Prognostic significance of ratio of positive lymph nodes in patients with operable major salivary ductal carcinoma
Abstract
Background
Major salivary duct carcinomas (MSDCs) often involve the regional lymph nodes (LNs). However, the clinical value of LN parameters in patients with MSDCs is unclear. We aimed to investigate the optimal cut-off points for number of positive LNs (PLNN) and ratio of positive LNs (PLNRs) and their prognostic value in patients with MSDC.
Study Design
Retrospective cohort.
Methods
We retrospectively reviewed relevant data extracted from the Surveillance, Epidemiology, and End Results database on patients with MSDC who had undergone surgery between 2004 and 2016. The optimal PLNN and positive lymph node ratio (PLNR) cut-off points were identified using the X-tile program. Kaplan–Meier and Cox regression analyses were performed to determine prognostic factors.
Results
Overall, 290 patients were enrolled, 57.6% of whom had LN metastases. Advanced T stage in the submandibular gland and unpaired lesions were associated with LN involvement. Positive LNs, late T stage, and submandibular gland location were associated with poor overall survival (OS). The 5-year OS rates of patients with negative and positive LNs were 74.3% and 36.5%, respectively. PLNN > 16 and PLNR > 0.48 were the best cut-off points. The 5-year OS of patients with PLNN ≤ 16 and PLNN > 16 was 42.8% and 15.4%, respectively. The 5-year OS rates were 46.8% for patients with PLNR ≤ 0.48 and 26.3% for patients with PLNR > 0.48. PLNR was a strong prognostic factor for patients with MSDC with LN metastases.
Conclusions
PLNR reflects both the effects of LN dissection and PLNN. Furthermore, its prognostic value in patients with MSDC exceeds that of PLNN. Patients with high PLNRs should be followed closely after surgery.
Abbreviations
-
- ELN
-
- examined lymph node
-
- LN
-
- lymph node
-
- LNR
-
- lymph node ratio
-
- MSDC
-
- major salivary duct carcinoma
-
- OS
-
- overall survival
-
- PLNN
-
- positive lymph node number
-
- PLNR
-
- positive lymph node rate
-
- SEER
-
- surveillance, epidemiology, and end results
1 BACKGROUND
Salivary duct carcinomas (SDCs) are highly malignant tumors. They have a low incidence, accounting for approximately 1%–3% of malignant salivary gland tumors, but are highly invasive [1, 2]. SDCs can occur in all salivary glands, the parotid gland being the most commonly involved, accounting for approximately 80% of all SDCs, followed by the submandibular gland, which accounts for approximately 15% [2, 3]. Early stage major salivary duct carcinomas (MSDCs) are prone to relapse, distant metastasis, peripheral nerve invasion, and extravasation; they accordingly have a poor prognosis and are frequently lethal [4, 5].
MSDCs more frequently develop lymph node (LN) metastases than do other salivary gland tumors [1, 6]. LN metastasis is generally considered a risk factor for long-term survival of patients with head and neck carcinomas [3, 7]. Accurate LN staging is therefore important for selection of postoperative therapy and evaluation of prognosis. The term “ratio of positive LNs” (PLNR) denotes the ratio of number of positive LNs (PLNN) to the total number of examined LNs (ELN); it has been proposed as an independent predictor of survival outcomes in patients with multiple cancers [8, 9]. Although the clinical value of PLNN and PLNR in head and neck carcinomas has been established [10-12], their prognostic roles in patients with MSDCs have not been separately studied.
In this study, we explored and analyzed the clinical characteristics and survival outcomes of patients with operable MSDCs. To the best of our knowledge, this is the first large-scale cohort analysis of the prognostic significance of PLNN and PLNR in patients with MSDC.
