Regional evidence and international recommendations to guide lipid management in Asian patients with type 2 diabetes with special reference to renal dysfunction
对亚洲2型糖尿病患者特别是有肾功能障碍患者的血脂管理的地区实证和国际建议
Abstract
enThe anticipated increase in the prevalence and incidence of type 2 diabetes in Asia, and its associated cardiovascular–renal complications, will place a significant burden on patients, caregivers, and society. Despite the proven effectiveness of lipid management in reducing these complications, there are major treatment gaps, especially in Asian patients with young-onset diabetes and chronic kidney disease (CKD). Recent international guidelines recommended the adoption of absolute risk estimation of atherosclerosis and cardiovascular disease to guide treatment intensity. These recommendations replaced the previous strategy of using low-density lipoprotein cholesterol targets to guide initiation and intensification of lipid lowering, albeit still widely practiced in Asia. The latest guidelines also highlight the high risk of atherosclerosis and cardiovascular disease (ASCVD) for people with diabetes, who should be protected with statins, except for young patients without other risk factors, who will need yearly monitoring of blood lipid levels. Given the propensity of Asian patients with diabetes to develop CKD and the amplifying effect of CKD on ASCVD, the use of statins in Asian patients is particularly important. Due to interethnic differences in drug metabolism, rosuvastatin, which is largely cleared by the kidney, should be prescribed in low dosages (5–10 mg daily) in Asian populations. Conversely, epidemiological and experimental data confirm pleotropic and organ-protective effects of atorvastatin, with proven safety in Asian populations within a daily dose range of 10–40 mg. Thus, there is a need for Asian countries to review and align their lipid-lowering treatment guidelines to reduce the substantial burden of diabetes in the Asian region.
摘要
zh亚洲2型糖尿病及其相关的心血管-肾病并发症的患病率和发病率, 预计在未来将持续增加, 这往往会为患者、照顾者及社会带来沉重负担。虽然血脂管理已被证实能有效减低上述并发症的发病率, 但是在亚洲的早发性糖尿病及慢性肾病(chronic kidney disease, CKD)患者中, 仍然存在着巨大的治疗缺口。最近的国际指南建议, 使用动脉粥样硬化和心血管疾病(atherosclerosis and cardiovascular disease, ASCVD)的绝对风险评估来指导治疗强度。这些建议取代了以往使用低密度脂蛋白胆固醇为目标来指导启动和增强降脂治疗的策略, 虽然这种策略在亚洲地区仍被广泛使用。最新的指南同时强调由于糖尿病患者属于ASCVD高风险人群, 所以应该使用他汀类药物预防相关的并发症, 至于没有其他风险因素的年轻患者, 则须每年监测自己的血脂水平。此外, 考虑到亚洲糖尿病患者倾向于发展为CKD, 而CKD的发展亦会大幅增加ASCVD的发病率, 所以在亚洲患者的治疗方案中使用他汀类药物尤为重要。由于药物代谢中存在种族差异, 瑞舒伐他汀往往会被肾脏大幅度地清除, 因此对于亚洲患者应处方低剂量(每日5-10 mg)。相反地, 流行病学和实验数据均证实了阿托伐他汀的多效性及器官保护作用, 所以在亚洲患者的处方中每日剂量可为10-40 mg。因此, 亚洲国家需要检视和修订其降脂治疗指南, 以减轻亚洲地区糖尿病管理的巨大经济负担。
Introduction
Worldwide, diabetes is reaching epidemic proportions, especially in Asia, which harbors nearly two-thirds of people with diabetes, mainly type 2 diabetes (T2D). In Asia, almost one-half of those diagnosed with diabetes reside in China and India combined, with the majority of them undiagnosed.1 Due to rapid lifestyle and environmental changes, which interact with genetic factors, the phenotypes of Asian people with diabetes are characterized by central obesity, early onset of disease, and high rates of renal disease.2-4 Of major concern is the rising prevalence of young-onset diabetes (YOD) globally, especially in Southeast Asia and the Western Pacific region. Between 2007 and 2014, the number of people with diabetes worldwide increased by 74% in those aged 20–39 years, compared with increases of 63% in those aged 40–59 years and 43% in those aged 60–79 years.1
In the Joint Asia Diabetes Evaluation (JADE) Registry, which recruited over 40 000 people with T2D from 11 countries, using HbA1c <7%, blood pressure (BP) <130/80 mmHg and low-density lipoprotein cholesterol (LDL-C) <2.6 mmol/L (ABC goals) as widely accepted treatment goals, only 20%–30% of patients attained any one of these goals. Among these patients, one in five had YOD diagnosed before the age of 40 years. Despite an age difference of 20 years, patients with YOD had, at best, similar risk factor control and were less likely to attain treatment goals, with only 6% of them attaining all three ABC goals, compared with 8% of patients with late-onset diabetes.5 In addition, less than 50% of these YOD patients were treated with statins or renin–angiotensin system (RAS) inhibitors, despite having cardiovascular–renal complications.5 Driven by long disease duration and suboptimal risk factor control, Asian patients with YOD had a 50% higher risk of cardiovascular–renal complications and death rates at any given age than individuals with late-onset diabetes.6
