
© 2020 by Ruijin Hospital, Shanghai Jiaotong University School of Medicine and John Wiley & Sons Australia, Ltd. All rights reserved.
This article is being provided to you free to access and is subject to Wiley’s Article Sharing Guidelines for subscription articles (Version of Record).
For permission to re-use or reproduce this content, please visit this link
Sleep disorders and risk of dementia in patients with new-onset type 2 diabetes: A nationwide population-based cohort study
新发2型糖尿病患者的睡眠障碍和痴呆风险:一项基于全国人群的队列研究
Funding information: The National Research Foundation of Korea, Grant/Award Number: 2020R1I1A1A01053104; The Yonsei University Research Fund (Post Doc. Researcher Supporting Program) of 2019, Grant/Award Number: 2019-12-0129
Abstract
enBackground
This study examined the relationship between sleep disorders and the risk of dementia in patients with newly diagnosed type 2 diabetes.
Methods
This study used the Korean Health Screening Cohort data and included 39 135 subjects aged ≥40 years with new-onset type 2 diabetes between 2004 and 2007, with follow-up throughout 2013. Sleep disorders were measured by F51(sleep disorders not due to a substance or known physiological condition) or G47(sleep disorders) under International Classification of Diseases, Tenth Revision (ICD-10) codes as a primary diagnosis, and the adjusted hazard ratio (AHR) and 95% CI of all-cause dementia, Alzheimer disease, vascular dementia, and other dementia were estimated using multivariable Cox proportional hazards regression models.
Results
In the patients with type 2 diabetes with an age range between 42 and 84 years (M = 57.8, SD = 9.5), this study identified 2059 events of dementia during an average follow-up time of 5.7 years. In patients with type 2 diabetes, subjects with sleep disorders were associated with an increased risk of all-cause dementia (AHR, 1.46; 95% CI, 1.19-1.80), Alzheimer disease (AHR, 1.39; 95% CI, 1.02-1.88), and other dementia (AHR, 1.69; 95% CI, 1.23-2.31) compared to those without sleep disorders. Men (AHR, 1.93; 95% CI, 1.42-2.62) and older adults (AHR, 1.70; 95% CI, 1.35-2.15) with sleep disorders were associated with an increased risk of dementia than their counterparts without sleep disorders among patients with type 2 diabetes.
Conclusions
These findings suggest that sleep disorders are significantly associated with an increased risk of dementia in patients with new-onset type 2 diabetes.
摘要
zh背景
本研究探讨了新发2型糖尿病患者睡眠障碍与痴呆风险之间的关系。
方法
本研究使用韩国健康筛查队列数据,纳入了2004年至2007年之间39135名年龄≥40岁的新发2型糖尿病患者,并在2013年进行了随访。根据国际疾病分类(ICD-10)代码作为主要诊断依据F51(不是物质或已知生理状况引起的睡眠障碍)或G47(睡眠障碍)来测量睡眠障碍,并使用多变量Cox比例风险回归模型估算了全因痴呆、阿尔茨海默病、血管性痴呆和其他痴呆的调整危险比(adjusted hazard ratio ,AHR)和95%置信区间。
结果
在年龄42至84岁之间的2型糖尿病患者(M = 57.8,SD = 9.5)中,该研究确定了平均随访时间为5.7年的2059例痴呆事件。在2型糖尿病患者中,与没有睡眠障碍的人相比睡眠障碍患者与全因痴呆(AHR,1.46; 95%CI,1.19-1.80)、阿尔茨海默病(AHR,1.39; 95%CI,1.02-1.88),及其他痴呆症(AHR,1.69; 95%CI,1.23-2.31)的患病风险增加相关。患有睡眠障碍的男性2型糖尿病患者(AHR,1.93; 95%CI,1.42-2.62)和老年人(AHR,1.70; 95%CI,1.35-2.15)与没有睡眠障碍的患者相比,患有痴呆症的风险增加。
结论
这些发现表明睡眠障碍与新发2型糖尿病患者痴呆风险增加显著相关。
1 INTRODUCTION
Dementia is a broad category of diseases characterized by an irreparable decline of cognitive function,1 and it was estimated that the number of individuals with dementia in the world was approximately 47 million in 2015 and is expected to reach 136 million in 2050.2 Type 2 diabetes is related with cognitive dysfunction and the development of dementia.3, 4 This includes subtle cognitive changes, also referred to as diabetes-associated cognitive decrements, as well as an increased risk of severe stages of cognitive dysfunction, including mild cognitive impairment and dementia.5 The processes that account for the excess mild cognitive impairment and dementia risk in patients with type 2 diabetes are still largely unclear. Furthermore, since anti-dementia medications have shown limited efficacy in treating dementia6 and there is no particular treatment for dementia among patients with type 2 diabetes,7 it is important to explore potential risk factors for dementia in patients with type 2 diabetes.
