without self-reported CVD. Compared to individuals without self-reported CVD, individuals with self-reported CVD had a 24% increased risk for incident DM (odds ratio = 1.24, 95% confidence interval = 1.01, 1.51). The association between self-reported CVD and DM was mediated by the use of beta-blockers (proportion explained = 25.4%), statins (proportion explained = 18%), and diuretics (proportion explained = 8%). We found that weight gain did not explain the observed association.
Conclusions
Prevalent cardiovascular disease was associated with a significant increased risk of incident diabetes. The observed association was partially explained by some medications used to manage CVD.
摘要
zh背景
已有研究报道流行的心血管疾病(CVD)与糖尿病(DM)风险之间存在关联。然而, 可能解释这种联系的因素仍不清楚。我们分析了流行的心血管疾病与糖尿病发病的关系, 并评估了体重增加和药物使用是否可以解释这种联系。
方法
使用西班牙裔社区健康研究/拉丁裔研究(HCHS/SOL) 2008-2011及2014-2017的数据, 比较前者中有和没有自我报告心血管疾病个体的糖尿病发病率。共有1899名自我报告心血管疾病的患者, 采用1:1倾向得分匹配的方法与无自我报告心血管疾病的对照组进行配对。倾向性模型中的协变量包括社会人口学特征, 生活方式因素, 并发症情况和研究地点。使用通用的估计方程来检验自我报告的心血管疾病对糖尿病的影响。评估体重增加和心血管药物使用的中介作用。
结果
在有和没有自我报告的心血管疾病的个体中, 协变量分布是相似的。自报心血管病患者的糖尿病发病率为15.3%, 非自报心血管病患者的糖尿病发病率为12.7%。与没有自我报告心血管疾病的个体相比, 有自我报告心血管疾病的个体发生糖尿病的风险增加24%(优势比=1.24, 95%可信区间=1.01, 1.51)。自我报告的心血管疾病和糖尿病之间的联系是通过使用β-受体阻滞剂(解释比例=25.4%), 他汀类药物(解释比例=18%)和利尿剂(解释比例=8%)来调节的。我们发现体重增加并不能解释观察到的关联。
结论
常见的心血管疾病与糖尿病发病风险显著增加相关。一些用于治疗心血管疾病的药物部分解释了观察到的关联。
1 INTRODUCTION
The past two decades have seen a dramatic increase in the number of people with diabetes mellitus (DM) worldwide. In 2010, there were 150-220 million people with DM worldwide, and this number is expected to reach 300 million by 2025.1, 2 As of 2018, estimates showed that 34.2 million US residents had DM,3 and the prevalence of DM in the United States is expected to increase 120% by 2050.4 The increasing prevalence of DM over the next decades is alarming and calls for public health attention.
DM is a well-established and widely recognized risk factor for cardiovascular disease (CVD). However, studies suggest that the relationship may be reciprocal, that is, that CVD also increases the risk of developing DM.5, 6 The mechanisms underlying the latter association are not well understood. Several posited mechanisms for the association of CVD with DM include gluconeogenesis and glycogenolysis resulting from neurohormonal activation usually present in CVD, catecholamine-induced increase insulin resistance, shared inflammatory pathways in CVD and DM, and physical inactivity.6 None of these hypothesized explanations have been formally tested and several other mechanisms may be involved as well. For instance, medications used for secondary prevention of CVD might sometimes have unintended health consequences.7-9 Prior mechanistic, clinical, and epidemiologic research suggests that commonly used pharmacological agents such as beta-blockers, statins, and diuretics may cause metabolic derangements leading to increased risk for incident DM but findings have been inconclusive.7-9 In addition, studies have reported higher risk for incident DM after coronary artery bypass graft surgery or transplant.10, 11 Furthermore, the physical impairment associated with CVD may limit the capacity of people with CVD to engage in physical activity12 and promote weight gain,13 which are both associated with elevated risk of DM. The extent to which the association of CVD with incident DM can be attributed to weight gain or cardiovascular medications is not known.
In the current study, we analyzed data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), using propensity score (PS) methods to test the hypothesis that CVD was associated with increased risk of incident DM. We further investigated whether this association was explained by weight gain or the use of beta-blockers, statins, and diuretics. This analytical framework may provide supportive evidence for the association of CVD with subsequent DM and elucidate potential underlying mechanisms.