On a global basis, atherosclerosis and cardiovascular disease (ASCVD) continue to be the leading cause of death in T2D.7 Due to the abnormal metabolic milieu characterized by hyperglycemia and inflammation, people with T2D and no prior coronary artery disease (CAD) often have a similar risk of future coronary events compared with a non-diabetic person with a prior history of CAD, albeit with considerable heterogeneity depending on the combination of various risk factors.8 In 2009, 46.4% of all Chinese patients in Singapore admitted with their first acute myocardial infarction (MI) were found to have diabetes upon presentation.9
In the Multiple Risk Factor Intervention Trial (MRFIT), lowering total cholesterol by 1 mmol/L was associated with a 21%–23% relative risk reduction of CAD.10 In people with diabetes, statins have been proven to reduce ASCVD in both primary and secondary prevention studies (Table 1).11-19 Using data from randomized clinical trials (RCTs), there were close correlations between ASCVD risk reduction and lowering of LDL-C, due primarily to the use of statins, with less robust data from other lipid-modifying drugs.7 That said, control of hypertension, hyperglycemia, and obesity, as well as smoking cessation are also important, with multifactorial management resulting in a 40%–60% risk reduction of cardiovascular–renal complications and related deaths in T2D.7, 20, 21
| Trial | Number of patients with diabetes | Total number of patients | Dose/day | Risk reduction in MACE |
|---|---|---|---|---|
| Primary prevention trials | ||||
| ASCOT-LLA11 | 2532 | 10 305 | Atorvastatin 10 mg vs placebo | 23% (P = 0.036) |
| CARDS12 | 2838 | 2838 | Atorvastatin 10 mg vs placebo | 37% (P = 0.001) |
| Secondary prevention trials | ||||
| Intensive versus less intensive statin therapy | ||||
| TNT13 * | 1231 | 10 001 | Atorvastatin 10 mg vs 80 mg | 29% (P < 0.0001) |
| PROVE-IT14 | 978 | 4162 | Atorvastatin 80 mg vs pravastatin 40 mg | 25% (P = 0.03) |
| A to Z15 | 1059 | 4497 | Simvastatin 40/80 mg vs simvastatin 0/20 mg | 12% (NR) |
| Other secondary prevention trials vs placebo | ||||
| 4S16 | 483 | 3720 | Simvastatin 20–40 mg | 42% (P = 0.001) |
| LIPID17 | 782 | 9014 | Pravastatin 40 mg | 29% (P < 0.001) |
| CARE18 | 586 | 4159 | Pravastatin 40 mg | 25% (P = 0.05) |
| HPS19 | 5936 | 20 536 | Simvastatin 40 mg | 27% (P < 0.0001) |
- * This cohort includes patients with diabetes and those with metabolic syndrome.
- 4S, Scandinavian Simvastatin Survival Study; ASCOT-LLA, ASCOT-Lipid Lowering Arm; CARDS, Collaborative Atorvastatin Diabetes Study; CARE, The Cholesterol And Recurrent Events; HPS, The Heart Protection Study; LIPID, Long-Term Intervention with Pravastatin in Ischaemic Disease; MACE, major adverse cardiac events; NR, not reported; PROVE-IT, Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction; TNT, Treating to New Targets.
Cardiovascular disease risk estimation in patients with T2D
With increasing knowledge about the natural history of ASCVD and the interactive effects between non-modifiable (e.g. age, sex, disease duration) and modifiable (e.g. HbA1c, BP, LDL-C, obesity, smoking) risk factors, many experts recommend the use of risk equations to estimate 5- or 10-year incidences of complications to stratify risk and prioritize treatment in people with or without diabetes. Despite having relatively normal LDL-C levels, people with T2D often have qualitative changes in lipid particles with increased proportions of smaller and more dense LDL particles,7 which contribute to a high risk of ASCVD.22 In the 2016 Standards of Care Guidelines,22 although the American Diabetes Association (ADA) recommended the use of the American College of Cardiology (ACC)/American Heart Association (AHA) risk calculator to estimate 10-year ASCVD, they acknowledged that this calculator may have limited use for assessing cardiovascular risk in people with diabetes.
In a recent review,23 the risk equations of the Framingham Heart Study, the USA–People's Republic of China Collaborative Study of Cardiovascular and Cardiopulmonary Epidemiology cohort (PRC), the UK Prospective Diabetes Study (UKPDS), and the JADE program have been shown to be valid and relevant to Chinese patients with diabetes in primary care (Table 2). The Framingham and PRC equations had the strongest correlation in predicting total annual coronary heart disease (CHD) risks (r = 0.8794, P < 0.001). Both the Framingham Heart Study and UKPDS showed a strong correlation with other risk functions. However, there was only a modest correlation between the JADE and PRC equations (r = 0.6840, P < 0.001), whereas the total annual CHD risk predicted by the UKPDS risk engine and the JADE function were similar (3.09% vs 2.96%, respectively; P = 0.13). These differences were not unexpected due to the different attributes of the UKPDS and JADE cohorts, which were clinic based and consisted of patients with T2D. Conversely, the Framingham and PRC cohorts were community based, consisting of relatively healthy subjects with documentation of cardiovascular risk factors.
