Pseudomonas lipopeptide: An excellent biomedical agent
Abstract
Lipopeptides are surface active molecules with hydrophilic and hydrophobic regions and are kenned to be engendered by different species of Bacillus and Pseudomonas. These lipopeptides can be applied in different domains because of their remarkable properties like antibacterial, antifungal, anticorrosion, antitumor, and antiviral. They act by engendering pores in the cell membrane to perforate and conclusively disrupt them. This property of lipopeptide is valuable as an antimicrobial agent. In 2003, lipopeptides were approved as an antibiotic drug in the United States by the USA Food and Drug Administration (FDA) for the purpose of skin and blood infections caused by bacterial species. The biosynthetic genes for these lipopeptides are regulated by the nonribosomal peptide synthetase system. Amphisin, Tolaasin, Viscosin, and Syringomycin are the four main types of lipopeptides produced by Pseudomonas species. Since these lipopeptides are nontoxic, biodegradable, and environmentally cordial, they can abbreviate undesirable ecological perturbances. They can be considered a multifarious weapon for their application in different domains such as biocontrol agents in plants, emulsifiers in cosmetic and food industries, anticorrosion agents in petroleum industries, and antimicrobial agents in pharmaceutical and biomedical industries.
1 INTRODUCTION
Pseudomonas is a gram-negative bacteria that belong to the Pseudomonadaceae family, which has roughly 191 species. They are cosmopolitan in nature, thus they can be found in a number of environments, such as rhizosphere, soil, organic debris, animals, and plants. Pseudomonas species show great metabolic versatility and induce a variety of secondary metabolites in both in vivo as well as in vitro conditions. Bacteria belonging to the genus Pseudomonas engender several surface-active molecules, that is, lipopeptides (LPs) that have paramount functions for the engendering cells. Several LPs engendering Pseudomonas spp. strains have been isolated from the soil and a few strains have additionally been isolated from water. Because of the release of a range of LPs, different Pseudomonas species, such as Pseudomonas fluorescens, have been noticed and studied in recent years for their potential as biocontrol agents. Few Pseudomonas species are already registered with the United States Environmental Aegis Agency (EPA) for their role as biocontrol agents in the field of agriculture.1 The functional roles and activities of these LPs are considerably broader and will perpetuate to expand with the more recent revelations. While there are just three structurally comparable groups of LPs in Bacillus, the structural diversity of LPs in Pseudomonas is substantially greater.2, 3
Microorganisms create LPs, which are surface-active biomolecules with a wide variety of uses. They are amphipathic molecules having hydrophilic and hydrophobic areas, causing them to combine in fluids of varying polarity. LPs are a group of biosurfactants that are produced mainly by the genus of Bacillus, Pseudomonas, and Streptomyces. A lipid tail is attached to a short cyclic or linear peptide to form these LPs. They are distinguished by differences in their fatty acid chains or peptide moiety. These LPs are gaining popularity due to their antifungal, anticancer, antibacterial, antiviral, and Immunosuppressive effects.4 They act by penetrating the plasma membrane by creating pores in the membranes, which leads to disparity in transmembrane ion fluxes and finally death of the cell.5, 6
LPs in Pseudomonas and Bacillus are synthesized by nonribosomal peptide synthetases (NRPSs) by a thiotemplate mechanism via Adenylation (A), Thiolation (T), and Condensation (C) domains.7, 8 Of the lipopeptide-producing genera, Pseudomonas and Bacillus have gained the utmost attention. They have significant structural diversity due to differences in the composition and length of their lipid tails along with differences in the amino acids in their peptide chains. Surfactin, Iturin, and Fengycin are among the main lipopeptide families secreted by Bacillus of which Surfactin is found to have both Antibacterial plus Larvacidal activity while Fengycin and Iturin have antifungal activities.
The LPs secreted by Pseudomonas were initially categorized into four major groups, namely Viscosin, Amphisin, Tolaasin, and Syringomycin.9 The Viscosin group contains 9 amino acids and the Amphisin group contains 11 amino acids with both the groups having 3-hydroxydecanoic acid as their lipid tail. The Tolaasin group contains 19–25 amino acids and usually 3-hydroxydecanoic acid or 3-hydroxyoctanoic acid as their lipid tail, thus is more diverse than other groups. The Syringomycin group contains nine amino acids which include unusual amino acids such as 2, 4-diaminobutyric acid, and C terminal chlorinated threonine residues.5, 10 Over the past few years, numerous structurally new LPs have been recognized in Pseudomonas such as arthrofactin, put solving I and II, and Pseudodesmin A and B. Tlipopeptidestides were quite different from the four main groups. Several LPs are recognized as co-produced by Pseudomonas syringae from mycin, peptin, and factin families. Syringomycin, syringotoxin, pseudomycin, and synringostatin are representatives of the mycin family, which are composed of a fatty acid tail with 10–16 carbons that is linked to a fully cyclized peptide with 9 amino acids and chlorinated threonine as the last amino acid, thus known as chlorinated cyclic LPs.11 The pectin family comprises the largest known cyclic LPs where the fatty acid chain is joined to a partially cyclized peptide with either 22 amino acids as in the case of synringopeptin SP22 or 25 amino acids as in the case of synringopeptin SP25. The factin family includes linear LPs composed of eight amino acids with synrigofactin as the solitary member.12
Most of the LPs are secreted by P. syringae and P. fluorescens complex. These LPs are known to have antibacterial activity versus soil-borne pathogens and can be used for the management of various plant diseases. Linear LPs were also discovered from some strains of Pseudomonas like syringofactin from P. syringae tomato strain DC300012 and peptin31 which was a derivative of synringopeptin produced by P. syringae strain31R1.13
The main function of lipopeptide secreted by Pseudomonas includes incompatible activity against other microorganisms, role in biofilm construction, and motility. These LPs have been tested against various human pathogenic bacteria. Antibacterial activity was shown against Bacillus megaterium and Mycobacteria, and so forth. LPs like Viscosin, Orfamide, and Putisolvin have the potential to lyse zoospores and thus have a critical effect on Oomycetes like Pythium and Phytophthora species. High concentrations of massetolide A, Putisolvins, and Orfamide were set up to immobilize and cause lysis of zoospores from various oomycetes.14-17 Viscosin was found to only inhibit the motility of zoospores, for its lysis, a higher concentration of Viscosin was required18 (Table 1).
