Nonviral vector system for cancer immunogene therapy
Wen Nie and Jing Chen contributed equally to this study.
Abstract
Immunogene therapy has become an effective and significant clinical strategy for cancer therapy. Many immunogene therapies are approved for cancer treatment, and more are undergoing clinical or preclinical trials. Even though most patients benefit greatly from immunogene therapy, the strategies may simply activate the systemic immune response against tumors while also pushing the immune system to supraphysiological levels along with a subsequently increased risk of immune-related adverse events. Enhancing the response rate to immunogene therapy is key to controlling side effects and improving efficacy. Improved delivery systems can efficiently deliver genes to the desired tumor cells while alleviating adverse reactions and immunogenicity. Thereinto, nonviral vectors improve the permeability, retention, and pharmacokinetic characteristics of drugs, thereby reducing side-effects and providing broad prospects for enhancing the efficiency of immunotherapy and becoming a leading anticancer candidate. Here, this review highlights the types of common functional nonviral vectors, discusses their advantages and disadvantages, as well as the latest applications in different cancers. Undoubtedly, this review proves that nonviral vectors combined with immunogene therapy are promising treatments for cancers. Nevertheless, further research is needed to solve safety concerns and improve the efficacy of nonviral vectors-based cancer immunogene therapy for future clinical application.
1 INTRODUCTION
According to the latest estimates of cancer incidence and mortality provided by Globocan, the number of new cancer cases worldwide reached 19.3 million in 2020 and nearly 10 million died of cancer.1, 2 Compared to 2020, the global burden of cancer is projected to increase by 47% in 2040.3 At present, the most widespread clinical application is the traditional cancer treatment strategy of surgical removal combined with radiotherapy or chemotherapy, which have strong tumor-killing ability. However, they have poor tumor tissue selectivity, multidrug resistance, and significant toxicity and side effects, limiting the clinical application of chemotherapeutic drugs. In recent years, successful immune checkpoint therapy cases have transformed people's concept of tumor treatment and promoted the development of new antitumor medications from relying on external methods to kill tumor cells to depending on the immune system to kill tumor cells.2, 4 Furthermore, gene editing, the development of next-generation sequencing technology, and CRISPR-Cas9 technology have improved new tumor molecular biology research methods.5, 6
Immunogene therapy, as an important immunotherapy, introduces the target gene into immune response cells or tumor cells, and then back into the body or directly into the body, improves the immune system's ability to recognize or kill tumor cells, and stimulates or enhances antitumor immunity, which holds great promise for cancer therapy.7, 8 The success of immunogene therapy largely depends on whether the gene is effectively delivered to target cells and tissues in vivo or in vitro. Naked gene delivery suffers from low transfection efficiency, nuclease sensitivity, renal clearance, low cell selectivity, and stimulation of immune responses.9, 10 Therefore, it is critical to use stable vectors to protect nucleic acids from circulating nucleases, avoid attack by the immune system, and ensure that therapeutic vectors effectively target tumor cells.11, 12 The vectors available for gene delivery are divided into viral and nonviral systems.13 Compared with viral vectors, nonviral vector systems have lower immunogenicity, provide a larger gene payload, and are not limited by the size of the introduced gene molecules.14-17 Nonviral vectors can protect antigens and adjuvants from the surrounding biological environment, increase half-life, minimize systemic toxicity, and promote drug delivery to tumor sites.18, 19 Nonviral vectors can also improve drug permeability, retention, and pharmacokinetic characteristics, thereby reducing side effects.20, 21 Modified nonviral vectors have drug-controlled release properties, which can precisely release drug doses within a controllable time and reduce drug resistance.22 Improved delivery technology can increase the accumulation of immunotherapy in diseased tissues, more effectively target the desired tumor and immune cells, and reduce off-target adverse reactions.23, 24 Research is underway to develop new nonviral delivery platforms for immunotherapy, including liposomes, exosomes, polymer nanoparticles, inorganic nanoparticles, and hydrogels.25 Some of them have been applied in immunogene therapy of diseases. For example, researchers have reported that polymer nanoparticles containing cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) small interfering RNA (siRNA) could directly and effectively modify T cells.26 Another study coupled IL-2 to the surface of hydroxyethyl starch nanocapsules.27 It has been reported that silica, poly (lactic-glycolic acid copolymer), and polystyrene nanoparticles were injected intraperitoneally and selectively accumulated in tumor-associated macrophages (TAMs).28 At present, a large number of nano-drug carriers have been applied to cancer treatment, such as drug conjugates, lipid nanocarriers, and polymer nanocarriers.29 Since 2016, the number of patents related to nonviral vectors has increased, of which the United States ranked first with 24.04%, while Europe and China ranked second with 14.8%.30 According to statistics, as of April 2022, ClinicalTrials. gov (https://www.clinicaltrials.gov/) has received 331 clinical trials on “nanoparticles” and “cancer,” of which 180 clinical trials are in “Recruiting” or “Completed.” Increasing evidence proves that nonviral vectors have become a beneficial tool for cancer treatment.