| Framingham Heart Study | PRC | UKPDS | JADE | |
|---|---|---|---|---|
| Setting | North American | Mainland Chinese | White, Afro-Caribbean, Asian-Indian | Hong Kong Chinese |
| Primary population | General population (no CVD) | General population (no MI, stroke) | Patients with diabetes (no CHD or HF) | Patients with diabetes (no CHD or HF) |
| Predicted years | 10 | 10 | 1–10 | 5 |
| Predictors | ||||
| Age | ✓ | ✓ | ✓ | |
| Gender | ✓ | ✓ | ✓ | ✓ |
| Ethnicity | ✓ | |||
| Blood pressure | ✓ | ✓ | ✓ | |
| Smoking | ✓ | ✓ | ✓ | ✓ |
| Diabetes | ✓ | ✓ | – | – |
| Age at diagnosis of diabetes | ✓ | |||
| Duration of diabetes | ✓ | ✓ | ✓ | |
| Total cholesterol | ✓ | |||
| LDL-C | ✓ | |||
| HDL-C | ||||
| Non-HDL-C | ✓ | |||
| Total cholesterol/HDL-C | ✓ | |||
| HbA1c | ✓ | ✓ | ||
| eGFR | ✓ | |||
| Spot urine ACR | ✓ | |||
- ACR, albumin: creatinine ratio; CHD, coronary heart disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; JADE, Joint Asia Diabetes Evaluation; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PRC, USA–People's Republic of China Collaborative Study of Cardiovascular and Cardiopulmonary Epidemiology cohort; UKPDS, United Kingdom Prospective Diabetes Study.
Against this background, the Framingham equation predicted lower total annual CHD risks than those predicted by either the JADE or UKPDS equations (2.52% vs 2.96% and 3.09%, respectively; P < 0.001). Taking feasibility, convergent validity, and sensitivity into consideration, the authors concluded that the UKPDS risk engine using age, sex, BP, LDL-C, HbA1c, and smoking as predictors may be the most useful cardiovascular risk prediction tool for Chinese people in the primary care setting.23 Conversely, the Framingham and PRC risk equations, which were derived using community-based data, tended to underestimate ASCVD risks in Chinese patients with diabetes.23 Using a hospital clinic-based population, the JADE equation identified age, male gender, disease duration, non-high-density lipoprotein cholesterol (HDL-C), smoking, estimated glomerular filtration rate (eGFR), and urinary albumin: creatinine ratio (ACR) as predictors for first CHD event with better performance than the UKPDS equation. These data highlight the importance of the context in which these risk equations were developed. As such, their utility in different settings will need revalidation and calibration of the effect size, although all these risk factors had confirmed prognostic significance.24 Researchers from Asia also first reported the multiple risk factors for predicting chronic kidney disease (CKD), which included age, sex, disease duration, retinopathy, HbA1c, smoking, and metabolic syndrome in addition to eGFR and ACR.24 These findings have subsequently been confirmed in several meta-analyses regarding the importance of metabolic syndrome in CKD25 and that of CKD in ASCVD.26, 27
Prevention of ASCVD in T2D: Current therapeutic recommendations
In addition to lifestyle modifications, such as medical nutrition therapy, increased physical activity, weight loss, and smoking cessation, which form the core for diabetes management,7 the majority of guidelines also recommend statin therapy in patients with diabetes.22, 28-30 Until recently, many guidelines used LDL-C goals for initiating and intensifying statin therapy. Given the growing body of evidence regarding the efficacy of statins in reducing ASCVD in people with or without diabetes, the latest international guidelines28-30 have transitioned from this target-based approach to a drug- and dose-based approach. In 2013, the ACC/AHA Cholesterol Treatment Recommendation Panel updated its guidelines28 and adopted a statin-centric approach without referring to LDL-C goals. In 2016, the ADA24 adopted the recommendations from the 2013 ACC/AHA guidelines29 for cholesterol management in diabetes; they recommended high-intensity statins for all people with diabetes with established ASCVD. In addition, moderate- to high-intensity statins were recommended in people with diabetes aged between 40 and 75 years with ASCVD risk factors for primary prevention. Although the ADA did not recommend the use of statins in patients aged <40 years without additional risk factors (such as obesity, hypertension, and smoking), blood lipid levels should be monitored yearly in these young patients with early intervention as indicated.22 Although the ADA guideline states that moderate-intensity stains and lifestyle therapy should be considered in patients aged 40–75 years with diabetes without additional ASCVD risk factors, they were published before the results of the simvastatin/ezetimibe-based Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) Study were released.31
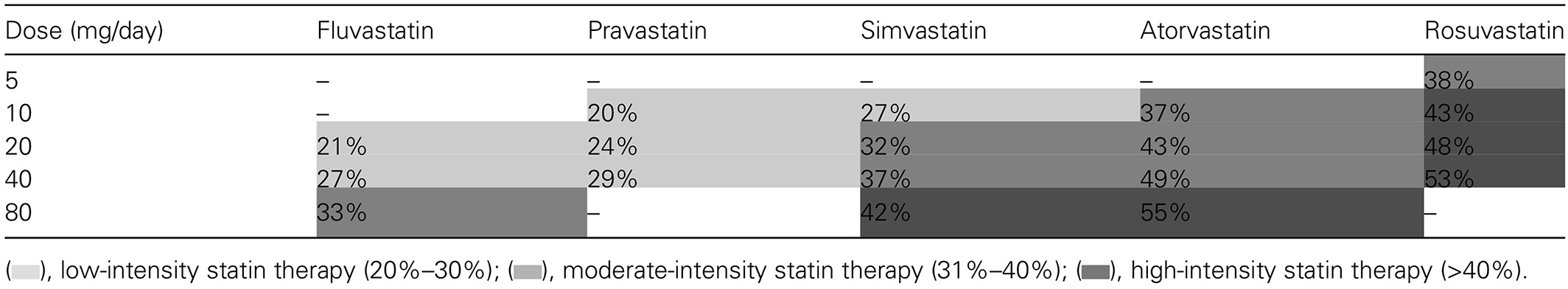
According to the 2013 ACC/AHA Cholesterol Treatment Guidelines, high-intensity statin therapy was defined as atorvastatin 40–80 mg or rosuvastatin 20–40 mg.28 The 2014 National Institutes of Clinical Excellence (NICE) lipid guidelines29 recommended only atorvastatin 20 mg for primary prevention of ASCVD in high-risk patients (including patients with diabetes) and atorvastatin 80 mg for secondary prevention of ASCVD. Given its high potency, a dose of 20 mg atorvastatin was considered to be high intensity according to the NICE guidelines (Table 3).