| S. no | Lipopeptide | Producing strain | Action | References |
|---|---|---|---|---|
| 1. | Viscosinamide | Pseudomonas fluorescens DR54 | Antifungal | [19-21] |
| 2. | Tolaasin | Pseudomonas tolasii | Antifungal | [20, 21] |
| 3. | Syringomycin | Pseudomonas syringae pv. syringae | Antifungal | [19, 21] |
| 4. | Putisolvin | Pseudomonas putida strain PCL1445 | Antiadhesive | [22] |
| 5. | Pseudofactin II | Pseudomonas fluorescens BD5 | Emulsifying | [23] |
| 6. | Viscosin | Pseudomonas fluorescens SBW25 | Causes lysis of Trypomastigotes of human pathogen Trypanozoma cruzi (causative agent of Chagas disease) | [24] |
| Mediates in vitro spreading motility and promotion of plant growth | ||||
| 7. | Massetolide | Pseudomonas fluorescens SS101 | Biological control of late blight pathogen Phytophthora infestans | [17] |
| 8. | Tensin | Pseudomonas fluorescens Strain 96.578 | Antifungal activity against Rhizoctonia solanii | [25] |
| 9. | Milkisin | Pseudomonas species UCMA 17988 | Antimicrobial activity against Staphylococcus aureus, Salmonella enterica | [26] |
| 10. | Orfamide | Pseudomonas protegens strain CMR5C | Action against various fungal plant pathogens | [27] |
| 11. | Orfamide H | Pseudomonas protegens CHAO | Inhibiting activity on Magnaporthe oryzae (causative agent of Blast disease in rice) | [28] |
| 12. | Xantholysin | Pseudomonas putida BW11M1 | Antifungal activity and facilitation of surface colonies by swarming | [26] |
| 13. | Xantholysin A | Pseudomonas soli strain F-279,208T | Cytotoxic against several human cancer lines | [29] |
| 14. | Brasmycin | Pseudomonas sp.11K1 | Antibacterial activity against Xanthomonas oryzae | [21] |
| 15. | Bananamide | Pseudomonas sp.COW3 | Antimicrobial activity against Pyricularia oryzae and Pythium myriotylum | [30] |
| 16. | Poaemide | Pseudomonas poae RE*1-1-14 | Shows action against Rhizoctonia solani | [31] |
- Abbreviation: LP, lipopeptide.
Drop collapse test, tensio-metric analysis, hemolysis assays, and spectrophotometric analysis are the various qualitative test to detect the occurrence of these LPs in Pseudomonas. However, the conformation of the LPs is done by various analytical techniques such as high-performance liquid chromatography (HPLC), thin layer chromatography, crystallography, nuclear magnetic resonance, and mass spectrometry Chiral gas chromatography.32 In a plant-affiliated environment where low amounts of LPs are secreted and where the detection is affected by plant-derived compounds, Immunological assays are considered to be a more sensitive technique to quantify and detect LPs. It has been applied in the detection of LP synringopeptin by P. syringae pv. lachrymans where competitive ELISA assay proved to be approximately 100 times more sensitive than HPLC analysis.18
LPs are surface active biomolecules engendered by a number of microorganisms spp. specially Bacillus and Pseudomonas. A number of research and review articles are present on LPs engendered from Bacillus sp. but very little information is available about Pseudomonas LPs and their applications in different sectors. This article presents a piece of concise information about LPs from Pseudomonas sp. and their applications in biomedical and other sectors. Also, further research activities can be performed to use Pseudomonas sp. for a more wide range of applications in association with nanoparticles-based synthesis of LPs.
2 GENE REGULATION OF LPS IN PSEUDOMONAS
The biosynthetic genes for LPs in Pseudomonas like syringopectin, syringofactin, Syringomycin or famideentolysin, Putisolvin, and Viscosin have been fully sequenced.12, 33, 34 The NRPS system is a building block for the incorporation of the amino acids in a stepwise manner in the LPs. The number and the order of the NRPS system are deemed to be collinear with the amino acid peptide (collinearity rule). The NRPS is further divided into an Initiation domain and Elongation modules. The initiation domain further comprises A, C, and T domains. The A domain (Adenylation) is in charge of selecting amino acids and then activating them. The thioesterification of activated amino acids is carried out by the T domain (Thiolation). The N acetylation of the first amino acid is catalyzed by the C domain (Condensation), which links the lipid moiety to the oligopeptides.35, 36 It is also answerable for the epimerization of the amino acids. The elongation domain also consists of the A, C, and T domains where the C domain is responsible for catalyzing the construction of peptide bonds between two amino acids. The Lipopeptide generated thus is cleaved at the terminal by an enzyme called Thiosesterase. This enzyme also cyclizes the mature peptide which affects the release of cyclic LPs.37, 38 These cyclic LPs are well thought-out to be more active than linear LPs because they reduce conformational freedom and thus provide stability.39 A type II TE is also present which acts as a repair enzyme for the proper functioning of the NRPS system.