Sleep disorders have a multifaceted impact on health and increase the risk of morbidity of physical and psychiatric diseases.8 Furthermore, sleep plays a significant role in cognitive processing, and sleep disorders can result in impairments of cognitive performance.9, 10 A few meta-analysis studies showed that sleep problems were associated to increased risk of all-cause cognitive disorders or dementia.11-13
Sleep disorders are common in patients with type 2 diabetes,14 and a previous study reported the high prevalence of sleep dysfunction in patients with type 2 diabetes and demonstrated that a high proportion (69%) of type 2 diabetics had a Pittsburgh Sleep Quality Index score ≥ 5, suggesting reduced and disturbed sleep.15 Although a cross-sectional study examined role of sleep in cognitive function of patients with prediabetes and type 2 diabetes,16 the cross-sectional design could not determine the direction of causality in the associations and only examined cognitive function, not dementia. The role of sleep disorders in dementia incidence of patients with type 2 diabetes is unclear, and more data are needed.17 This study hypothesized that sleep disorders were associated with an increased risk of dementia among patients with type 2 diabetes.
The present study therefore aimed to identify the relationship between sleep disorders and the risk of all-cause dementia in patients with newly diagnosed type 2 diabetes using data of a nationwide population-based cohort. This study also examined the incidence of subtypes of dementia (Alzheimer disease, vascular dementia, and other dementia) according to sleep disorders and explored the association between sleep disorders and risk of dementia stratified by sex and age group.
2 METHODS
2.1 Data and study sample
The Korean National Health Insurance Service-Health Screening Cohort (NHIS-HEALS) data were used in this study.18 The NHIS is the sole national insurance provider in South Korea, and almost all citizens are health insurance subscribers. All subscribers (≥40 years) of the national health insurance are required to receive standardized medical examinations biennially that include bioclinical laboratory results (eg, blood pressure [BP] and fasting glucose) and questionnaires (eg, smoking, alcohol drinking, and exercise). The NHIS-HEALS data were obtained from information of 2002 to 2003 health screening participators who were aged between 40 and 79 years and were followed up to 2013. Data were obtained by a simple random sampling method to construct a representative sample. This resulted in 514 866 health screening participators being randomly selected, a sample which constituted about 10% of all health screening participators in 2002 and 2003. The NHIS-HEALS data include demographics and clinical information about diagnoses, treatments, and prescribed medications for all visits to health care facilities (inpatient, outpatient, and pharmacy visits) of the cohort subjects. The death registration information (eg, dates and causes of deaths) of Statistics Korea was linked to the NHIS-HEALS. The proportion of men and the mean age (SD) of the sample are 54.2% and 52.6 years old (9.6), respectively.
Figure 1 shows the flowchart of the selection of the study participants. From 513 268 study participants from 2004 to 2007, this study included patients with newly diagnosed type 2 diabetes (n = 39 135); type 2 diabetes was defined as the existence of any one of the following criteria: (a) fasting blood glucose level ≥ 7 mmol/L (126 mg/dL), (b) at least one further diagnosis of type 2 diabetes within 6 months after the first date of the diagnosis under the International Classification of Diseases, Tenth Revision (ICD-10) codes (E11-E14), and (c) prescription of antidiabetes medication.