2 METHODS
2.1 Study population
The HCHS/SOL is a population-based longitudinal study in the United States that enrolled participants between 2008 and 2011.14 Participants were self-identified Hispanic/Latino individuals aged 18-74 years at baseline, randomly selected from households in the Bronx, New York; San Diego, California; Chicago, Illinois; and Miami, Florida. A stratified, two-stage sampling method was used to select households. The design oversampled individuals aged 45 to 74 years. A total of 16 415 participants were enrolled in the study. Each participating institutional review board approved the study, and written informed consent was obtained from all participants.
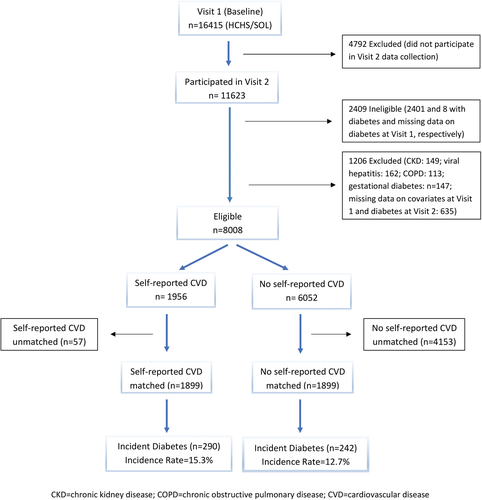
We restricted our study population to individuals without DM at baseline (Visit 1) and who participated in Visit 2 data collection. Of the 16 415 participants at Visit 1, we excluded 4792 (29.2%) individuals who did not participate in the Visit 2 data collection. Of those who participated in Visit 2 data collection (N = 11 623), we excluded 2401 with diabetes and 8 with missing data on diabetes at Visit 1. We further excluded 149 individuals with chronic kidney disease (defined as estimated glomerular filtration rate <60 mL/min/1.73 m2), 162 individuals seropositive for hepatitis C virus or hepatitis B virus, 113 individuals who self-reported chronic obstructive pulmonary disease (diagnosed by a doctor), 147 individuals with history of gestational diabetes, and 635 individuals because of missing information on covariables at Visit 1 and diabetes at Visit 2, thus yielding 8008 eligible individuals (Figure 1). We restricted our main analysis to a subset of 3798 individuals: 1899 individuals who self-reported prior CVD at baseline, and 1899 individuals who did not report CVD but had similar probability or propensity to self-report CVD at baseline, as described later. This approach was taken to address differential loss to follow-up because 3163 (31.6%) participants free of self-reported CVD were lost to follow-up compared to 798 (25.4%) participants with self-reported CVD (P < 0.0001). By matching, we created a control group of individuals without self-reported CVD that are comparable to individuals with self-reported CVD with respect to observed covariates.
2.2 Exposure and outcome assessment
Self-reported CVD at baseline (Visit 1) was defined as the presence of self-reported coronary heart disease, cerebrovascular events, peripheral artery disease, or heart failure. Incident DM at 6-year follow-up (visit 2) was defined by any of the following American Diabetes Association criteria: fasting time >8 hours and fasting blood glucose of 126 mg/dL or greater; fasting time less than 8 hours and fasting glucose of 200 mg/dL or greater; post-oral glucose tolerance test glucose of 200 mg/dL or greater; hemoglobin A1C of 6.5% or greater; or use of antihyperglycemic medications.15
2.3 Covariables
At HCHS/SOL Visit 1, sociodemographic data including age, sex, and Hispanic/Latino background were self-reported. Additional self-reported information assessed at Visit 1 included smoking status, the average number of cigarettes smoked per day, alcohol use, family history of diabetes, and 24-hour dietary recalls. Blood pressure, height, weight, and waist circumference were ascertained on physical examination. Physical activity was assessed using Actical accelerometers and categorized as high, moderate, and low. Venous blood specimens were also collected at Visit 1 and analyzed to measure serum creatinine, blood glucose, hemoglobin A1C, total and high-density lipoprotein (HDL) cholesterol, and triglycerides. Alternate Healthy Eating Index 2010 (AHEI-2010), a measure of dietary quality, was calculated from the 24-hour dietary recalls.16 Height and weight were used to calculate body mass index as weight (in kilograms) divided by height (in meters) squared. Hypertension was defined as systolic blood pressure 140 mm Hg or greater, diastolic blood pressure 90 mm Hg or greater, or use of antihypertensive medications.17 Prediabetes was defined as fasting time >8 and fasting blood glucose in range 100-125 mg/dL, or post-oral glucose tolerance test glucose in range 140-199 mg/dL, or 5.7% ≤ A1C < 6.5%. The diagnosis of metabolic syndrome was made when three or more of the following factors were present: hypertension, triglyceride ≥150 mg/dL, low HDL (HDL cholesterol <40 mg/dL in men and <50 mg/dL in women), fasting blood glucose ≥100 mg/dL, and waist circumference ≥102 cm in men and ≥88 cm in women.18
2.4 Mediating variables
We examined whether the association between self-reported CVD and incident DM was explained by medication classes such as beta-blockers, statins, and diuretics as well as by weight gain. HCHS/SOL obtained information on medications taken in the past 4 weeks before the baseline examination. Participants were asked to bring in all prescribed or over-the-counter medications. The medication information was used to uniquely identify drug products based on their generic ingredients and then assigned to their medication classes. Data were also gathered on weight at both Visit 1 and Visit 2, and weight gain was defined as the difference in weight between the two visits.