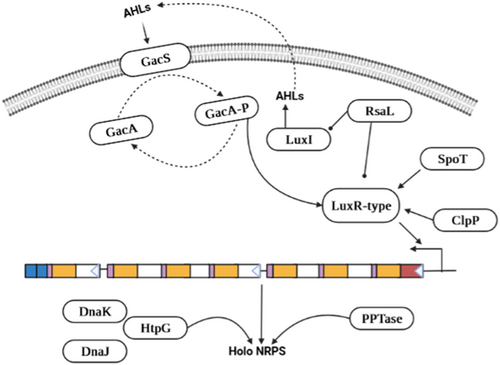
In Pseudomonas sp., two components of transcription regulatory systems have been identified (Figure 1). This system contains GacS as a sensory kinase and GacA as a regulatory response. GacS is activated by autophosphorylation on the interaction with a signal molecule whereas GacA acts as a phosphoryl activator.40 GacA promotes the regulatory gene's transcription following trans-phosphorylation, which in turn regulates the expression of the target genes. Syringomycin, Amphisin, Putisolvin, and entolysin are four genes necessary for the synthesis of LPs, and the GacS/GacA system positively regulates their expression since a mutation in either gene reduces the ability to produce LPs.36, 41 The lipopeptide Putisolvin is synthesized via a quorum-sensing mechanism that causes GacA/GacS phosphorylation under high cell density. The link between the transcriptional regulator LuxR and the signaling chemical N-acyl homoserine lactones (AHLs), which are produced by the LuxI protein in Gram-negative bacteria, is crucial for the quorum-sensing system. ppuI and ppuR are two expression genes that are required for the biosynthesis of AHLs. However, AHL-based quorum sensing does not control the manufacture of the LPs massetolide, Amphisin, and Syringomycin.36 The associated transcriptional regulator LuxR controls the synthesis of various LPs that bind to the NRPS gene operator downstream of the Gac system.
3 TYPES OF LPS
Broadly there are four main types of LPs formed by different species of Pseudomonas, namely Viscosin, Amphisin, Tolaasin, and Syringomycin.
3.1 Viscosin
Viscosin was first obtained from Pseudomonas viscose as an antibiotic compound. It is also known to be manufactured by P. fluorescens. The Viscosin group comprises cyclic LPs which contain nine amino acids linked at the N terminus to 3-hydroxydecanoioc acid. The three presumed genes for the cyclic peptide production under the Viscosin group include viscA, viscB, viscC, and also regulatory gene gacS plays a key role in the regulation of lipopeptide synthesis. The gene viscA is positioned at approximately 1.62 Mb from viscB and viscC genes. These LPs are known to have antiviral properties, inducing g trypomastigotes of the human pathogen Trypanosoma cruzi, which causes Chagas disease, to lyse.42 They are also effective against Phytophthora infestans zoospores, which cause late blight or potato blight, a major potato and tomato disease.43 Viscosinamide is also well-known to have antifungal activity against Rhizoctonia solani where it damages the mycelial growth and thus can prevent the damping off disease in sugar beet.44 They are also known to inhibit the metastatic prostate cancer line without showing any side effects or toxicity. Massetolide is structurally related to Viscosin and is produced by P. fluorescens SS101.
Structure of Viscosin—FA-β-OH-L-Leu-D-Glu-D-aThr-D-Val-L-Leu-D-Ser-L-Leu-D-Ser-L-Ile.
Structure of Massetolide A—FA-β-OH-L-Leu-D-Glu-D-aThr-D-aIle-L-Leu-D-Ser-L-Leu-D-Ser-L-Ile.
3.2 Amphisin
These LPs are secreted by P. fluorescens in the soil environment, especially in the rhizosphere of germinating sugar beet seeds.45 They consist of 11 amino acids in their peptide chain which is coupled to 3-Hydroxy decanoic acid of the adipose acid tail. The study of the crystal structure of members like tensin and ampisin revealed that their structure was mainly helical with the water molecule wrapped around by the cyclic peptide. It was further found that in Amphisin, the 3-hydroxy decanoic acid in the fatty tail bulges from the hydrophilic side of the structure.46 These LPs are engendered by Pseudomonas when they enter the stationary phase and GacA/GacS are associated in their regulation mechanism with amsY as the synthetase gene. Because of the engendering of chitinase and HCN by these LPs, they play a role in antagonism and have the potential for the biological regulation of plant root pathogenic fungi.47 Another cyclic lipoundecapeptide in this family, arthrofactin, is required for swarming activity and blocks the early attachment of planktonic cells in biofilm development. The synthesis of the ester bond between the carboxyl group of the C-terminal Asp and the β-hydroxyl group of d-allo-Thr mediated the cyclization in its structure.48 The gene responsible for its biosynthesis was found to be arthrofactin synthetase (Arf).49 It has remarkable biosurfactant and antifungal properties and thus is one of the best efficacious cyclic LPs.