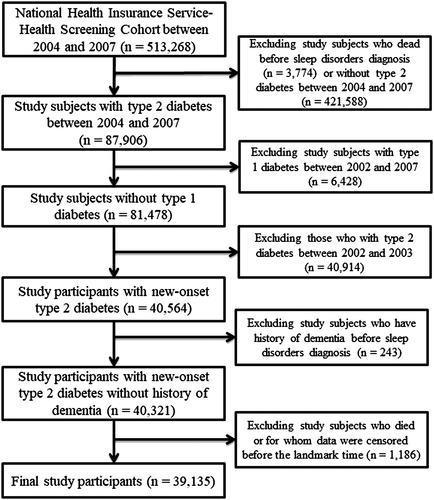
The exclusion criteria for study participants were the following: (a) death before sleep disorder diagnosis (n = 3774); (b) no type 2 diabetes, to recruit subjects having type 2 diabetes between 2004 and 2007 (n = 421 588); (c) type 1 diabetes between 2002 and 2007 (n = 6428); (d) type 2 diabetes between 2002 and 2003, to enroll individuals with new-onset type 2 diabetes (n = 40 914); (e) history of dementia before sleep disorder diagnosis, to minimize reverse causality (n = 243); and (f) census of study subjects before the landmark time (1 January 2008), to resolve a potential immortal time bias (n = 1186).
2.2 Measurement
The dependent variable in the present study was dementia, and dementia was estimated by ICD-10 codes (F00, F01, F02, F03, G30, and G31) as primary diagnosis.19 Dementia was further classified as Alzheimer disease (ICD-10 codes F00 and G30), vascular dementia (ICD-10 code F01), and other dementia (ICD-10 codes F02, F03, and G31), and the definition of dementia included inpatients as well as outpatients in the present data. This study used sleep disorders as independent variable, and the sleep disorders were measured by F51 (sleep disorders not due to a substance or known physiological condition) or G47 (sleep disorders) under ICD-10 codes as primary diagnosis.20 Table S1 presents the number of incidents of dementia according to subtypes of sleep disorders; 138 participants with sleep disorders were diagnosed with dementia during the follow-up period among 1059 subjects with sleep disorders, and insomnia accounted for approximately 76% of all sleep disorders in the present study.
This study measured sex, age, the number of medical visits from diagnosis of type 2 diabetes, body mass index (BMI), BP, total cholesterol, fasting glucose, hypoglycemia, family history of diabetes, exercise, smoking, heavy drinking, residential area, household income, and comorbidities as potential confounding factors. Sex, age, residential area, and household income were estimated using information on years, including index date.
Age was categorized into middle-aged adults (40 ~ 64 years), and older adults (≥ 65 years). The number of medical visits from diagnosis of type 2 diabetes was measured using all records for medical visits (inpatient and outpatient) from diagnosis of type 2 diabetes to final date. Household income was categorized as (a) low (<40th percentile), (b) middle (41th-80th percentile), or (c) high (81th-100th percentile). Residential area was categorized as metropolitan (capital), urban (local government where >1 million individuals live), or rural (otherwise). BMI, BP, total cholesterol, fasting glucose, family history of diabetes, exercise, smoking, and, heavy drinking were measured by information of national health screening closest to the index date. The recommendations of the World Health Organization for Asian populations were utilized to classify subjects into five BMI groups: <18.5 kg/m2 (underweight), 18.5 to 22.9 kg/m2 (normal), 23.0 to 24.9 kg/m2 (overweight), 25.0 to 29.9 kg/m2 (class I obese), or ≥ 30 kg/m2 (class II obese).21 Study subjects who consumed ≥30 g/d of alcohol were defined as heavy alcohol drinkers.22 Exercise was defined as doing exercise at least once a week. Smoking was categorized as nonsmoking, former smoking, or current smoking. Systolic and diastolic BP were estimated with the person seated following at least 5 minutes of rest. Blood samples were gained following overnight fasting to measure total cholesterol and serum glucose. Hypoglycemia was defined as a plasma glucose level less than 70 mg/dL.23 Comorbidities were estimated by screening before index date using medical records, and the comorbidities were composed of dyslipidemia (ICD-10: E78), hypertension (ICD-10: I10-I15), chronic kidney disease (ICD-10: N18), ischemic heart disease (ICD-10: I20-I25), stroke (ICD-10: I60-I63), cancer (ICD-10: C00-C99), and depressive disorders (ICD-10: F32-F33).