2.5 Selection of the analytical sample
We matched HCHS/SOL participants based on their probability or propensity to self-report CVD at baseline. The PS is the conditional probability of being exposed (eg, CVD) given a vector of measured covariates and can be used to adjust for selection bias when assessing causal effects in observational studies.19 We estimated the PS for self-reported CVD for each participant using a multivariable logistic regression model, in which self-reported CVD was modeled using all baseline covariates in Table 1, as well as interaction effects. We then used the estimated PS to match participants without self-reported CVD to participants with self-reported CVD who had very similar PS using greedy algorithms (nearest best match).20 Under the greedy algorithms, matches with the highest digit on PS are the best ones and selected first, and then the next-best matches are selected in a sequential process until no further matches can be made.20 In our matching algorithm, we first attempted to match each individual without self-reported CVD with a self-reported CVD individual who had a similar PS to eight decimal places. Then we removed those matched pairs of individuals and repeated the process matching to seven, six, five, four, three, two, and one decimal places.
| Baseline characteristics | Before matching (n = 8008) | After matching (n = 3798) | ||||
|---|---|---|---|---|---|---|
| Self-reported CVD | Self-reported CVD | |||||
| Yes (n = 1956) | No (n = 6052) | Standardized difference (%) | Yes (n = 1899) | No (n = 1899) | Standardized difference (%) | |
| Age, years, mean (SD) | 52.3 (10.4) | 42.7 (13.1) | 81.7 | 52.0 (10.3) | 51.9 (10.7) | 0.6 |
| Sex (male), n (%) | 607 (31.0) | 233 (38.6) | −15.9 | 593 (31.2) | 697 (36.7) | −11.6 |
| Hispanic/Latino Background, n (%) | ||||||
| Dominican 0 | 243 (12.4) | 478 (7.9) | 15.0 | 231 (12.2) | 232 (12.2) | −0.2 |
| Central American 1 | 192 (9.8) | 689 (11.4) | −5.1 | 190 (10.0) | 208 (10.9) | −3.1 |
| Cuban 2 | 279 (14.3) | 897 (14.8) | −1.6 | 274 (14.4) | 259 (13.6) | 2.3 |
| Mexican 3 | 661 (33.8) | 2670 (44.1) | −21.3 | 654 (34.5) | 670 (35.3) | −1.8 |
| Puerto Rican 4 | 396 (20.2) | 650 (10.7) | 26.5 | 367 (19.3) | 326 (17.2) | 5.6 |
| South American 5 | 142 (7.3) | 485 (8.0) | −2.8 | 141 (7.4) | 157 (8.3) | −3.1 |
| More than one/Other heritage 6 | 43 (2.2) | 183 (3.0) | −5.2 | 42 (2.2) | 47 (2.5) | −1.7 |
| Cigarette use, n (%) | ||||||