3.3 Milkisin
It is a novel antibacterial cyclic lipopeptide that is secreted by Pseudomonas species UCMA 17988 and constituted of 10 carbons in the lipid tail and 11 amino acids in the peptide chain and is named Milkisin C. It is isolated from bovine raw milk which has three isoforms namely Milkisin A, B, and D name which belong to the Amphisin family.50 This cyclic lipopeptide is usually active against gram-positive bacterial strains and has shown hostile activity against Listeria monocytogenes, Salmonella enterica Newport, and Staphylococcus aureus. Isoforms C and D of milkisin have longer acyl chains and thus are more potent than isoforms A and B. Thus milkisin can act as a barrier and prevent spoilage of milk by acting against Salmonella or S. aureus in raw milk.51
Milkisin A—CH3(CH2)4 CH(OH)CH2CO-Leu-Asp-Thr-Leu-Ile-Glu-Leu-Ser-Leu-Gln-Leu.
Milkisin B—CH3(CH2)5 CH(OH)CH2CO- Leu-Asp-Thr-Leu-Ile-Glu-Leu-Ser-Leu-Gln-Leu.
Milkisin C—CH3(CH2)6 CH(OH)CH2CO- Leu-Asp-Thr-Leu-Ile-Glu-Leu-Ser-Leu-Gln-Leu.
Milkisin D—CH3(CH2)7 CH(OH)CH2CO- Leu-Asp-Thr-Leu-Ile-Glu-Leu-Ser-Leu-Gln-Leu.
3.4 Tolaasin
In comparison to cLPs in the Amphisin and Viscosin group, LPs in the Tolaasin group are much more diverse for the variation in composition as well as the length of the peptide chain and the lipid tail. These sets of LPs are usually engendered by plant pathogenic strains of Pseudomonas and play a consequential role in the virulence of plants. Tolaasin, a lipodepsipeptide (one or more amide bonds superseded by ester bonds) engendered by Pseudomonas tolaasii, has a molecular weight of 1985 Da with 18 amino acids of which 11 are kenned to be in the D form.52 The primary structure of Tolaasin I, explained by Nutkins et al.,53 bears a B-hydroxyoctanoic acid moiety which is connected to the N-terminus with a sequence of seven consecutive D-amino acids at the N-terminal region of the peptide (Pro2-Val8), with a Ser-Leu-Val reiterate, and then alternate D- and L-amino acid. It also consists of a 2, 3-dehydro-2-aminobutyric acid residue at positions 1 and 13, a D-homoserine (Hse16), and a D-2, 4-diaminobutyric acid (D-Dab17). Finally, the hydroxyl of D-Thr14 and the C-terminal L-Lys18 create a lactone ring. All the analogs of Tolaasin (Tolaasin I, Tolaasin II, Tolaasin A, B, C, D, E) show differences only in peptide moiety and maintaining the B-hydroxyoctanoyl chain at the N-terminus except for Tolaasin A, in which the acyl moiety was a γ-carboxybutanoyl chain.54 These groups of LPs show hemolytic activity and reason for the lysis of red blood cells but their activity is dependent on the temperature and they are recognized as more active at a temperature of 37°C. They are known to be active against fungi plus inhibit the growth of various fungi like Agaricus bisporus, and Pleurotus spp. They also show antibacterial activity toward gram-positive bacteria like B. megaterium and gram-negative species consisting of the genera Escherichia, Erwinia, Agrobacterium, Pseudomonas, and Xanthomonas.20
3.5 Syringomycin
This group harbors LPs that have a unique conserved structure and are structurally quite homogeneous to the Viscosin family of LPs. They are secreted by Pseudomonas syringae. However, these groups of LPs contain some uncommon amino acids like 2, 4-diamino butyric acid (Dab), dehydrobutyrine (Dhb), and the C-terminal 4-chlorothreonine. For the antifungal activity exhibited by Syringomycin, the C-terminal 4-chlorothreonine plays a consequential role. The fatty acid tail is constituted of either a 3, 4-hydroxy or 3-dihydroxy adipose acid with 10-14 carbon atoms. While in the Viscosin group, the lactone ring is formed at the third amino acid position between C-terminal and D-allo-Thr in the peptide chain, in the Syringomycin group the lactone ring is formed between the N-terminal Ser and C-terminal Thr giving rise to the second largest macrocycles of all pseudomonal cLPs. All the members of this group differ only in the length of the fatty acid moiety which might be decanoic, dodecanoic, or tetradecanoic acid.5 In plants, they are recognized to function as virulence factors and substantially increase the disease severity of plants.25
3.6 Putisolvin
Putisolvin is a cyclic lipopeptide that is synthesized by Pseudomonas putida extracted from soil that is heavily tainted with aromatic hydrocarbons. It consists of a 12 amino acid peptide connected to a hexanoic lipid chain where the ester formation occurs among the ninth serine residue and the C-terminal carboxyl group. The genes psoA, psoB, and psoC were set up to encode NRPS which is associated with the biosynthesis of Putisolvin.55, 56 Two isoforms Putisolvin I and Putisolvin II are well-known to have a difference in only the second amino acid from C-terminus with valine at the second position in Putisolvin I and leucine/isoleucine at the second position in Putisolvin II. They were the first LPs to have a longer amino acid moiety and a shorter fatty acid chain. Interestingly both of these LPs were able to break down the existing Pseudomonas biofilms and thus have the potential of reducing the hazardous effects of biofilms in medical or technical equipment.22
4 APPLICATIONS OF LPS
LPs expressed by Pseudomonas and Bacillus have been extensively studied for their several roles including activity against different microorganisms.