Similarly, the European Society of Cardiology (ESC) also recommends the prompt implementation of statin therapy in patients with target organ damage, as well as in those with diabetes to reduce ASCVD risk.32 In adults aged ≥50 years with an eGFR of <60 mL/min per 1.73 m2, but not treated with chronic dialysis or kidney transplantation, treatment with a statin or statin/ezetimibe combination is recommended.32 In patients with diabetes, pharmacological therapy should be used to ensure attainment of HbA1c <7% (53 mmol/L) and BP below 140/80 mmHg and 140/90 mmHg according to the respective ESC and ADA guidelines.22, 32 Taking into consideration the recent results on the efficacy of ezetimibe,31 in a recent consensus statement, experts stated that clinicians treating patients with diabetes aged 40–75 years who had a less-than-anticipated response to statins, who were unable to tolerate a less-than-recommended intensity of a statin, or who were completely statin intolerant may consider the addition of a non-statin cholesterol-lowering therapy (i.e. ezetimibe or bile acid sequestrants).33
Current lipid-lowering guidelines and practice in Asia
Despite these international recommendations, many Asia-specific guidelines still follow a target-based approach for preventing ASCVD (Table 4).34-43 The recent Singapore Clinical Practice Guidelines (CPG)34 recommended that statin therapy should be started concurrently with therapeutic lifestyle modification for most patients with T2D. Patients with diabetes can be stratified into two levels of risk (very high or high risk) based on the presence or absence of CKD and/or cardiovascular disease (CVD). For patients with diabetes without established chronic CVD and/or CKD, moderate-intensity statin therapy is recommended, whereas high-intensity statin therapy is recommended for individuals with diabetes and with CVD and/or CKD. In patients with high triglyceride and low HDL-C levels, the Singapore CPG recommend clinicians consider the use of a fibrate in addition to statin therapy.34
| Patient population | Statin therapy | ||
|---|---|---|---|
| Primary prevention | Secondary prevention | ||
| ADA22 (2016) | Patients with diabetes with ASCVD risk factors | Moderate to high intensity statin | |
| ACC/AHA28 (2013) | Patients with diabetes aged 40–75 years with ASCVD risk factors | Atorvastatin 40–80 mg or rosuvastatin 20–40 mg | |
| NICE29 (2014) | All adults with T1D aged >40 years | Atorvastatin 20 mg | Atorvastatin 80 mg |
| All patients with T2D with ≥10% risk of CVD | |||
| ESC/EAS30 (2016) | T1D in the presence of microalbuminuria and/or renal disease | Statin therapy (specific statins not recommended) | |
| T2D and CVD or CKD, and in those without CVD who are >40 years of age with ≥1 other CVD risk factor or marker of target organ damage | |||
| Singapore34 (2014) | Individuals with diabetes mellitus without established ASCVD and/or CKD are categorized as high CV-risk | High CV risk: moderate-intensity statin therapy (target LDL-C < 2.6 mmol/L [100 mg/dL]) | High CV risk: moderate-intensity statin therapy (target LDL-C < 2.6 mmol/L [100 mg/dL]) |
| Individuals with diabetes mellitus with CKD are categorized as very high CV-risk patients | Very high CV-risk diabetes patients: high-intensity statin therapy (<2.1 mmol/L [80 mg/dL]) | Very high CV-risk diabetes patients: high-intensity statin therapy (<2.1 mmol/L [80 mg/dL]) | |
| Korea36 (2015) | T2D plus LDL-C > 100 mg/dL (>2.6 mmol/L) | Statin therapy (specific statins not recommended) | |
| Malaysia37 (2011) | All patients with diabetes aged >40 years (regardless of baseline LDL-C) | Statin therapy (specific statins not recommended) | |
| Hong Kong38 (2014) | T2D plus dyslipidemia | Statin therapy (specific statins not recommended) | |
| Taiwan40 (2017) | Patients with diabetes aged ≥40 years plus additional CV risk factors | Statin therapy (specific statins not recommended) | |
| Thailand41 (2016) | Diabetes plus LDL-C ≥ 190 mg/dL (≥4.9 mmol/L) | Moderate intensity statins (specific statins not recommended) | |
| Diabetes plus LDL-C < 190 mg/dL (<4.9 mmol/L) plus a Thai ASCVD risk score ≥ 10% | Low or moderate statin therapy (specific statins not recommended) | ||
| India42 (2016) | Diabetes with ≥2 other major ASCVD risk factors or evidence of target organ damage plus LDL-C ≥ 100 mg/dL (classified as moderate risk) | Statin therapy (target LDL-C < 100 mg/dL) | |
| Japan43 (2012) | Individuals with diabetes with established ASCVD and/or CKD are categorized as high CV riskDiabetes plus dyslipidemia (classified as high risk) | Statins are the drug of choice. Resin, probucol ± ezetimibe are recommended in combination with statins or when selected statins cannot be administered | |
| Statin treatment is recommended but not mandatory (target LDL-C < 120 mg/dL) | LDL-C < 100 mg/dL | ||
- ADA, American Diabetes Association; ASCVD, atherosclerotic cardiovascular disease; ACC, American College of Cardiology; AHA, American Heart Association; CAD, coronary artery disease; CKD, chronic kidney disease; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; LDL-C, low-density lipoprotein cholesterol; NICE, National Institute of Clinical Excellence; T1D, type 1 diabetes; T2D, type 2 diabetes.