2 METHODS
2.1 Data collection
The clinicopathological characteristics and survival information of patients with MSDC from 2004 to 2016 were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database. The following inclusion criteria were applied: (1) diagnosis of invasive ductal carcinoma; (2) location of primary tumor in a major salivary gland, including the parotid, submandibular, and major salivary, and not otherwise specified glands; (3) no evidence of distant metastasis; and (4) had undergone excision of the primary lesion. Patients with duplicated information and those for whom definite T, N, or M stages had not been supplied were excluded. The flowchart of patient selection is shown in Figure 1. To further explore the clinical value of LN parameters, we selected ELN ≥ 1 and PLNN ≥ 1 to be the main criteria for further screening the enrolled population. Because all studied data were extracted from a public SEER database, no ethics approval or informed consent was required.

Flow chart of patient selection. ELN, number of examined lymph nodes; NA, not available; PLNN, number of positive lymph nodes.
2.2 Statistical analysis
We defined PLNR as the ratio of PLNN to ELN and overall survival (OS) as the interval from the initial diagnosis of MSDC to death due to any reason or the last visit. Patient characteristics are presented as proportions and frequencies and were compared between groups using the χ2 test. Survival curves were constructed using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate analyses were performed to evaluate the association of each variable with OS. X-tile software (version 3.6.1; Yale University) was used to determine the appropriate thresholds for PLNR and PLNN. Statistical analyses were performed using SPSS software (version 23.0; IBM), and figures were produced using GraphPad Prism (version 9.0). Statistical significance was set at p < 0.05.
3 RESULTS
3.1 Patient characteristics
Overall, 290 patients who had undergone surgery for MSDC between 2004 and 2016 were enrolled in the SEER database (Table 1). The median age at diagnosis was 68 years and 72.1% of the patients were men. More than half of the patients had LN metastases, patients with N1, N2, and N3 stage disease accounting for 12.1%, 44.5%, and 1.4% of all patients, respectively. All patients had undergone surgery for the primary lesion, 226 (77.9%) had received radiotherapy, and 75 (25.9%) had received chemotherapy. The patients' basic characteristics are summarized in Table 1. The median follow-up time was 48 months and the median OS 73 months for the whole study cohort. The 1-, 3-, 5-, and 8-year OS rates were 93.2%, 72.2%, 53.4%, and 44.3%, respectively (Figure 2a).
| Characteristic | Total (n = 290) | % |
|---|---|---|
| Sex | ||
| Female | 81 | 27.9 |
| Male | 209 | 72.1 |
| Age at diagnosis (year) | ||
| ≤ 68 | 150 | 51.7 |
| > 68 | 140 | 48.3 |
| Race | ||
| White | 225 | 77.6 |
| Black | 33 | 11.4 |
| Others | 32 | 11.0 |
| Insurance | ||
| Insured | 243 | 83.8 |
| No/Unknown | 47 | 16.2 |
| Marital status at diagnosis | ||
| Single/Widowed/Separated | 63 | 21.7 |
| Married | 189 | 65.2 |
| Unknown | 38 | 13.1 |
| Primary site | ||
| Parotid gland | 233 | 80.3 |
| Submandibular gland | 39 | 13.4 |
| Major salivary gland, NOS | 18 | 6.2 |
| Grade | ||
| I–II | 36 | 12.4 |
| III | 122 | 42.1 |
| IV | 78 | 26.9 |
| Unknown | 54 | 18.6 |
| Laterality | ||
| Left - origin of primary | 121 | 41.7 |
| Right - origin of primary | 160 | 55.2 |
| Not a paired site | 9 | 3.1 |
| T stage | ||
| T1 | 66 | 22.8 |
| T2 | 63 | 21.7 |
| T3 | 88 | 30.3 |
| T4 | 73 | 25.2 |
| N stage | ||
| N0 | 123 | 42.4 |
| N1–3 | 167 | 57.6 |
| AJCC stage | ||
| I | 48 | 16.6 |
| II | 34 | 11.7 |
| III | 49 | 16.9 |
| IVA/B | 159 | 54.8 |
| SEER stage | ||
| Localized | 78 | 26.9 |
| Regional | 121 | 41.7 |
| Distant | 91 | 31.4 |
| Radiotherapy | ||
| No | 64 | 22.1 |
| Yes | 226 | 77.9 |
| Chemotherapy | ||
| No | 215 | 74.1 |
| Yes | 75 | 25.9 |
- Abbreviations: AJCC, American Joint Committee on Cancer; NOS, not otherwise specified; SEER, Surveillance, Epidemiology, and End Results.