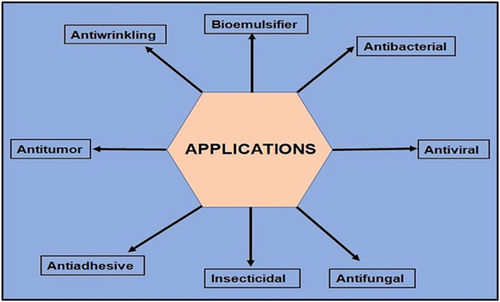
They have paramount applications in arrears to their antibacterial, antifungal, and antiviral activity in different sectors as discussed further. Attempts on utilizing these LPs in the cosmetic and food industries have incremented over the past few years. They additionally have the ability to be utilized as biocontrol agents in plants. The incomparable properties carried by these LPs offer their potential uses in pharmaceutical industries too (Figure 2).
4.1 LPs as antibacterial agents
Bacterial diseases of plants are conventionally very obstinate. Frequently, an amalgamation of control measures is required to combat a given bacterial disease. Antibiotics are customarily preferred to combat these diseases but they result in bacterial resistance after their exorbitant application. Thus the utilization of antagonistic or biological control products like LPs may be considered efficacious for controlling these bacterial diseases. LP s secreted by Pseudomonas are known to be more efficacious against gram-positive bacteria and usually ineffective against gram-negative bacteria mainly explained by the presence of a peptidoglycan layer which inhibits access to the inner plasma membrane. Although in the latest research all the LPs secreted by Pseudomonas were known to be active against the gram-negative bacteria Legionella pneumophila, which is the causative agent of legionellosis. This can also be employed in the biological control of water treatment. While accessing antibacterial activity, metal ions are given due consideration, however, most of the LPs secreted by Pseudomonas were found to be ineffective by the occurrence of metal ions(Ca2+) except for pseudofactin which showed increased activity in the presence of Ca2+, Mg2+, Zn2+ ions.57 Synringopeptin and Tolaasin were effective against B. megaterium.20, 58, 59 Synringopetin, and Viscosin showed activity against Staphylococcus species.60-63
4.2 LPs as insecticidal agents
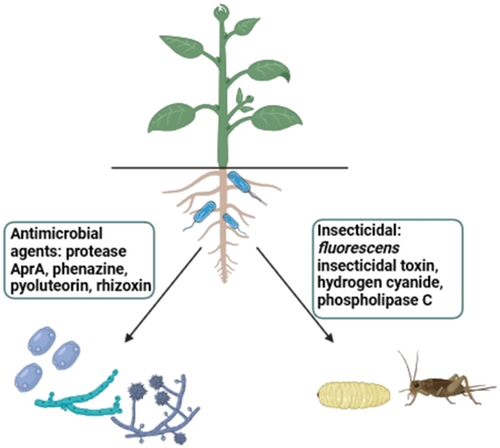
Cyclic LPs engendering Pseudomonas species are mostly kenned for their activity against bacterial, fungal, and protozoans however insecticidal properties have additionally been detected in various Pseudomonas species. The insecticidal property of these LPs is kenned to be associated with the engendering of an Insecticidal toxin, Fit. This toxin is authentically a sensor protein that has been detected in P. fluorescens and Pseudomonas chlororaphis and is accountable for oral insecticidal activity (Figure 3).64 For full insecticidal activity, global Regulator Gac is required. The toxin engenderment is activated upon infection of the host with the insects. Spraying the plant leaves with suspensions of Pseudomonas cells was ample to exterminate 70%–80% of Spodoptera and Heliothis larvae.65 Orfamide A and B, and sessilin-(T) all contribute to the lethal oral insecticidal activity where Orfamide A was active against aphid Myzus persicae of peach trees. In integration, activity against aphids is also testified in Viscosin. Thus, they have the ability to control insect pests and open an incipient perspective for their application in crop pest control in the field of agriculture. Since Pseudomonas are excellent root colonizers, they offer an incipient possibility to combat root pests that are arduous to control with traditional strategies.
4.3 LPs as antiviral agents
Most antivirals target specific viruses, whereas a broad-spectrum antiviral is efficacious against a large range of viruses. Unlike many other antibiotics, antiviral drugs do not eradicate their target pathogen, instead, they obviate its development. Since no definite link has been established between LPs engendering bacteria and the presence of viruses, very little information is available regarding the antiviral activity of these LPs. However, in 1951 the antiviral aspects of lipopeptide Viscosin against infectious influenza A virus, bronchitis virus, and Newcastle disease virus were reported. Antiviral activity of Xantholysin against chronic Hepatitis C has withal been reported.1 These LPs work by acting on the lipid membrane of the virus and finally leading to their disintegration. The capacity for the inactivation of the virus increases with the increase in fatty acid hydrophobicity. With the incrementation in the resistance of antiviral drugs, these LPs offer an incipient array of their utilization in pharmaceutical and biomedical industries.66
4.4 LPs as antifungal agents
Fungi constitute the most astronomically immense number of plant pathogens and are accountable for a range of earnest plant diseases. Once active, fungal diseases exploit plant impotencies, leaving plants more prone to disease and insect pests. Abundant research has been done and reported on the antifungal activities of various LPs. Antifungal activity has been seen in viscosinamide, pseudophomin, tensin, tolaasins, and entolysin. Intriguingly, no marked fungitoxic effect was reported from sensilin and Orfamide A, although their co-production by Pseudomonas sp. CMR12a showed antifungal activity against R. solani.67 They can be used as biopesticides for crop protection. These LPs have also been proven to be effective against various human pathogenic fungi like Aspergillus niger, Aspergillus fumigatus, Aspergillus flavus, Fusarium oxysporum that are the cause of aspergillosis and various other skin infections68 so they can be used as a potential source of antifungal drugs for these diseases.