| Never | 1195 (61.1) | 4059 (67.07) | −12.5 | 1140 (60.0) | 1163 (61.24) | 2.5 |
| Former | 399 (20.4) | 988 (16.33) | 10.5 | 425 (22.4) | 384 (20.22) | −5.3 |
| Current | 362 (18.5) | 1005 (16.61) | 5.0 | 334 (17.6) | 352 (18.54) | 2.5 |
| Cigarette pack years, mean (SD) | 6.2 (13.7) | 3.8 (10.6) | 19.5 | 6.1 (13.5) | 6.5 (15.1) | −3.2 |
| Alcohol use, n (%) | ||||||
| Never | 375 (19.17) | 1223 (20.21) | −2.6 | 370 (19.5) | 328 (17.3) | 5.7 |
| Former | 689 (35.22) | 1786 (29.51) | 12.2 | 660 (34.7) | 666 (35.1) | −0.7 |
| Current | 892 (45.60) | 3043 (50.28) | −9.4 | 869 (45.8) | 905 (47.6) | −3.8 |
| Physical activity level, n (%) | ||||||
| Low | 655 (10.8) | 178 (9.1) | −5.7 | 178 (9.4) | 185 (9.7) | −1.3 |
| Moderate | 2807 (46.4) | 834 (42.6) | −7.5 | 811 (42.7) | 861 (45.4) | −5.3 |
| High | 2590 (42.8) | 944 (48.3) | 11.0 | 910 (47.9) | 853 (44.9) | 6.0 |
| AHEI-2010, mean (SD) | 49.7 (7.5) | 48.9 (7.5) | 10.8 | 49.7 (7.6) | 50.1 (7.5) | −4.4 |
| Family history of diabetes, n (%) | 910 (46.5) | 2382 (39.4) | 14.5 | 881 (46.4) | 851 (44.8) | 3.2 |
| Metabolic syndrome, n (%) | 696 (35.6) | 1547 (25.6) | 23.6 | 692 (36.4) | 681 (35.9) | 1.2 |
| Hypertension, n (%) | 652 (33.3) | 1000 (16.5) | 39.6 | 613 (32.3) | 614 (32.3) | −0.1 |
| High total cholesterol, n (%) | 876 (44.8) | 2318 (38.3) | 13.2 | 847 (44.6) | 828 (43.6) | 2.0 |
| Prediabetes, n (%) | 1188 (60.7) | 2773 (45.8) | 30.2 | 1146 (60.4) | 1162 (61.2) | −1.7 |
| BMI categories, n (%) | ||||||
| Underweight (BMI < 18.5) | 14 (0.7) | 48 (0.8) | −0.9 | 13 (0.7) | 17 (0.9) | −2.4 |
| Normal (18.5 ≤ BMI < 25) | 333 (17.0) | 1318 (21.8) | −12.0 | 323 (17.0) | 400 (21.1) | −10.3 |
| Overweight (25 ≤ BMI < 30) | 737 (37.7) | 2479 (40.9) | −6.7 | 726 (38.2) | 674 (35.5) | 5.7 |
| Obese I (30 ≤ BMI < 35) | 523 (26.8) | 1474 (24.4) | 5.5 | 511 (26.9) | 525 (27.6) | −1.6 |
| Obese II (35 ≤ BMI < 40) | 251 (12.8) | 486 (8.0) | 15.8 | 235 (12.4) | 207 (10.9) | 4.6 |
| Obese III (BMI ≥ 40) | 98 (5.0) | 247 (4.1) | 4.5 | 91 (4.8) | 76 (4.0) | 3.8 |
| Hemoglobin A1C, mean (SD) | 5.6 (0.4) | 5.5 (0.3) | 32.7 | 5.6 (0.4) | 5.6 (0.4) | 4.0 |
- Note: Standardized difference is the mean difference divided by the pooled SD, expressed as percentage. A standardized difference > 10% is suggestive of a meaningful covariate imbalance.
- Abbreviations: AHEI 2010, Alternate Healthy Eating Index; BMI, body mass index; CVD, cardiovascular disease; HCHS/SOL, Hispanic Community Health Study/Study of Latinos.