There are a few guidelines for ASCVD issued by different professional organizations in Korea, with the recommendations generally following the Adult Treatment Panel III (ATP-III) guidelines.35 The 2015 Korean Society of Lipidology and Atherosclerosis guidelines considered people with diabetes as having high CVD risk and that drug treatment should be considered to attain an LDL-C level of 70–99 mg/dL (1.8–2.56 mmol/L).35 In addition to lifestyle modification, initiation of drug treatment was recommended in individuals with diabetes and an LDL-C level > 100 mg/dL (2.6 mmol/L).36
In the 2011 Malaysian CPG, diabetes was also recognized as a CHD risk equivalent condition in which therapeutic lifestyle changes should be implemented in all patients.37 Individuals should be stratified by global risk assessment for determining target lipid levels. Statins were recommended as the drug of choice for reducing LDL-C and should be initiated in all individuals >40 years of age with T2D regardless of baseline LDL-C, aiming at a target LDL-C < 100 mg/dL (<2.6 mmol/L). In patients with established CVD, a target level of LDL-C < 70 mg/dL (<1.8 mmol/L) was recommended.37
In Hong Kong, statins were also recommended as the drug of choice along with lifestyle modifications, with an LDL-C target of <100 or <70 mg/dL (<2.6 or <1.8 mmol/L, respectively) for patients with pre-existing CVD.38
The Chinese guidelines for lipid management are aligned with the 2016 ESC guidelines and categorize patients according to individual total CVD risk level. Importantly, using data from a Chinese population cohort, they provide a CVD risk stratification scheme to categorize patients into very high risk, high risk, moderate risk, and low risk.39 These guidelines recommend statin therapy as the first-line lipid-lowering therapy and define different LDL-C target goals based on risk stratification: <70 mg/dL (<1.8 mmol/L) for very high-risk patients, <100 mg/dL (<2.6 mmol/L) for high-risk patients, and <130 mg/dL (<3.4 mmol/L) for moderate- to low-risk patients.
The 2017 Taiwan Lipid Guidelines advocate statin therapy as the first-line treatment deemed necessary for people with diabetes and CVD, with an LDL-C target of <70 mg/dL in patients with diabetes and stable CAD. These guidelines highlight the increased risk of ASCVD in patients with diabetes and suggest that a lower LDL-C target of <55 mg/dL (<1.4 mmol/L) can be considered in patients with diabetes and acute coronary syndrome.40 A target of <100 mg/dL (<2.6 mmol/L) is recommended in individuals with diabetes aged ≥40 years or those aged <40 years with additional cardiovascular risk factors.40
The 2016 Thai Dyslipidaemia Guidelines recommend an LDL-C-lowering strategy to reduce CVD risk and, although these guidelines recommend a specific LDL-C target level, the primary aim is “the lower LDL-C level, the better”.41 Patients with diabetes and an LDL-C ≥ 190 mg/dL (>4.9 mmol/L) should be managed with moderate-intensity statins with a target of <130 mg/dL (<3.4 mmol/L). Treatment in those with an LDL-C < 190 mg/dL and a Thai ASCVD risk score ≥ 10% should receive low- or moderate-intensity statin therapy aiming at an LDL-C target of ≤130 mg/dL.
Using regional data and international guidelines to guide lipid management in Asia
The ADA, ACC/AHA, ESC and UK NICE guidelines were based on extensive review of experimental, epidemiological, and RCT data, primarily from Caucasian populations. In contrast, the Asian guidelines described above were based on available local data and the consensus of experts taking into consideration these international guidelines, and thus were more pragmatic and less current. There was a paucity of RCTs of lipid management in Asia. However, epidemiological and mechanistic studies in Asian populations supported the use of moderate- to high-dose statins in people with diabetes for primary and secondary prevention of ASCVD, due, in part, to the high prevalence of CKD in this population.