4.5 LPs as regulators of biofilm
Biofilm is a build-up of bacteria that composes on different surfaces by engendering a substance kenned as an extracellular polymeric substance which enables the bacteria to cohere. These biofilms act as a protective barrier and avert dehydration as well as serve as a defense system against antimicrobials, and UV lights. Since LPs play a role in the regulation of these biofilms, they can be exploited in biomedical applications and can obviate biofilm formation by other microorganisms. A lot of literature data is available regarding the involution of LPs in biofilm regulation. Based on the type of LPs found in Pseudomonas, biofilm formation is either fortified or inhibited.2 This attachment or detachment of bacteria by LPs is stimulated after influencing the outer membrane hydrophobicity of the bacterial cell surface. Xantholysin, senssilin, massand etolide fortifies the production of biofilms,69-71 while arthrofactin, Orfamide, Viscosin, and Putisolvin were found to inhibit the biofilm formation.16, 24
Whereas another lipopeptide Xantholysin identified from P. putida BW11M was discovered to contribute to the biofilm formation. LPs were found to facilitate the distribution of nutrients and oxygen in the biofilms by maintaining the liquid-filled channels in them.72, 73 Thus, these LPs which have intriguing properties of inhibiting the hazardous biofilm formation or breakdown of subsisting biofilms can be employed in the sterilization of medical instruments like catheters which are arduous to emaculate and consequently harbor pathogenic bacteria. These catheters accommodate as superhighways for bacteria and enter the bloodstream and cause serious health quandaries. Catheters that were preincubated with pseudofactin showed a pronounced capability to truncate the adhesion and biofilm formation of Candida albicans, S. aureus, Staphylococcus epidermidis, and Streptococcus agalactiae.74 Hence, LPs have proven to be useful as antibiofilm and anti-adhesive compounds and protect various surfaces from the contamination of microbes.
4.6 LPs as antitumor agents
Resistance to chemotherapeutic drugs and their hazardous side effects are urging the perpetuated revelation of incipient antitumor agents. Multiple research findings have indicated that tumors are kenned to be inhibited by these LPs thus they accommodate as the possible broad-spectrum agents for cancer chemotherapy. The process of apoptosis is blocked in cancer cells which get activated by the LPs. The stimulation of apoptosis in the cell inhibits the spread of cancer cell lines the cell membrane further gets disrupted and causes cellular lysis. Regulation of akt (Protein kinase B) pathway which plays a crucial role in the proapoptotic process is also mediated by LPs75 (Figure 4).
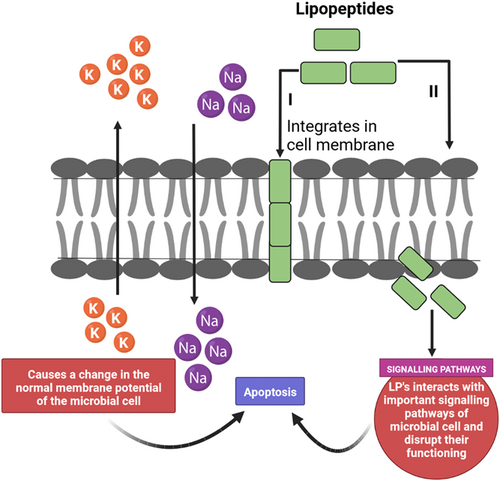
Fragmentation or the condensation of the nuclei, and DNA nicking corroborated the potential of these LPs as antitumor agents.23 Different LPs engendered by Bacillus, Pseudomonas, and Serratia species exhibit antitumoral activity against sundry cancer cell lines. Surfactin, a potent lipopeptide produced by Bacillus, showed a cytotoxic effect on the growth of human colon carcinoma cell lines. Viscosin, a cyclic lipopeptide secreted by Pseudomonas, inhibited breast cancer cell lines (MDA-MD-231) and a metastatic prostate cancer cell line (PC-3M) without causing any paramount side effects and toxicity.76 Pseudofactin II, another lipopeptide showed initiation of apoptosis of human melanoma cells. The phenomenon behind apoptosis was thought to be the interaction of the LPs with the plasma membrane causing cell permeabilization.23 The major compound Xantholysin A, secreted by Pseudomonas soli species, showed intriguing activity against kidney cell lines without causing any cytotoxic effect even when higher concentrations were utilized. It causes the deactivation of mutation of the VHL gene and since VHL and HIFs interact proximately, it induces the HIF pathway which plays a major role in homeostasis.29 In another study, it was found that Syringomycin group cyclic LPs (Nunamycin and Nunapeptin) induced apoptosis in different cancer cell lines like melanoma cancer cell lines, mantle cell lymphoma, and T cells leukemia, while no effect of the amalgamation of these LPs was shown on healthy cells. The occurrence of apoptosis was optically canvassed only when the cancer cell lines were treated with a coalescence of these two LPs. No antitumor activity was visually perceived when they were applied alone for the treatment.77 Lack of cognizance of the underlying mechanism of the antitumor activity of these LPs is the reason for the shortcomings of their potential as antitumor agents.