2.6 Assessment of baseline covariate balance
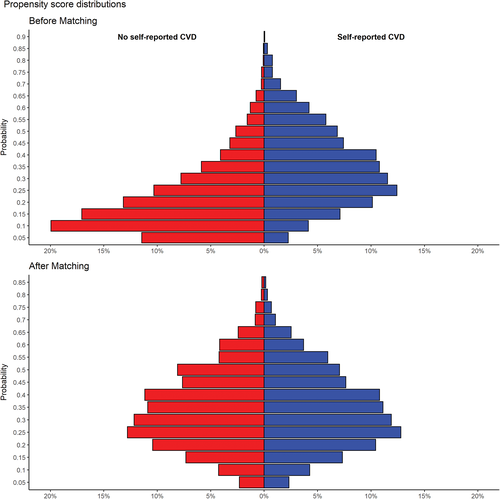
We compared the balance of all baseline covariates in Table 1 between individuals with and without self-reported CVD before and after PS matching using the standardized differences. A standardized difference greater than 10% is suggestive of a meaningful covariate imbalance.21 We also used the standardized difference to compare the mean PS score before and after matching, which directly quantifies the bias in the mean PS across the two groups (individuals with and without self-reported CVD), expressed as a percentage of the pooled SD. Before matching, the mean PS for people without self-reported CVD (n = 6052) was 0.193 (SD = 0.142) and in those with self-reported CVD (n = 1956) was 0.328 (SD = 0.163), with an associated standardized difference of 87.8% (t test P value, <0.0001). After matching, the mean PS for individuals without self-reported CVD (n = 1899) was 0.318 (SD = 0.154) and for those with self-reported CVD (n = 1899) was 0.319 (SD = 0.155), which yields a standardized difference of 0.7% (t test P-value 0.81).
2.7 Statistical analysis
A generalized estimating equation (GEE) was used to calculate the odds ratio (OR) and corresponding 95% confidence intervals (CIs) for the association between self-reported CVD and incident DM. This method allowed accounting for the lack of independence induced by PS matching. To assess the robustness of our findings, we analyzed data from the unmatched sample (N = 8008) by performing weighted logistic regression adjustment to estimate the association of self-reported CVD with incident DM; these analyses adjusted for covariates used in the logistic regression model for PS. Because individuals were matched regardless of their sampling units, analyses for the matched data were not weighted. We also evaluated the association between self-reported CVD and incident DM among males and females. The interaction between sex and self-reported CVD was assessed. Finally, we examined the associations between CVD subtypes (heart failure and myocardial infarction) and incident DM.
We further examined whether the association between self-reported CVD and incident DM was explained by either weight gain or cardiovascular medication classes such as beta-blockers, statins, and diuretics. For each mediating factor, we decomposed the total effect into two different pathways: (a) the effect of self-reported CVD on DM through the mediating pathway (ie, the natural indirect effect) and (b) the effect of self-reported CVD on DM that is not through the mediating pathway (ie, the natural direct effect). For each mediator, we determined the proportion mediated by calculating the ratio of the log indirect effect and the log total effect. The mediation analysis was conducted using semiparametric methods.22, 23 For each medication class, we estimated the total effect by regressing DM on self-reported CVD using GEE model accounting for matching and follow-up time between Visit 1 and Visit 2. To estimate the natural direct effect of self-reported CVD, we regressed DM on self-reported CVD using GEE model in a subsample of individuals who were not on that medication (individuals who were not on beta-blockers, statins, and diuretics were 3486, 3437, and 3377, respectively). Restricting the analysis to medication free individuals ensured that we isolated the effect of self-reported CVD that is not mediated by the use of that medication (ie, direct effect). Finally, the natural indirect effect was determined by subtracting the direct effect from the total effect.22, 23 All analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
3 RESULTS
The mean (+SD) age of the 3798 PS-matched individuals was 52 (SD = 10.5) years (median: 53; range: 18-74), and 34.0% were males. Table 1 compares baseline characteristics by CVD status before and after PS matching. Before matching, individuals with self-reported CVD were more likely to be female and older as compared to individuals without self-reported CVD. Individuals with self-reported CVD were more likely to have DM risk factors including prediabetes, metabolic syndrome, obesity, family history of DM, high cholesterol, and cigarette smoking at baseline. They more often had hypertension at baseline. Of the 1956 individuals with self-reported CVD and 6052 individuals without self-reported CVD at baseline, 296 (15.1%) and 581 (9.6%) developed incident DM respectively at 6-year follow-up (P < 0.0001).