Overall survival in the whole study cohort and indicated subgroups. (a) Overall survival curves for the entire population. (b) Survival curves stratified by sex. (c) Survival curves stratified by T stage. (d) Survival curves stratified by N stage. (e) Survival curves stratified by TNM stage. (f) Survival curves stratified by site of disease onset. TNM, Tumor Node Metastasis.
3.2 Risk factors for lymph node metastasis
As shown in Table 2, advanced T stage (χ2 = 52.921, p < 0.001), submandibular gland location (χ2 = 7.166, p = 0.028), and unpaired lesions (χ2 = 6.271, p = 0.043) were significantly associated with LN involvement. Moreover, patients with positive LNs were more likely to have received adjuvant radiotherapy (χ2 = 3.858, p = 0.0495) and chemotherapy (χ2 = 28.897, p < 0.001) than those with negative LNs. Nevertheless, LN metastasis was not associated with age, sex, race, insurance coverage, or pathological grade (all p > 0.05) (Table 2).
| Characteristic | N0 (n = 123) | N + (n = 167) | χ2 | p |
|---|---|---|---|---|
| Age at diagnosis | 0.22 | 0.883 | ||
| ≤68 | 63 | 87 | ||
| >68 | 60 | 80 | ||
| Sex | 0.932 | 0.334 | ||
| Female | 38 | 43 | ||
| Male | 85 | 124 | ||
| Race | 1.737 | 0.420 | ||
| White | 91 | 134 | ||
| Black | 17 | 16 | ||
| Others | 15 | 17 | ||
| Insurance | 1.609 | 0.205 | ||
| Insured | 107 | 136 | ||
| No/Unknown | 16 | 31 | ||
| Primary site | 7.166 | 0.028 | ||
| Parotid gland | 93 | 140 | ||
| Submandibular gland | 17 | 22 | ||
| Major salivary gland, NOS | 13 | 5 | ||
| Laterality | 6.271 | 0.043 | ||
| Left - origin of primary | 55 | 66 | ||
| Right - origin of primary | 61 | 99 | ||
| Not a paired site | 7 | 2 | ||
| T stage | 52.921 | <0.001 | ||
| T1 | 49 | 17 | ||
| T2 | 34 | 29 | ||
| T3 | 26 | 62 | ||
| T4 | 14 | 59 | ||
| Grade | 7.693 | 0.053 | ||
| I–II | 21 | 15 | ||
| III | 42 | 80 | ||
| IV | 34 | 44 | ||
| Unknown | 26 | 28 | ||
| Radiotherapy | 3.858 | 0.0495 | ||
| No | 34 | 30 | ||
| Yes | 89 | 137 | ||
| Chemotherapy | 28.897 | <0.001 | ||
| No | 111 | 104 | ||
| Yes | 12 | 63 |
- Abbreviations: MSDC, major salivary duct carcinoma; NOS, not otherwise specified.
3.3 Prognosis analysis of whole study cohort
Univariate analysis showed that male sex (p = 0.039), T3–4 stage (p < 0.001), positive LNs (p < 0.001), and stage III–IV disease (p < 0.001) were significantly associated with poor OS (Figure 2b–e). The 5-year OS rate of patients with negative LNs was 74.3% and that of patients with positive LNs 36.5%. Location of lesion in the parotid gland was associated with a survival benefit compared with location of lesion in the submandibular gland (Figure 2f). Multivariate Cox regression analysis showed that T stage (p = 0.002), LN metastases (p = 0.004), and primary site (p = 0.021) were independent prognostic factors for OS (Table 3).