In the Hong Kong Guidelines, the authors concurred that high-dose statins were moderately effective in individuals with hypertriglyceridemia and high LDL-C.38 In the Korean guidelines, weight reduction, regular physical exercise, and reduced alcohol intake were all recommended to reduce triglyceride (TG) levels.36 If TG levels are in the range 200–500 mg/dL with elevated LDL-C, statin treatment is recommended to lower LDL-C to the target level. In very high- and high-risk patients, if TG levels remain >200 mg/dL after lifestyle modification and statin treatment, drug treatment, such as fibrate, nicotinic acid and omega-3 fatty acid, can be added to prevent CVD.36
Evaluation of macrovascular disease and its risk factors should also be included in the clinical assessment of patients with diabetes.44 A detailed history should screen for the presence of angina, neurological symptoms, claudication, and past episodes of vascular events.44 Subclinical atherosclerosis screening (e.g. pedal pulses or carotid bruit) could be included as an integral component of any comprehensive management program in order to improve cardiovascular risk prediction and improve cardiovascular outcomes.
Diabetic nephropathy and effects of statins on renal function
Diabetes is a major cause of CKD and end-stage renal disease (ESRD), which is particularly prevalent in the Asian population. In a subanalysis of the JADE Registry, involving over 28 000 patients from seven countries or areas in Asia, 15% had CKD defined by eGFR <60 mL/min per 1.73m2 and 40% had increased albuminuria. Compared with those without CKD, patients with CKD were less likely to attain ABC goals, with approximately only 50% receiving statins and/or RAS inhibitors.45 In a clinic-based cross-sectional study in Singapore, among patients with T2D aged 21–89 years, 53% had evidence of CKD defined according to the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines.46 In Singapore, the proportion of incident cases of kidney failure due to diabetes had increased from 46% in 1999 to 62% in 2009.9 Among patients on dialysis, those with diabetes were twofold less likely to survive than those without diabetes.9, 45 An annual mortality rate of 64.1 per 1000 individuals has been reported in patients with diabetic kidney disease (Stage 3–5), with an increasing mortality rate observed in parallel with increasing CKD severity.47, 48
The role and pathogenesis of dyslipidemia in CKD are still not clearly understood. However, LDL-C levels were implicated in atherogenesis in vascular walls and mesangial cell proliferation in the kidney, both of which were closely associated with inflammation.49 Several lines of evidence suggest that oxidative stress and inflammation may provide the link between ASCVD and CKD.50 Experimental studies suggest that statins could attenuate oxidative stress, reduce oxidized LDL-C, and prevent downregulation of endothelial nitrous oxide synthase, with improved endothelial function.50 Furthermore, hyperlipidemia could worsen inflammation of the kidney, whereas lowering LDL-C levels may potentially preserve renal function.50 In support of these findings, atorvastatin treatment has been shown to dose-dependently decrease not only LDL-C levels, but also oxidative stress and inflammatory markers, atheromatous plaques and the rate of decline in renal function.49, 51-54
Although the biological and genetic mechanisms underlying the increased incidence of diabetic kidney disease in Asia remain to be clarified, there is an urgent need to implement diabetes detection and prevention strategies to reduce disease burden. It is recommended that patients with diabetes, hypertension, a family history of CKD, or a past history of acute kidney injury should undergo regular screening for detection of CKD.55
Statin therapy in CKD and ESRD
In the latest international guidelines, high absolute risk of ASCVD rather than elevated LDL-C was used to initiate or adjust lipid-lowering treatment, including in patients with CKD. The KDIGO guidelines recommended evaluation of a full lipid profile (total cholesterol, LDL-C, HDL-C, TG) in patients with newly diagnosed CKD, including those on chronic dialysis or kidney transplantation.56 In patients with diabetes and CKD aged 18–49 years but not receiving chronic dialysis or a kidney transplantation, statin treatment was recommended. In most adults with CKD (including those on chronic dialysis or receiving kidney transplantation), follow-up measurement of lipid levels was not specifically indicated, although periodic measurement may be useful to check treatment adherence.