After matching, individuals with and without self-reported CVD were very similar with regards to baseline covariates (Table 1). The standardized difference for the mean PS was 0.7% in absolute value, thus demonstrating an excellent balance in measured covariates across the two groups. The distributions of the PS score for individuals with and without self-reported CVD matched were nearly identical (Figure 2). In the matched cohort, two-thirds of beta-blocker users and about three in five statin and diuretics users self-reported CVD (Table 2). Significantly higher proportions of people who were on beta-blockers, statins, or diuretics at baseline developed DM at 6-year follow-up (beta-blockers and statins, P < 0.0001; diuretics, P = 0.0043) (Table 2).
| Self-reported CVD at Visit 1 (baseline) | Incident diabetes at Visit 2 (Follow-up) | |||||
|---|---|---|---|---|---|---|
| Medications | Yes | No | P value | Yes | No | P -value |
| Beta-blocker use, n (%) | <0.0001 | <0.0001 | ||||
| Yes | 216 (69.2) | 96 (30.8) | 69 (22.1) | 243 (77.9) | ||
| No | 1645 (48.3) | 1762 (51.7) | 451 (13.2) | 2956 (86.8) | ||
| Statin use, n (%) | <0.0001 | <0.0001 | ||||
| Yes | 224 (62.1) | 137 (37.9) | 75 (20.8) | 286 (79.2) | ||
| No | 1637 (48.7) | 1721 (51.2) | 445 (13.3) | 2913 (86.7) | ||
| Diuretics use, n (%) | 0.0118 | 0.0043 | ||||
| Yes | 235 (55.8) | 186 (44.2) | 78 (18.5) | 343 (81.5) | ||
| No | 1626 (49.3) | 1672 (50.7) | 442 (13.4) | 2856 (86.6) | ||
- Note: Seventy-nine matched individuals had missing information on medication use. Row percentages are reported.
- Abbreviation: CVD, cardiovascular disease.
At 6-year follow-up, 290 (15.3%) of the 1899 individuals with self-reported CVD and 242 (12.7%) of the 1899 individuals without self-reported CVD in the propensity-matched cohort developed incident DM (Figure 1). Compared to individuals without self-reported CVD, individuals with self-reported CVD had a 24% increased risk for incident DM (OR = 1.24, 95% CI = 1.01, 1.51) after adjusting for baseline hemoglobin A1C (Table 3). When we adjusted for baseline covariables in the full (unmatched) cohort (n = 8008), we observed similar but nonsignificant effect of CVD on incident DM (OR = 1.22, 95% CI = 0.93, 1.58). We found no evidence of interaction between self-reported CVD and sex (P = 0.4273). The data showed that individuals with myocardial infarction and heart failure had increased odds for incident DM, but the associations were not significant (myocardial infarction: OR = 1.45, 95% CI = 0.87, 2.41; heart failure: OR = 1.33, 95% CI = 0.71, 2.48).
| Propensity-matched cohort (n = 3798) | Overall cohort (n = 8008) | |||||
|---|---|---|---|---|---|---|
| Model 1a | Model 2a | Model 1b | Model 2b | Model 3b | ||
| CVD | ||||||
| All | 1.23 (1.03, 1.48) | 1.24 (1.01, 1.51) | 2.15 (1.71, 2.69) | 1.30 (1.01, 1.69) | 1.22 (0.93, 1.58) | |
| Male | 1.32 (0.97, 1.79) | 1.36 (0.98, 1.89) | 2.45 (1.70, 3.55) | 1.54 (1.03, 2.29) | 1.49 (0.98, 2.28) | |
| Female | 1.20 (0.96, 1.51) | 1.16 (0.91, 1.49) | 1.94 (1.48, 2.53) | 1.10 (0.79, 1.54) | 1.05 (0.76, 1.44) | |
| Heart failure | 1.21 (0.68, 2.17) | 1.33 (0.71, 2.48) | 2.05 (0.99, 4.23) | 1.57 (0.66, 3.75) | 1.60 (0.70, 3.65) | |
| Myocardial infarction | 1.43 (0.89, 2.29) | 1.45 (0.87, 2.41) | 2.04 (1.13, 3.67) | 1.22 (0.58, 2.53) | 1.15 (0.55, 2.39) | |
| Mediators | Proportion explained (%) | |||||
| Beta-blockers | 25.4 | |||||
| Statins | 18.0 | |||||
| Diuretics | 8 | |||||
- Note: Heart failure: n = 93 (propensity-matched cohort); n = 309 (overall cohort). Myocardial infarction: n = 134 (propensity-matched cohort); n = 384 (overall cohort). Model 1a and Model 1b: Adjusted for follow-up time between Visit 1 and Visit 2. Model 2a: Adjusted for follow-up time between Visit 1 and Visit 2, sex, and baseline hemoglobin A1c. Model 2b: Adjusted for follow-up time between Visit 1 and Visit 2, age, sex, and baseline hemoglobin A1C. Model 3b: Adjusted for follow-up time between Visit 1 and Visit 2, age, sex, Hispanic/Latino background, cigarette smoking, alcohol use, physical activity level, healthy eating index (AHEI-2010), body mass index, family history of diabetes, hypertension, high cholesterol, metabolic syndrome, prediabetes, and baseline hemoglobin A1C. Interaction sex and CVD: P = 0.4273 (matched cohort).