| Characteristics | Univariate | p | Multivariate | p |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age at diagnosis (year) | ||||
| ≤68 | 1 | |||
| >68 | 1.47(0.988–2.188) | 0.057 | ||
| Race | ||||
| White | 1 | 0.603 | ||
| Black | 0.925(0.492–1.739) | 0.809 | ||
| Others | 0.691(0.334–1.43) | 0.319 | ||
| Sex | ||||
| Female | 1 | |||
| Male | 1.653(1.026–2.662) | 0.039 | ||
| Insurance | ||||
| Insured | 1 | |||
| No/Unknown | 1.474(0.951–2.285) | 0.082 | ||
| Primary site | ||||
| Parotid gland | 1 | 0.174 | 1 | 0.021 |
| Submandibular gland | 1.635(0.975–2.741) | 0.062 | 2.203(1.25–3.882) | 0.006 |
| Major salivary gland, NOS | 1.161(0.535–2.521) | 0.706 | 1.446(0.648–3.224) | 0.368 |
| Grade | ||||
| I–II | 1 | 0.823 | ||
| III | 1.204(0.605–2.395) | 0.596 | ||
| IV | 0.989(0.475–2.061) | 0.976 | ||
| Unknown | 0.994(0.465–2.126) | 0.987 | ||
| Laterality | ||||
| Left - origin of primary | 1 | 0.269 | ||
| Right - origin of primary | 1.366(0.899–2.076) | 0.144 | ||
| Not a paired site | 1.678(0.657–4.287) | 0.280 | ||
| T stage | ||||
| T1 | 1 | <0.001 | 1 | 0.002 |
| T2 | 1.356(0.618–2.975) | 0.447 | 1.135(0.511–2.52) | 0.756 |
| T3 | 3.41(1.766–6.583) | <0.001 | 2.324(1.157–4.669) | 0.018 |
| T4 | 3.656(1.907–7.01) | <0.001 | 3.089(1.555–6.137) | 0.001 |
| N stage | ||||
| N0 | 1 | 1 | ||
| N1–3 | 2.707(1.741–4.209) | <0.001 | 2.042(1.251–3.331) | 0.004 |
| AJCC stage | ||||
| I | 1 | <0.001 | ||
| III | 0.986(0.342–2.844) | 0.979 | ||
| III | 3.337(1.475–7.548) | 0.004 | ||
| IV | 3.377(1.616–7.057) | 0.001 | ||
| Radiotherapy | ||||
| No | 1 | |||
| Yes | 0.843(0.529–1.345) | 0.474 | ||
| Chemotherapy | ||||
| No | 1 | |||
| Yes | 1.366(0.88–2.12) | 0.165 | ||
- Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; HR, hazard ratio; NOS, not otherwise specified; OS, overall survival; PLNR, ratio of positive lymph nodes.
3.4 Identification of the optimal cutoff point for PLNN and PLNR
Next, the clinical value of LN parameters in patients with PLNN ≥ 1 and ELN ≥ 1 was further explored. The optimal cutoff point for PLNN was 16 and the maximum χ2 value was 4.0032. The 5-year OS rate of patients with PLNN ≤ 16 was 42.8% and that of patients with PLNN > 16 was 15.4% (p = 0.046). Moreover, the optimal cut-off point for PLNR was 0.48 and the maximum χ2 value was 7.3602. The 5-year OS rate was 46.8% for patients with PLNR ≤ 0.48 and 26.3% for patients with PLNR > 0.48 (p = 0.007) (Figure 3).
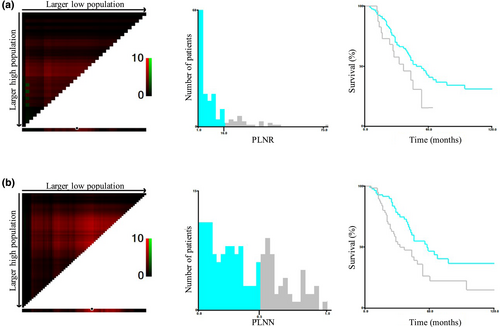
Optimal cut-off values for indicated lymph node parameters. (a) The optimal cutoff value for PLNN was determined to be 16 (χ² = 4.0032). Survival curves based on different PLNN values. (b) The optimal cutoff value for PLNR was determined to be 0.48 (χ² = 7.3602). Survival curves based on different PLNR values. PLNN, number of positive lymph nodes; PLNR, ratio of positive lymph nodes.