2.3 Statistical analysis
Sociodemographic and clinical characteristics in the study participants were compared by sleep disorders using a Pearson's chi-square test for categorical variables and an independent t test for continuous variables. Values are presented as number with percentage for categorical variables or as mean ± SD for continuous variables.
This study calculated adjusted hazard ratio (AHR) and 95% CI for the relationship of sleep disorders and risk of dementia using Cox proportional hazards regression models. Detection bias arises when patients in one exposure group have a higher probability of having the study outcome detected, due to increased surveillance, screening, or testing of the outcome itself, or an associated symptom. To reduce the detection bias, this study measured the number of medical visits per study subject from diagnosis of type 2 diabetes to final events and adjusted the number of medical visits in a Cox proportional hazards model. Since a potential immortal time bias could occur because of different index dates set for the two study groups, this study introduced landmark analysis to resolve immortal time bias or problems likely to occur by other length of follow-up between the two groups. All patients with type 2 diabetes regardless of having sleep disorders were followed up from the landmark time instead of the index date, and this study designated the landmark time as 1 January 2008 for all subjects. The length of follow-up was estimated in days, and all study subjects were followed up until incidence of dementia, withdrawal from the insurance system, death from any cause, or until the end of 2013, whichever occurred first. First, this study identified the effect of sleep disorders on the incidence of all-cause dementia in patients with newly diagnosed type 2 diabetes. Second, this study explored the association between sleep disorders and risk of subtypes of dementia (Alzheimer disease, vascular dementia, and other dementia). Finally, this study analyzed the relationship between sleep disorders and incidence of dementia stratified by sex and age group. Given the significant interaction effect of sleep disorders and BMI on dementia incidence, this study analyzed the association between sleep disorders and incidence of dementia by BMI and comorbidities (Tables S4 and S5).
All data extraction and statistical analyses were conducted using the SAS 9.4 software (SAS Institute Inc, Cary, North Carolina). Proportional hazards assumptions were assessed and satisfied for all models. Since this study used administrative cohort data, the study was exempt from ethical approval from the Yonsei University Institutional Review Board. The data used in the study are not sensitive, and there is no risk of disclosure of the identity of individuals as the NHIS-HEALS database was constructed after anonymization according to strict confidentiality guidelines.