Despite the high ASCVD risk in patients with CKD with or without diabetes,56 there have been few studies examining the effects of lipid lowering in these populations. In the Study of Heart And Renal Protection (SHARP), which enrolled 9270 patients with CKD including 3023 patients on dialysis randomized to receive either placebo or simvastatin/ezetimide, followed-up for a median of 4.9 years, the intervention produced a 17% proportional reduction in major atherosclerotic events (Table 5).57 In another RCT involving 2776 patients on maintenance hemodialysis, treatment with rosuvastatin reduced LDL-C levels by a mean of 43%, but there was no effect on the composite primary endpoint of death from cardiovascular causes, non-fatal MI, or non-fatal stroke compared with placebo.58 The results of the Die Deutsche Diabetes Dialyse Studie (4D),59 showed no benefit from atorvastatin on cardiovascular death, non-fatal MI, or stroke in patients with T2D on hemodialysis. In a post hoc analysis of the 4D study involving this patient population, atorvastatin reduced the risk of fatal (hazard ratio [HR] 0.58; 95% confidence interval [CI] 0.34–0.99) and non-fatal cardiac events (HR 0.68; 95% CI 0.47–0.98) and all-cause mortality (HR 0.72; 95% CI 0.52–0.99) if pretreatment LDL-C was >145 mg/dL (3.76 mmol/L).59
| Study design | Study subjects | Treatment regimen | Primary endpoint | |
|---|---|---|---|---|
| AURORA58 | Multicenter prospective randomized double-blind | 2775 patients undergoing maintenance dialysis | Rosuvastatin 10 mg/day for a median of 3.8 years | 4% reduction in composite of death from CV causes, non-fatal MI, or non-fatal stroke (95% CI 0.84–1.11; P = 0.59); 9.2 and 9.5 events per 100 patient-years for rosuvastatin vs placebo, respectively |
| SHARP57 | Randomized double-blind | On dialysis: 3023 | Ezetimibe/simvastatin 10/20 mg/day for a median of 4.9 years | 17% proportional reduction in major atherosclerotic events (11.3% for ezetimibe/simvastatin vs 13.4% for placebo; RR 0.83, 95% CI 0.74–0.94; P = 0·0021)* |
| Not on dialysis: 6247 |
- * Proportional effects on major atherosclerotic events were similar between patients on dialysis and those not on dialysis.
- AURORA, A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events; CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; RR, rate ratio; SHARP, Study of Heart and Renal Protection.
Evidence from large clinical trials indicates that statin therapy is effective and well tolerated in patients with a mean eGFR of 50 mL/min per 1.73 m2.60 A meta-analysis of data from individual participants from 28 RCTs (n = 183 419) concluded that in patients with CKD, statin-based therapy reduced the risk of a first major vascular event by 21% for every 1.0 mmol/L reduction (39 mg/dL) in LDL-C (P < 0.0001; Table 5); however, the effect size became attenuated with declining eGFR, which became non-significant in patients on dialysis.61
Pleiotropic effects of different statins
Recent published findings have raised concerns about potential renal adverse effects of some statins, particularly rosuvastatin, especially when given in high doses.62, 63 In the Prospective evaLuation of proteinuriA and reNal function in diabETic patients with progressive renal disease (PLANET),62 the authors compared the renal effects of rosuvastatin (10 and 40 mg daily) with those of atorvastatin (80 mg daily) over a 12-month period in patients with high fasting LDL-C (≥90 mg/dL or 2.33 mmol/L) and moderate proteinuria (500–5000 mg/g), with or without diabetes (PLANET II and PLANET I, respectively). In the latter group, there was a twofold higher incidence of adverse renal events (i.e. any adverse renal event 9.8% vs 4.5%; acute renal failure 4.1% vs 0.9%) in rosuvastatin-treated patients than in patients treated with atorvastatin.62 In the combined analysis of PLANET I and PLANET II trials, treatment with high-dose atorvastatin significantly reduced proteinuria by 20%, with no adverse effects on renal function, whereas rosuvastatin caused more rapid decline in eGFR with no reduction in proteinuria (Table 6), albeit the lack of a placebo in that study limited the full interpretation of these findings.62 Given that the plasma concentrations of rosuvastatin in Japanese and Chinese are approximately double those in Caucasians given the same dose, the US Food and Drug Administration recommend lower starting doses in Asian patients (5 or 2.5 mg in Japan).64
| Rosuvastatin | Atorvastatin 80 mg (n = 102†) | ||
|---|---|---|---|
| 10 mg (n = 107) | 40 mg (n = 116*) | ||
| Change in UPCR | 1.02 (P = 0.83) | 0.96 (P = 0.53) | 0.87 (P = 0.033) |
| Change in eGFR (mL/min per 1.73m2) | −3.70 (P = 0.0098) | −7.29 (P = 0.0002) | −1.61 (P = 0.21) |
- Data are mean changes from baseline at Week 52 (LOCF).
- * Estimated glomerular filtration rate (eGFR) was evaluated in only 115 patients.
- † Estimated glomerular filtration rate was evaluated in only 101 patients.
- UPCR, urine protein: urine creatinine ratio.