3.5 Analysis of prognosis of patients with lymph node involvement
The results of univariate and multivariate analysis of characteristics of patients with MSDC with LN involvement are presented in Table 4. Univariate analysis indicated that age >68 years (p = 0.046), location in the submandibular gland (p = 0.022), and PLNR > 0.48 (p = 0.009) were associated with worse OS. In addition, multivariable Cox regression analysis showed that patients with PLNR > 0.48 had a 1.87-fold greater risk of death than patients with PLNR ≤ 0.48 (p = 0.014). After adjustment for other variables, PLNR remained a strong prognostic factor for patients with MSDC with LN metastasis.
| Characteristics | Univariate | p | Multivariate | p |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age at diagnosis (year) | ||||
| ≤ 68 | 1 | |||
| > 68 | 1.637(1.009–2.655) | 0.046 | ||
| Race | ||||
| White | 1 | 0.944 | ||
| Black | 0.903(0.361–2.257) | 0.827 | ||
| Others | 1.100(0.5–2.418) | 0.813 | ||
| Sex | ||||
| Female | 1 | |||
| Male | 1.383(0.768–2.492) | 0.280 | ||
| Insurance | ||||
| Insured | 1 | |||
| No/Unknown | 1.100(0.655–1.847) | 0.719 | ||
| Primary site | ||||
| Parotid gland | 1 | 0.048 | 1 | 0.012 |
| Submandibular gland | 2.003(1.103–3.636) | 0.022 | 2.917(1.434–5.935) | 0.003 |
| Major salivary gland, NOS | 2.041(0.633–6.577) | 0.232 | 1.456(0.444–4.769) | 0.535 |
| Grade | ||||
| I-II | 1 | 0.460 | ||
| III | 0.49(0.202–1.186) | 0.114 | ||
| IV | 0.521(0.204–1.33) | 0.173 | ||
| Unknown | 0.501(0.189–1.334) | 0.167 | ||
| Laterality | ||||
| Left - origin of primary | 1 | 0.205 | ||
| Right - origin of primary | 0.984(0.597–1.62) | 0.949 | ||
| Not a paired site | 3.598(0.841–15.399) | 0.084 | ||
| T stage | ||||
| T1 | 1 | 0.034 | 1 | 0.004 |
| T2 | 1.990(0.581–6.814) | 0.273 | 1.480(0.408–5.364) | 0.551 |
| T3 | 3.070(1.071–8.8) | 0.037 | 2.941(0.978–8.841) | 0.055 |
| T4 | 4.077(1.43–11.627) | 0.009 | 6.229(1.991–19.482) | 0.002 |
| PLNN | ||||
| ≤ 16 | 1 | |||
| > 16 | 1.786(0.998–3.194) | 0.051 | ||
| PLNR | ||||
| ≤ 0.48 | 1 | 1 | ||
| > 0.48 | 1.900(1.178–3.065) | 0.009 | 1.868(1.138–3.067) | 0.014 |
| AJCC stage | ||||
| III | 1 | 1 | ||
| IV | 0.904(0.493–1.659) | 0.745 | 0.411(0.197–0.858) | 0.018 |
| Radiotherapy | ||||
| No | 1 | |||
| Yes | 0.659(0.365–1.188) | 0.165 | ||
| Chemotherapy | ||||
| No | 1 | |||
| Yes | 1.004(0.606–1.665) | 0.986 | ||
- Abbreviation: AJCC, American Joint Committee on Cancer; CI, confidence interval; ELN, examined lymph node; HR, hazard ratio; NOS, not otherwise specified; OS, overall survival; PLNN, positive lymph node number; PLNR, positive lymph node ratio.