- Abbreviations: AHEI 2010, Alternate Healthy Eating Index; CVD, cardiovascular disease.
Our results showed evidence of mediation of the effect of self-reported CVD through a pathway involving cardiovascular medication use (Table 3). The largest indirect effect was for beta-blockers (proportion explained = 25.4%), followed by statins (proportion explained = 18%), and diuretics (proportion explained = 8%). Weight gain did not appear to explain the excess risk of DM associated with self-reported CVD: from baseline to the six-year follow-up visit, individuals with self-reported CVD had a mean weight gain (in kilograms) that was similar to that observed in individuals without self-reported CVD (0.87 ± 6.66 and 0.80 ± 6.61, respectively, P = 0.72).
4 DISCUSSION
Our findings provide evidence that CVD is associated with increased risk of DM. This study showed that the association between CVD and incident DM was partially explained by cardiovascular medications such beta-blockers, statins, and diuretics.
Both PS matching and regression adjustment method showed similar results for the association between CVD and incident DM, which was not significant for the latter. Methods based on PS have theoretical advantages over traditional regression methods used to adjust for baseline differences between exposed and nonexposed groups in observational studies.24, 25 A common concern in covariate adjustment methods is that such models might be overfitted when there are too many covariates. Furthermore, the statistical literature argues that covariate adjustment methods cannot reliably adjust for differences in observed covariates across groups when the differences in the distribution of these covariates are substantial.24, 25 In our study, individuals with and those without self-reported CVD (full sample) were considerably different as evidenced by the approximatively 87.8% standardized difference in their PS. Thus, individuals with and those without self-reported CVD markedly differed on baseline characteristics and regression adjustment may not reliably account for these differences because the fitted regression model might be interpolating between two nearly distinct groups.
A similar relationship between CVD and incident DM has been found in other studies. A previous study reported that individuals with CVD and without comorbid DM have 35% increased risk of subsequently developing incident DM after controlling for other risk factors.5 A large cohort study followed a population free of diabetes after acute cardiovascular event and found a significant increased risk of incident DM associated with heart failure, with the strength of the association proportionate to the severity of heart failure.26 The same study reported that mild, moderate, and severe heart failure was associated with 34%, 63%, and 68% increased risk of incident DM, respectively. Findings from other studies also concur with the results of the current study, showing that CVD significantly increases the risk of incident DM.6, 27
Several mediators in the pathway between self-reported CVD and incident DM were examined in this study. Our results showed that the observed association between self-reported CVD and incident DM was partly attributed to medications used in the management of CVD. Beta-blockers, statins, and diuretics accounted for 25.4%, 18%, and 8% of the association between self-reported CVD and incident DM, respectively. We also noted that weight gain did not explain the association between self-reported CVD and incident DM. These findings are important as beta-blockers, diuretics, and statins remain among the most commonly used medication classes for treating CVD.