This review highlights the recent advances in the development of nonviral vectors for gene delivery and discusses the advantages and limitations. We also summarize the application of nonviral vector-mediated immunogene delivery in solid tumor treatment.
2 IMMUNOGENE THERAPIES
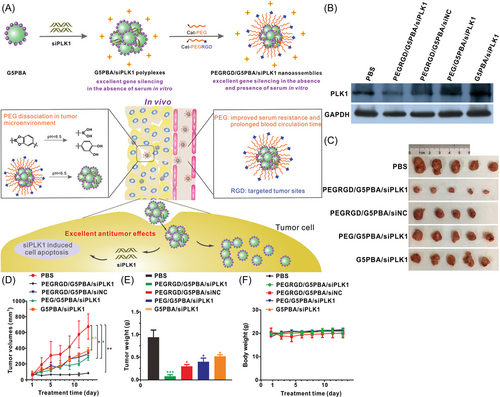
Gene therapy refers to the introduction of normal or therapeutic genes (DNA, RNA, or oligonucleotides) into target cells through molecular biology methods to restore the normal expression of genes, thereby treating diseases.31, 32 Improvements in the design of gene delivery systems allow the safe delivery of cytokines (CKs), siRNA, and several other gene replacement strategies and are effective in both preclinical and clinical applications.33, 34 Studies show that most gene therapy research is still at the clinical or preclinical stage. As of April 2022, ClinicalTrials.gov (https://www.clinicaltrials.gov/) showed 5274 “gene therapy” studies in clinical trials, of which 2781 were “cancer.” Thus, gene therapy has great potential for cancer treatment. Therefore, many researchers are devoted to modifying gene delivery carriers to better realize their antitumor effects. Hongmei Liu et al.35 reported that a poly (ethylene glycol) (PEG)-modified dendrimer/siRNA nanoassembly was constructed by coating phenylboronic acid (PBA)-modified Generation 5 (G5) poly(amidoamine) dendrimers (G5PBA) (Figure 1A). The results showed that PEG/G5PBA/siRNA had a better gene silencing effect and serum resistance than the other groups (Figure 1B). PEG/G5PBA/siRNA significantly inhibited tumor growth (Figure 1C–E), while the body weight of each group remained unchanged (Figure 1F).
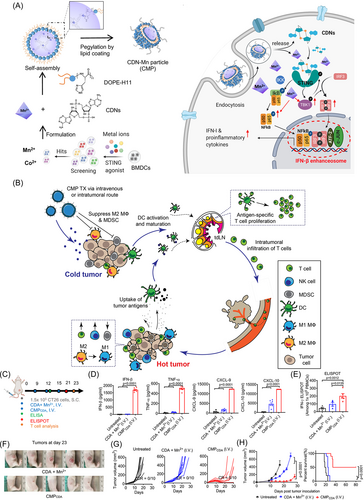
Current immunotherapy is divided into immune checkpoint therapy, CK therapy, adoptive cell transfer, agonistic antibodies against costimulatory receptors, cancer vaccines, oncolytic viruses, and bispecific antibodies.36-39 There are now more than a dozen immunotherapies approved for disease treatment, and more are in clinical trials. The application of immunotherapy in cancer treatment is also increasing.40, 41 Cyclic dinucleotide (CDN) of stimulator of interferon genes (STING) agonists and Mn2+ activate innate immunity by increasing tumor immunogenicity. However, CDNs have delivery obstacles, such as poor cell targeting, fast clearance speed, and inefficient transport to the STING-located cytoplasm.42, 43 As shown in Figure 2A, Mn2+ and CDN STING agonists collaborate to self-assemble nanoparticles (CDN-Mn2+ particles [CMPs]), which can effectively deliver STING agonists to immune cells. CMP exerts anti-tumor efficacy by reversing immunosuppressive tumor microenvironment (Figure 2B). The mouse tumor model was treated with trace STING agonists (Figure 2C). Compared with the free CDA+ Mn2+ mixture, the CMP group significantly improved immune activation, increased the levels of interferon-β (IFN-β), tumor necrosis factor-α (TNF-α), C-X-C motif chemokine ligand 10 (CXCL-10), and C-X-C motif chemokine ligand 9 (CXCL-9) (Figure 2D), and significantly enhanced the AH1-specific CD8+ T-cell response (Figure 2E). CMP significantly inhibited tumor growth (Figure 2F–H).44
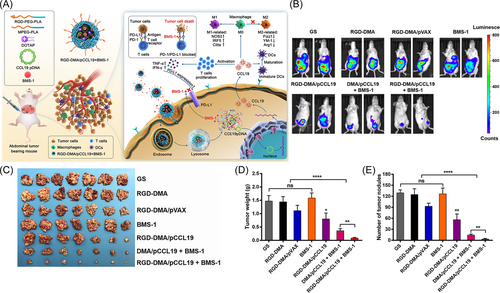