3 RESULTS
This study recruited 39 135 patients with newly diagnosed type 2 diabetes aged ≥40 years and identified 2029 incidents of dementia during an average follow-up time of 5.7 ± 1.1 years. Table 1 presents the general characteristics of the study participants according to sleep disorders. The proportions of women (46.6% vs 39.5%), older adults (45.6% vs 25.5%), underweight (2.4% vs 1.6%), low household income (33.9% vs 31.6%), and comorbidities (hypertension [62.9% vs 44.5%], dyslipidemia [57.3% vs 29.9%], chronic kidney disease [1.7% vs 0.5%], ischemic heart disease [28.8% vs 14.9%], stroke [14.0% vs 5.0%], depressive disorders [30.3% vs 8.2%], and cancer [17.4% vs 7.2%]) of patients with sleep disorders were significantly higher compared to those without sleep disorders among patients with type 2 diabetes. The average of medical visits from diagnosis of type 2 diabetes (287 vs 195) of patients with sleep disorders was significantly higher compared to those without sleep disorders in patients with type 2 diabetes. The average of systolic (129.0 mm Hg vs 131.1 mm Hg) and diastolic (79.5 mm Hg vs 81.4 mm Hg) BP, and fasting glucose (114.0 mg/dL vs 117.2 mg/dL) and the proportions of current smoking (16.5% vs 21.6%) and exercise (34.7% vs 41.2%) of patients with sleep disorders were significantly lower compared to those without sleep disorders in patients with type 2 diabetes. There is no significant difference between subjects with sleep disorders and without sleep disorders in the average of total cholesterol (201.7 mg/dL vs 203.1 mg/dL), the proportions of family history of diabetes (6.1% vs 6.2%), heavy drinking (4.1% vs 5.5%), metropolitan as residential area (14.9% vs 15.5%), and hypoglycemia (0.5% vs 0.5%). Missing data for each confounding factor among study subjects have been presented in Table S2. The results using multiple imputations for missing data were similar to the main findings in this study (Table S3).
| Variables | Total | Sleep disorders | P value | |||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| N | % | N | % | |||
| Total | 39 135 | 1059 | 2.7 | 38 076 | 97.3 | |
| Women | 15 532 | 494 | 46.6 | 15 038 | 39.5 | <.001 |
| Age (years) | <.001 | |||||
| 40-64 | 28 951 | 576 | 54.4 | 28 375 | 74.5 | |
| ≥ 65 | 10 184 | 483 | 45.6 | 9701 | 25.5 | |
| Average number of medical visits from diagnosis of type 2 diabetes | 197.3 ± 168.5 | 287 | 221.6 | 195 | 166.0 | <.001 |
| BMI (kg/m2) | <.001 | |||||
| ≤18.5 | 623 | 25 | 2.4 | 598 | 1.6 | |
| 18.5-23 | 9584 | 305 | 28.8 | 9279 | 24.4 | |
| 23-25 | 9429 | 234 | 22.1 | 9195 | 24.1 | |
| 25-30 | 13 954 | 318 | 30.0 | 13 636 | 35.8 | |
| ≥30 | 1664 | 37 | 3.5 | 1627 | 4.3 | |
| BP (mm Hg) | ||||||
| Systolic | 131.1 ± 17.5 | 129.0 | 17.4 | 131.1 | 17.5 | <.001 |
| Diastolic | 81.4 ± 11.2 | 79.5 | 11.0 | 81.4 | 11.2 | <.001 |
| Fasting glucose (mg/dL) | 117.1 ± 42.9 | 114.0 | 46.2 | 117.2 | 42.8 | .035 |
| Total cholesterol (mg/dL) | 203.1 ± 39.7 | 201.7 | 40.8 | 203.1 | 39.6 | .283 |
| Family history of diabetes | 2408 | 65 | 6.1 | 2343 | 6.2 | .580 |
| Current smoking | 8411 | 175 | 16.5 | 8236 | 21.6 | .001 |
| Heavy drinking | 2143 | 43 | 4.1 | 2100 | 5.5 | .073 |
| Exercise | 16 060 | 368 | 34.7 | 15 692 | 41.2 | <.001 |
| Household income | .003 | |||||
| Low | 12 402 | 359 | 33.9 | 12 043 | 31.6 | |
| Middle | 14 793 | 347 | 32.8 | 14 446 | 37.9 | |
| High | 11 940 | 353 | 33.3 | 11 587 | 30.4 | |
| Residential area | .220 | |||||
| Metropolitan | 6054 | 158 | 14.9 | 5896 | 15.5 | |
| Urban | 10 647 | 267 | 25.2 | 10 380 | 27.3 | |
| Rural | 22 434 | 634 | 59.9 | 21 800 | 57.3 | |
| Hypoglycemia | 203 | 5 | 0.5 | 198 | 0.5 | .831 |
| Comorbidities | ||||||
| Hypertension | 17 627 | 666 | 62.9 | 16 961 | 44.5 | <.001 |
| Dyslipidemia | 12 006 | 607 | 57.3 | 11 399 | 29.9 | <.001 |
| CKD | 212 | 18 | 1.7 | 194 | 0.5 | <.001 |
| Ischemic heart disease | 5989 | 305 | 28.8 | 5684 | 14.9 | <.001 |
| Stroke | 2053 | 148 | 14.0 | 1905 | 5.0 | <.001 |
| Depressive disorders | 3444 | 321 | 30.3 | 3123 | 8.2 | <.001 |
| Cancer | 2939 | 184 | 17.4 | 2755 | 7.2 | <.001 |
- Note: Values are presented as mean ± SD or n (%).