4.7 LPs as emulsifying agents
Among the sundry functional properties that LPs carry, emulsification and de-emulsification are other major properties that can be exploited in different industries. Emulsification, which is the formation of emulsions from two immiscible liquid phases is probably the most multifarious property of surface-active molecules for practical applications and, as a result, has been extensively studied. These LPs work as an emulsifier or emulsifying agent that acts as a stabilizer by lowering the interfacial tension, resulting in surface energy reduction between two phases and obviates liquids that customarily do not cumulate from separating and can be profoundly utilizable in different food industries along with health care industries. Lipopeptide with the chemical composition of lipid (49.8%) and protein (50.2%) isolated from Pseudomonas aeruginosa exhibited higher emulsifying activity toward peanut oil, crude oil, waste motor lubricant oil, kerosene, diesel, naphthalene and anthracene, xylene than with Triton X-100 under the same concentration (1 mg/ml) opening new possibilities for their application in bioremediation of environment polluted with hydrocarbon to treat oily soils and petroleum wastes. This Pseudomonas-derived lipopeptide showed maximum emulsification activity against discarded motor lubricant oil.78 In another study, it was found that Pseudomonas-derived LPs showed higher emulsifying activity for petroleum than Bacillus-derived LPs. Furthermore, in the same study, Pseudomonas-derived lipopeptide showed emulsifying activity for petroleum, while Bacillus species did not.79 A novel lipopeptide bio emulsifier created by Pseudomonas nitroreducens TSB.MJ10 formed stable emulsions with various hydrocarbons (xylene, gasoline, petroleum, diesel, crude oil, etc.) under pH, temperature, and NaCl stress and consequently revealed their potential in bioremediation of hydrocarbons in the marine environment and also in oil recovery.80 PF2 (C16-Leu), an analog of pseudofactin produced by P. fluorescens BD5 showed good emulsification and foaming activities compared to synthetic surfactants.74 Thus, microbial-derived emulsifiers can replace synthetic surfactants in a wide range of industrial applications such as emulsifiers, solubilizers, wetting agents, and detergents. Because of the increasing environmental concerns and health, consumers tend to prefer natural over synthetic additives in food products. For the solubilization and dispersion of food formulations plus for the steadiness, texture, and appearance of food products, emulsifying agents are consequential. These LPs can ameliorate the consistency of food, amend the shelf life and also revise the rheological properties of the food products. The texture, volume, and crumb structure of bread were kenned to be enhanced by the addition of a lipopeptide emulsifier.81 These biosurfactants are additionally capable of emulsifying oil in creams, gels, and conditioners and thus have a huge potential in cosmetic industries too. Supernatants of the P. putida (B1) strain showed stable emulsions with sunflower oil. The emulsion formed by the strain grown in the KB medium was less stable than that of the strain grown in the LB medium and therefore could have application in the preparation of oil-based cosmetics.82 As they present great biotechnological potential with respect to their emulsifying properties, LPs derived from P. aeruginosa have already been exploited for their utilization as an emulsifier in the cosmetic industry.83
4.8 LPs as plant growth regulatory agents
LPs engendering Pseudomonas species can be found in bulk in the rhizosphere where they act as a pathogen and cause diseases like canker disease in peach trees. However, many Pseudomonas species isolated from different rhizospheres describe their part in biocontrol capabilities too. Many studies have fixated on the potential of these LPs for Phytosanitation and a hefty quantity of data for their direct or indirect role in this respect is additionally available. LPs have a positive impact on plants through the stimulation of their immune system. Many plant-beneficial Pseudomonas species are kenned to actively colonize the soil portion under the influence of plant roots called the rhizosphere and thus forfend the plants from invading pathogens.84 In an investigation, it was found that Viscosin, secreted by P. fluorescens SBW25 protected the germinating seedlings in the soil infected by pathogen Pythium, thus Viscosin could be a useful target for the development of plant growth regulatory agents.85 In a similar study, strain SBW25 and CHAO of P. fluorescens showed the greatest beneficial characteristics by increasing the root length and lateral root length, resulting in a significantly improved pea plant growth, thereby reducing the effects of Pythium infection.86 Similar observations were displayed by the lipopeptide massetolide A-(V).87 Many species of Pseudomonas are shown to form biofilm on the root surfaces. In the rhizosphere of barley roots Pseudomonas sp. DR54, producing viscocinamide was found to produce micro colonies with the indigenous bacteria. In a mutualistic relationship between sugar beets and the Pseudomonas poae, lipopeptide poeamide penetrates the root to form biofilm and absorbs the nutrition from the roots. In exchange for nutrients, the lipopeptide protects the plant from invading pathogens.31 Additionally, there is a generous amount of evidence that states that these LPs induce systemic resistance in plants which is a resistance mechanism in plants that are activated by infection and provide an additional defense system against pathogens. The mode of action depends on increasing the physical or chemical barrier of the host plant.16 As a result, they can act as utilizable targets for the formulation of a seed or treatment compound for engendering plant PGPRs (Figure 5).