Despite the inconclusive findings of the PLANET trial, the renal effects of atorvastatin were consistent with previous analyses. In the post hoc analyses of the Treat to New Targets (TNT) trial,63 a progressive increase in eGFR was reported in patients with CKD treated with atorvastatin for a median of 4.9 years, with a 5.6% increase with 10 mg daily and an 8.3% increase with 80 mg daily. The respective mean change from baseline eGFR was an increase of 3.5 mL/min per 1.73 m2 in the 10-mg group versus an increase of 5.2 mL/min per 1.73 m2 in the 80-mg group.63 In a 1-year prospective study in 56 patients with CKD, treatment with atorvastatin at doses of 20–40 mg/day reduced proteinuria and the rate of CKD progression in patients already treated with RAS inhibitors.65 Data from the Collaborative Atorvastatin Diabetes Study (CARDS) study in patients with T2D without prior CVD also confirmed the beneficial effect of atorvastatin therapy on eGFR, especially in those with albuminuria at baseline.12 Apart from LDL-C lowering, these renal effects of atorvastatin may be attributed to its pleiotropic effects, including amelioration of oxidative stress, endothelial dysfunction, and inflammation.66 In support of these mechanistic and experimental data, large-scale prospective data in Chinese patients with T2D also confirmed the cardiovascular- and renoprotective effects of statins in real-world settings.67, 68
Pitavastatin also has a range of pleiotropic effects that may play a role in reducing cardiovascular morbidity and mortality beyond that achieved by pitavastatin-induced LDL-C reduction.69 These effects include increasing HDL-C, decreasing markers of platelet activation, improving cardiac, renal and endothelial function, and reducing endothelial stress and lipoprotein oxidation, all culminating in improved signs and symptoms of atherosclerosis.69
Pharmacokinetic properties of different statins
Atorvastatin and rosuvastatin are among the most potent and popular statins for LDL-C lowering. However, in Asian people, there are interethnic differences in drug metabolism that call for adjustment of drug dosage. The pharmacokinetic profiles of atorvastatin in Asian and Caucasian volunteers are similar, making the efficacy and safety data of different dosages in Caucasian subjects relevant to Asian populations.70 In a pooled analysis of RCTs comparing atorvastatin versus placebo or comparators, Caucasian (n = 47 455) and Asian (n = 2519) participants had similar adverse events and serious adverse events (e.g. myalgia, rhabdomyolysis or creatinine kinase levels, renal or liver dysfunction) within the dose range 10–40 mg daily.71 Due to the limited number of Asian participants receiving 80 mg daily in these RCTs, the safety of high-dose atorvastatin requires further evaluation in these subjects.71 In these RCTs conducted in controlled settings, discontinuation due to treatment-related adverse events was infrequent.71 Myalgia, elevations in liver transaminases, and renal adverse events occurred with an incidence of <2% and no cases of rhabdomyolysis were reported.71
Conversely, Asian subjects exhibited different pharmacokinetic profiles with rosuvastatin with higher systemic drug exposure compared with Caucasians for the same dosage.72 In addition, rosuvastatin has a predominant renal clearance route, which calls for dose adjustment in people with reduced renal function to avoid adverse renal events, especially in patients with CKD with or without type 1 diabetes or T2D.73 In a 12-month comparative study in Asian patients with diabetes treated with either moderate-intensity atorvastatin (10–20 mg/day) or rosuvastatin (5–10 mg/day), both drug regimens improved serum lipid levels with a greater reduction in eGFR observed with rosuvastatin (−1.6 [P = 0.012] vs −3.0 mL/min per 1.73 m2 [P = 0.001], respectively).74 Review of the evidence also indicated the heterogeneous properties of different statins with regard to LDL-C lowering, with atorvastatin showing more favorable pharmacokinetic and renoprotective effects over rosuvastatin.75
Conclusions
The growing burden of T2D, especially YOD, in Asia is expected to drive the growing incidence of ASCVD in the region with CKD as a major disease amplifier. Based on decades of epidemiological and experimental data, the latest international guidelines recommend the use of high- to moderate-dose statins in most people with diabetes, regardless of their LDL-C levels. This compelling evidence calls for revisit of these treatment guidelines in Asian countries in order to align with international recommendations. Given the importance of CKD in Asian patients with diabetes, the consistent evidence regarding the pleiotropic and the cardiovascular- and renoprotective effects of atorvastatin is particularly relevant to Asians. Based on pooled RCT data analysis, interethnic differences in pharmacokinetics, and results from regional studies, Asian patients with diabetes who have target organ damage or ASCVD risk factor(s) should benefit from moderate- to high-intensity statins (e.g. 10–40 mg atorvastatin, 20–40 mg simvastatin, 5–10 mg rosuvastatin). In patients who cannot tolerate high-dose statins or show reduced response to statins, ezetimibe can be considered as an adjunct therapy. Although the benefits of starting lipid-lowering treatment in dialysis-dependent patients with or without diabetes remain uncertain, lipid-lowering treatment should be continued in patients who are already treated with statin at the time of dialysis initiation. At present, there is a lack of large-scale RCTs on statins in Asian populations. Therefore, outcome modeling to create scenarios relevant to Asian populations for estimating the benefits of statins on ASCVD, renal events and mortality will be useful for decision making.
Acknowledgements
This paper was compiled based on discussions during an expert meeting convened in Singapore on 31 October 2015 attended by the coauthors and sponsored by Pfizer. The content reflects the opinion of the authors, with editorial support from Anna Battershill and See Mee Yen of Medica Comms Pte Ltd. This editorial support was funded by Pfizer Pte Ltd, Singapore. None of the authors received any honorarium for the preparation of the manuscript.
Disclosure
Juliana Chan is a consultant to Astra Zeneca, Bayer, Boehringer Ingelheim, Lilly, Novo Nordisk, MSD, Sanofi, and Pfizer with donation of the honoraria to the Chinese University of Hong Kong to support research and education. Her affiliated organizations, including the Chinese University of Hong Kong and Asia Diabetes Foundation, have received funding support from these companies for research and education purposes. Tavintharan S. has served on Advisory Boards for Sanofi, Amgen, and Pfizer, with donation of the honoraria to Khoo Teck Puat Hospital to support research and education. Jason C.J. Choo has served on Advisory Boards for Novartis and Pfizer, with donation of honoraria to the Singapore General Hospital to support research and education. Titus Lau, Kevin Tan and T.G. Ng have nothing to disclose.