- Abbreviations: BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease.
Table 2 shows the incidence of dementia per 10 000 person-years, and the incidence of all-cause dementia in subjects with sleep disorders (246.3) was higher than in those without sleep disorders (88.5). Table 2 also presents the AHR and 95% CI for risk of dementia by sleep disorders among patients with new-onset type 2 diabetes. After adjusting for sex, age, medical visits from diagnosis of type 2 diabetes, hypoglycemia, BP, BMI, fasting glucose, total cholesterol, family history of diabetes, current smoking, heavy drinking, exercise, household income, residential area, and comorbidities, patients with sleep disorders significantly were at increased risk of dementia (AHR, 1.46; 95% CI, 1.19-1.80) compared to those without sleep disorders in subjects with type 2 diabetes. In the results for subtypes of dementia of patients with type 2 diabetes, patients with sleep disorders were associated with increased risk of Alzheimer disease (AHR, 1.39; 95% CI, 1.02-1.88) and other dementia (AHR, 1.69; 95% CI, 1.23-2.31) compared to those without sleep disorders. However, there was no significant association between sleep disorders and vascular dementia (AHR, 1.03; 95% CI, 0.51-2.07). Hypoglycemia was associated with an increased risk of dementia (AHR, 1.33; 95% CI, 1.09-1.69) in subjects with type 2 diabetes.
| Variables | N | Events | Person-years | Incidence of dementia per 10 000 person-years | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||||||
| All-cause dementia | |||||||||
| Sleep disorders | |||||||||
| No | 38 076 | 1921 | 217 150 | 88.5 | 1.00 | ||||
| Yes | 1059 | 138 | 5603 | 246.3 | 1.76 (1.48-2.10)*** | 1.70 (1.39-2.09)*** | 1.46 (1.19-1.80)*** | ||
| AD | |||||||||
| Sleep disorders | |||||||||
| No | 38 076 | 947 | 217 150 | 43.6 | 1.00 | ||||
| Yes | 1059 | 68 | 5603 | 121.4 | 1.69 (1.32-2.17)*** | 1.63 (1.21-2.20)** | 1.39 (1.02-1.88)* | ||
| VD | |||||||||
| Sleep disorders | |||||||||
| No | 38 076 | 205 | 217 150 | 9.4 | 1.00 | ||||
| Yes | 1059 | 14 | 5603 | 25.0 | 1.66 (0.96-2.85) | 1.37 (0.69-2.72) | 1.03 (0.51-2.07) | ||
| Other dementia | |||||||||
| Sleep disorders | |||||||||
| No | 38 076 | 769 | 217 150 | 35.4 | 1.00 | ||||
| Yes | 1059 | 56 | 5603 | 99.9 | 1.89 (1.44-2.48)*** | 1.87 (1.38-2.54)*** | 1.69 (1.23-2.31)*** | ||
- Abbreviations: AD, Alzheimer disease; BMI, body mass index; BP, blood pressure; CI, confidence interval; HR, hazard ratio; VD, vascular dementia.
- a HRs were estimated after adjusting for sex and age.