4.9 LPs as anticorrosion agents
Corrosion causes serious damage to communication/data transfer facilities, industrial process control installations, sensitive engenderment, pipelines, and cultural heritage premises. The annual worldwide cost of metallic corrosion is estimated to be over $2 trillion, yet experts believe 25%–30% could be averted with congruous corrosion bulwark. Microbiologically Influenced Corrosion refers to the corrosion caused by the presence and the action of microorganisms. It is additionally referred as microbially induced corrosion or biocorrosion.88 Anticorrosion agents avail to forfend all types of metal against rust and corrosion to forfend industrial assets and elongate the life of costly machinery. However, these traditional anticorrosion agents pose serious health risks, and hazardous environmental hazards, are very toxic, and are not biodegradable thus their utilization has been stringently circumscribed.89 Research studies are consequently fixating on finding bio anticorrosion agents that are nontoxic, facilely biodegradable, environment cordial, and frugal. Previous readings have publicized that these LPs play a major role in anticorrosion and therefore can be utilized as fascinating corrosion inhibition implements to forfend steel pipelines in petroleum industries and in victuals and pharmaceutical industries.90 In a study, it was found that the biosurfactant was able to delay the corrosion of stainless steel where the oxide layer engendered by P. fluorescens acted as a barrier to the diffusion of compounds like dissolved oxygen and chloride. It was the adsorption capacity of these surfactants that decelerated the diffusion of dissolved oxygen and delayed the corrosion.91 These biosurfactants have the ability to aggregate and form micelles, composing a protective layer at the surface of the metal thus truncating and averting corrosion.92 A lipopeptide amalgamation of surfactin engendered by Bacillus species H2O-1 had antagonistic activity opposing sulfate-minimizing bacteria, which are the major bacterial group responsible for biocorrosion in petroleum reservoirs and hence was studied to be a potential alternative to the chemical biocides.93 In another such research, LPs engendered by Pseudomonas stutzeri F01 were known to have antibacterial activity against the corrosion-causing bacterial strains. Innovative research in this field can avail to supersede the conventional or the chemical synthetic biocides utilized as anticorrosion agents by these green solutions or the green biocides94 which are eco-friendly and additionally have no negative effects on the environment and human beings.
4.10 LPs in cosmetic industry
The exceptional surface properties, and biological activities along with antiwrinkling and moisturizing activities of LPs have led to their incrementing use in the past few years in cosmetic industries. They are utilized for different purposes in the cosmetic industry like in antidandruff shampoos, soaps, creams, hair conditioners, cleansers, and other dermatological products for the reason of their water-binding capacity, emulsifying, and demulsifying capabilities. The commercially available lipopeptide Matrixylt C16-KTTKS has already been utilized for its antiwrinkle activity in skin care applications. With the advancement in its studies, it has been remarked that it is additionally able to stimulate collagen engenderment by fibroblasts.95 The utilization of LPs has additionally been implicatively insinuated in whitening cosmetic products to distribute a melanocyte-stimulating hormone.96 They are already being utilized as antiwrinkling agents in cosmetics. They have been thus utilized in a variety of cosmetic products where they have demonstrated excellent washability as well as low skin irritation compared to synthetic chemical formulations. More recently, cosmetic compositions consisting of LPs have been utilized for anti-aging treatment too and also in the aversion of stretch marks.97 Monoglycerides engendered by P. fluorescens are commonly utilized in cosmetic industries as a surfactant.98 Surfactin, engendered by Bacillus subtilis has been studied the most for its role in a variety of cosmetic products. Since more research has been done on LPs isolated from Bacillus very little information about LPs isolated from Pseudomonas is available, however, because of their excellent surface properties and biological activities, they hold great potential to be utilized in cosmetic industries in future.
5 CONCLUSION AND FUTURE PERSPECTIVES
LPs are surface active molecules that have wide diversity and very alluring functional properties. They show more vigorous antimicrobial activity against gram-positive bacteria in comparison to gram-negative bacteria. With the extensive use of antibiotics, bacterial strains have developed antibiotic resistance toward several antibiotics. As a result, there is an urgent need to investigate new methods as antibacterial agents. Because of these numerous applications of LPs from Pseudomonas sp., it can be an excellent biomedical agent. Future studies can be made on the comparison of LPs extracted from different bacterial sources for more detailed studies on the mode of action, efficacy, and delivery mechanism in foreign as well as in vitro conditions. These LPs are applied in different domains and have a diverse range of applications not only in combating antimicrobial resistance against plants and animal pathogens but additionally in the cosmetics, food, and pharmaceutical fields. LPs are a class of low molecular weight lipoproteins that possess antibacterial, antifungal as well as anticancer activities. The use of LPs as antibacterial agents has several advantages such as: they are less toxic to plants and animals, having excellent biodegradability, and compatibility with human skin. Further with the incrementation in scientific and commercial interest and advancement in various biotechnological fields can avail to decipher their underlying molecular mechanism and increment their commercial use in the global sector. Future research studies can be performed to enhance the characteristic properties of LPs using different mechanisms such as capping the LPs on nanoparticles and attaching LPs with different antibiotic drugs to enhance the antibacterial activity of antibiotics. LPs have antibacterial activities thus it is suggested that LPs can be used as a preservative for fruits and vegetables also. LPs are the future of modern medicines, studies can be performed to understand the mechanism and target of LPs inside the cell through the Insilco method to enhance the drug targeting and drug delivery method with the help of LPs.
AUTHOR CONTRIBUTIONS
Vivek Chauhan: Conceptualization; methodology; investigation; reviewing. Shreya Mazumdar: Writing – original draft preparation. Akash Pandey: Writing – manuscript preparation; manuscript reviewing; and editing. Shamsher S. Kanwar: Supervision. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors are thankful to CSIR, New Delhi, as well as DBT, New Delhi, for continuous financial support. The authors are also thankful to the BioRender App for providing help with graphical illustration in the manuscript. This work has been funded by Council for Scientific and Industrial Research, New Delhi, under a CSIR-NET Senior Research Fellowship [File No. 09/237(0161)/2017-EMR-1] awarded to one of the authors (V. C.).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
All the authors hereby state that the research and study related to the manuscript has been done under proper norm of ethical code of conduct.
Open Research
DATA AVAILABILITY STATEMENT
The data for the manuscript are studied, collected and compiled from different research papers and review papers mentioned in the reference section.