4 DISCUSSION
MSDCs are one of the most aggressive salivary gland tumors, with high rates of local recurrence and metastasis [13]. Surgery with or without adjuvant therapy is the primary treatment for MSDC [14, 15]. More than half of MSDC patients reportedly have LN involvement at the time of initial diagnosis; the rate of occult LN metastasis can reach 24.4% [16]. Neck LN dissection aimed at completely removing occult metastatic LNs should therefore be performed during the initial surgery in patients suspected of having highly malignant MSDCs, whereas selective neck LN dissection combined with adjuvant radiotherapy and chemotherapy should be performed in patients with low-grade malignant tumors [17].
Notably, the status of LN metastasis is closely associated with the prognosis of patients with head and neck carcinomas [18-20]. In clinical practice, the accuracy of the PLNN is limited by the number of ELNs; an adequate number of ELNs may to some degree reduce the risk of residual LNs and LN micro-metastases. The higher the number of ELNs, the higher the possibility of pathological examination revealing LN metastasis; that is, the lower the possibility of missed diagnosis and the more accurate the pN stage. A variety of factors can lead to inadequate ELNs, such as a small surgical field, inadequate technology, inexperienced operators, small LNs, and inaccurate detection methods [21, 22]. Such factors limit the accuracy of prognoses based only on LN metastasis status. Too few ELNs can result in underestimation of the actual number of LN metastases, which in turn can directly affect the accuracy of pN stage [23]. In addition, incorrect assessment of the pN stage further affects the selection of postoperative adjuvant therapy and the accuracy of determination of a patient's prognosis.
PLNR, the ratio of PLNN to the total number of ELNs, reflects both of these prognostic factors and thus has great prognostic value. For the same number of LN metastases, the PLNR decreases in parallel with increasing number of ELNs. Conversely, for the same number of ELNs, the PLNR increases with increasing number of LN metastases, indicating that the tumor is prone to metastasize. Therefore, PLNR may provide a more accurate estimate of the pN stage than either PLNN of ELN alone in patients with head and neck carcinomas.
The main highlight of our study is that we first identified a significantly increased risk of death in patients with than without LN metastases and then focused on the subgroup of patients with LN metastases to further explore the potential value of PLNN and PLNR. The optimal cut-off values for PLNRs used in recently published studies evaluating the prognostic value of PLNR in patients with head and neck carcinoma have been inconsistent owing to differences in pathological type, sample size, inclusion and exclusion criteria, and statistical methods [24-26]. The cohort of the present study was limited to patients who had undergone surgery for MSDC. After adjustment for other variables (hazard ratio: 1.868, 95% confidence interval: 1.138–3.067, p = 0.014), we established an optimal cut-off value for PLNR of 0.48 and found that PLNR was closely associated with OS. These data suggest that PLNR is more reliable and accurate than other variables in guiding prognosis and helping to select high-risk patients for active treatment to prevent recurrence.
Our study had some limitations. We included only patients with complete clinicopathological data, which reduced the heterogeneity of samples in certain cities. In addition, the SEER database does not provide information about patient comorbidities, Eastern Cooperative Oncology Group scores, or tumor margins, lack of which may have affected the results of the present study.
5 CONCLUSION
We found that advanced T stage, submandibular gland location, and unpaired lesions were associated with LN involvement. PLNR can reflect both the effects of LN dissection and PLNN, and its prognostic value in patients with MSDC exceeds that of PLNN. The clinical application of PLNR requires further investigation.
AUTHOR CONTRIBUTIONS
Lixi Li: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); writing—review & editing (equal). Di Zhang: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing—original draft (equal).
ACKNOWLEDGMENTS
The authors acknowledge the efforts of the SEER Program tumor registries in the creation of the SEER database and thank all the patients whose data were examined in this study.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
If data are needed, they can be requested from the corresponding author.