- b HRs were estimated after adjusting for sex, age, medical visits from diagnosis of type 2 diabetes, hypoglycemia, BMI, BP, fasting glucose, total cholesterol, family history of diabetes, smoking, heavy alcohol drinking, exercise, household income, and residential area.
- c HRs were estimated after adjusting for sex, age, medical visits from diagnosis of type 2 diabetes, hypoglycemia, BMI, BP, fasting glucose, total cholesterol, family history of diabetes, smoking, heavy alcohol drinking, exercise, household income, residential area, and comorbidities.
- *P value<.05; **P value<.01; ***P value<.001.
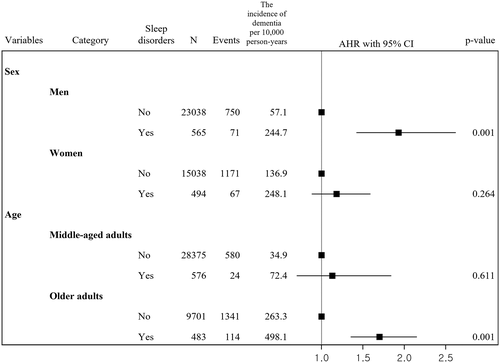
Figure 2 shows associations between sleep disorders and incidence of dementia by sex and age group. Among patients with type 2 diabetes, the incidence of all-cause dementia per 10 000 person-years in men with sleep disorders (244.7) was higher than that in those without sleep disorders (57.1), and, among men, the presence of sleep disorders was associated with an increased risk of dementia adjusted for covariates compared to that among those without sleep disorders (AHR, 1.93; 95% CI, 1.42-2.62). The incidence of all-cause dementia per 10 000 person-years in women with sleep disorders (248.1) was higher than that among those without sleep disorders (136.9), but the adjusted association between sleep disorders and dementia was not significant (AHR, 1.18; 95% CI, 0.88-1.59). In patients with type 2 diabetes, the incidence of all-cause dementia per 10 000 person-years in older adults with sleep disorders (498.1) was higher than in those without sleep disorders (263.3), and older adults (AHR, 1.70; 95% CI, 1.35-2.15) with sleep disorders had an increased risk of dementia compared to those without sleep disorders. The incidence of all-cause dementia per 10 000 person-years in middle-aged adults with sleep disorders (72.4) was higher than in those without sleep disorders (34.9), but the adjusted risk was not significant (AHR, 1.13; 95% CI, 0.70-1.84).
4 DISCUSSION
The present study has three main findings. First, sleep disorders are associated with an increased risk of dementia in patients with new-onset type 2 diabetes. Second, among patients with type 2 diabetes, those with sleep disorders had increased risk of Alzheimer disease and other forms of dementia compared to those without sleep disorders. Third, among patients with type 2 diabetes, men and older adults with sleep disorders had higher risk of dementia compared to those without sleep disorders.
A few potential mechanisms may underlie the relationships between sleep disorders and dementia. First, the glymphatic system is a macroscopic waste clearance system. It facilitates efficient removal of soluble proteins and metabolites from the central nervous system and operates to help distribute non-waste compounds in the brain.24 The glymphatic system functions mainly during sleeping, while it is inactive during wakefulness. Second, β-amyloid (Aβ) has a diurnal rhythm, where cerebrospinal fluid Aβ levels are increased during the waking hours and decreased during sleeping.25 Previous evidence implies that sleep disorders may affect the clearance and/or production of Aβ.26, 27
The present study found that sleep disorders are associated with an increased risk of dementia in patients with new-onset type 2 diabetes, which is consistent with previous results. Saetung et al examined the role of sleep in cognitive function of patients with abnormal glucose tolerance.16 The study included 162 patients (81 type 2 diabetes and 81 prediabetes patients) and measured sleep duration (the amount of actual sleep obtained at night), sleep efficiency (percentage of time in bed spent sleeping), and obstructive sleep apnea using a portable diagnostic device. Cognitive function was assessed by the Thai version of the Montreal Cognitive Assessment. The study demonstrated that sleep duration and obstructive sleep apnea were not related to cognitive function, but lower sleep efficiency was associated with lower cognitive function in patients with abnormal glucose tolerance.