The mediating effects of beta-blockers, statins, and diuretics on incident DM are consistent with previous studies reporting a significantly greater risk of DM with these pharmacological agents. Previous studies have linked new-onset DM to the use of beta-blockers7 and diuretics.8 A 3- to 6-year follow-up study has reported a 28% increased risk for incident DM among individuals receiving beta-blocker therapy.7 A meta-analysis has found that atenolol and metoprolol are associated with a 30% and 32% increased risk for incident DM, respectively.28 Diuretics were found to increase the risk of incident DM by 23%.8 Statin-based therapy was associated with a 9% increased risk of incident DM in pooled data from 13 trials.29 Other studies found no association between beta-blockers,8 diuretics,7 and statins30 and subsequent development of DM. The lack of association observed in those studies may be explained by the type of study designs, the type of beta-blocker used, dosages, and duration of follow-up. The results of this study also showed that the use of beta-blockers was a stronger mediator for incident DM than the use of statins and diuretics. This is consistent with prior research reporting that the association between beta-blockers and incident DM is greater than that for other antihypertensive agents.7 We found that the mediating effect of statins appears greater than that of diuretics. A previous study examining the diabetogenic effects of statins and diuretics has found a stronger effect of statins than diuretics.8
The mediating effect of beta-blockers on incident DM is biologically plausible. Insulin release from the pancreas is regulated by the autonomic nervous system.31 Thus, pharmacological agents that affect this system can affect insulin secretion. Beta-blockers can potentially inhibit beta-2 receptors involved in regulation of pancreatic autonomic pathways, thus attenuating insulin release from the pancreas. Studies have reported that propranolol infusion caused decrease in insulin secretion.32, 33 Beta-blockers have also been reported to decrease insulin sensitivity.34 The mechanisms underlying the increased risk of DM associated with the use of statins and diuretics are not fully understood. Hypotheses for statin-induced DM have focused on statin-induced insulin resistance, inhibition of insulin secretion and synthesis, and decreased insulin-mediated cellular glucose uptake.35, 36 The mechanism for diuretics-induced DM has been postulated to result from diuretics-induced hypokalemia leading to decreased insulin secretion and/or decreased insulin sensitivity.37, 38 However, a study has reported no association between diuretics-induced change in potassium and blood glucose.39
This study has several limitations. First, data were not available on doses of drugs, medication adherence, and the duration of treatment. Second, we did not examine differences in incident DM rates across specific types of beta-blockers and diuretics (eg, cardio-selective vs nonselective beta-blockers, thiazides vs other diuretics) because the information for these classifications was not available. Third, the present study might also be subject to self-report bias because CVD was self-reported. Fourth, the results of this study might be because of residual confounding related to unmeasured or hidden covariates. It is unlikely that a hidden covariate related to both CVD and DM could exist and be completely unrelated to any of the covariates used in our PS model. Furthermore, such confounding is unlikely to explain our results given that beta-blockers, statins, and diuretics have generally been thought to increase the risk of DM. Fifth, inadequate matching might also affect the findings of this study; however, individuals with and without self-reported CVD in our analytic sample were nearly identical with a mean PS difference of 0.7% in absolute value. Sixth, although the exclusion of unmatched individuals might have compromised to some degree the external validity of the results presented in this study, we believe that our matching procedure improves internal validity.
Notwithstanding these limitations, the findings from this study add to the growing concern that beta-blockers, statins, and diuretics may be diabetogenic. These drugs are recommended for secondary prevention of CVD because of their proven benefits. The increased risk of DM associated with the use of these drugs should not discourage clinicians from prescribing them, but rather, should be weighed against their proven cardiovascular benefits.
Our results suggest that the excess risk of on incident DM associated with self-reported CVD was not completely explained by the three potential mediators identified in our analysis. Thus, the results highlight the need for investigation of other factors that might account for the unexplained risk. Furthermore, additional studies are needed to more fully understand the mechanisms by which beta-blockers, diuretics, and statins increase DM risk.
5 CONCLUSIONS
Using data from the HCHS/SOL, we found that the association between CVD and incident DM was partly attributed to beta-blockers, statins, and diuretics. The current study suggests that further research is needed to examine the risk/benefit ratio of using these medications for their known cardiovascular benefit vs the potential risk for incident diabetes.
ACKNOWLEDGEMENTS
We would like to thank the staff and participants of HCHS/SOL for their important contributions. We would also like to thank the HCHS/SOL Publications Committee for reviewing our manuscript for scientific content and consistency of data interpretation with previous HCHS/SOL publications. Celestin Missikpode was supported by the National Heart, Lung, and Blood Institute (NHLBI) (T32-HL125294). The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the NHLBI to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes/centers/offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, National Institutes of Health Institution-Office of Dietary Supplements.
DISCLOSURES
Krista M Perreira is a co-investigator on the HCHS/SOL grant from NHLBI.