In the present study, sleep disorders had differential effects on subtypes of dementia. Sleep disorders increased the risk of Alzheimer disease, but had no influence on vascular dementia. Although a positive significant association between sleep disorders and Alzheimer disease is well established, previous evidence for vascular dementia is unclear. Elwood et al reported that sleep disorders were associated with increased risk of vascular dementia.28 Benedict et al showed that self-reported sleep disorders were unrelated to vascular dementia.29 Yaffe et al demonstrated that sleep disorders were associated with a modestly increased risk of vascular dementia.30 A recent meta-analysis suggested sleep disorders had a higher risk of incident vascular dementia.12 Previous studies commonly indicated that no significant independent association was found between insomnia and vascular dementia. Insomnia was not associated with subclinical atherosclerosis, which is a key process in vascular dementia.31 Additionally, white matter hyperintensity is extensively present in vascular dementia, and insomnia only may be insufficient to result in changes in white matter.32, 33 Considering insomnia accounted for approximately 76% of all sleep disorders in the present study, the high proportion of insomnia in the present study may influence the nonsignificant result for vascular dementia (Table S1).
In sex-specific analyses of patients with type 2 diabetes, the present findings showed that men with sleep disorders had an increased risk of dementia compared to those without sleep disorders, which implies the need for prevention of sleep disorders among men. This phenomenon is different from the study findings of the general population. The prevalence of sleep disturbance and dementia in women is generally more frequent than in men,34, 35 and previous studies implied that women with sleep apnea36 and sleep-related movement disorders37 had greater risk of dementia than men with these disorders. Our understanding of why the results differed by status of diabetes is limited, and further research is needed to clearly explain this difference. This study also showed that older adults with sleep disorders had a higher risk of dementia compared to those without sleep disorders in patients with type 2 diabetes. Given higher dementia incidence in older adults with type 2 diabetes,38 sleep management may be an important factor to prevent incidence of dementia in older adults with type 2 diabetes.
A few limitations to the present study need to be taken into account. First, this study could not identify the severity of dementia from the medical records. Second, this study could not assess the relationship between subtypes of sleep disorders and dementia due to limited data. Further research is warranted to examine risk of dementia by subtypes of sleep disorders using appropriate data. Third, although this study eliminated individuals with a dementia diagnosis made prior to sleep disorder diagnosis, there may have been reverse causality. Fourth, a total of 1186 patients with new-onset type 2 diabetes between 2004 and 2007 who were censored before the landmark were excluded from the analysis. Since patients with sleep disorders who were at greater risk of dementia were disproportionally excluded from the analysis in patients with type 2 diabetes, the present findings may include selection bias. Finally, the Korean population is predominantly of Asian descent, and future studies are needed to identify whether these results apply to other geographic regions and ethnicities.
In spite of these limitations, the strengths of the present study include its longitudinal design and plentiful data on sociodemographics, lifestyle, biomedical variables, and comorbidities. The present study, to our knowledge, is the first to examine the relationship between sleep disorders and risk of dementia in patients with newly diagnosed type 2 diabetes.
In conclusion, the present study of the Korean population verified that sleep disorders are significantly associated with an increased risk of dementia in patients with new-onset type 2 diabetes. This study suggests that fastidious investigation and adequate management of sleep disorders among patients with type 2 diabetes may be useful and relevant ways to prevent dementia partly.
ACKNOWLEDGEMENTS
This work was supported by the Yonsei University Research Fund (Post Doc Researcher Supporting Program) of 2019 (project no.: 2019-12-0129) and the National Research Foundation of Korea (grant no.: 2020R1I1A1A01053104).
CONFLICT OF INTEREST
The authors declare that there is no duality of interest associated with this manuscript.