Immunogene therapy is based on the principle of immunology, a new tumor treatment plan established by immunology technology and genetic engineering technology.45 Early immunotherapy mainly focused on enhancing the activity of CKs,46 such as IFN-α and interleukin-2 (IL-2), which are used in antitumor therapy,47, 48 and this stage is mainly nonspecific immunotherapy.49, 50 The emergence of ipilimumab and chimeric antigen receptor (CAR)-T-cell therapy is a turning point in the rapid development of cancer immunotherapy.11, 51 CAR-T-cell therapy has changed the cancer treatment strategy, using ex vivo-modified autologous T cells to treat relapsed or refractory cancer.52-54 Current immunogene therapy can be achieved through different methods55-57: encoding tumor-associated antigen (TAA),58, 59 major histocompatibility complex (MHC), or costimulatory signal gene60 to improve the immunogenicity of tumors and stimulate the body to produce antitumor immune responses; encoding related CKs,61 such as IL,62 granulocyte-macrophage colony-stimulating factor (GM-CSF),63, 64 IFN,65 and tumor antigen receptor genes65-67 to enhance the specificity of the body or nonspecific antitumor immune responses; editing genes based on immune checkpoints to inhibit the expression of tumor cell immunosuppressive factors or their receptors8, 68; and encoding tumor suppressor genes.69, 70 Yu Ting's team reported that the immunostimulatory chemokine C-C motif chemokine ligand 19 (CCL19) and the immune checkpoint ligand programmed death ligand 1 (PD-L1) inhibitor BMS-1 reshaped the immunosuppressive tumor microenvironment (TME) by increasing the likelihood of interactions between DCs, T cells, and B cells in secondary lymphoid tissue.71 A RGD-modified targeted gene delivery system consisting of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), methoxy poly(ethylene glycol)-poly(lactide) (MPEG-PLA), and RGD-poly(ethylene glycol)-poly(lactide) (RGD-PEG-PLA) was successfully developed by the self-assembly method (Figure 3A). RGD-DMA/CCL19 and BMS-1 significantly inhibited tumor growth (Figure 3B–E). There are two major application approaches for immune gene therapy: indirect in vivo application, such as in vivo transfusion of immune cells transfected in vitro, and direct injection in vivo application of gene expression bodies, thereby interfering with the gene expression of immune cells through gene editing systems and ultimately regulating the immune system to promote anticancer immunity.72 As of April 2022, ClinicalTrials. Gov (https://www.clinicaltrials.gov/) shows 12 clinical trials of immunogene therapy studies on cancer research. This shows that immunogene therapy technology is developing rapidly and is gradually being translated into clinical practice.
However, in terms of safety, immunotherapy can cause autoimmune side effects in some patients, leading to attacks on healthy tissues.72 Second, immunotherapy is not as effective as lymphoma for solid tumors because these cancers produce abnormal extracellular matrix (ECM), which is difficult to penetrate. Nuclease attack, cell diffusion, biofilm formation, and endocytosis are also obstacles to immunogene delivery.73 Enhancing the response rate to immunotherapy is key to controlling side effects and improving efficacy. In these clinical trials, the lack of safe and effective delivery vectors hinders the clinical application of immunogene therapy. The low transfection efficiency of the vector makes the immunogene perform poorly in achieving the desired biological effect. Recently, studies have used delivery technology to solve the limitations of existing cancer immunotherapy.56, 74 Currently, 16 gene therapy products are in the market, including 13 viral vectors and 3 nonviral products.33 Nonviral vectors are less mutagenic and cytotoxic than viral vectors, attracting an increasing number of researchers to explore promising delivery systems that will drive the development of immunogene therapy.75 With the development of vector research, immunogene therapy is expected to become a first-line therapy for cancer diseases.
3 NONVIRAL CARRIERS
Physical and chemical nonviral systems have been developed for therapeutic DNA/RNA delivery.76-78 Physical methods include microinjection, electroporation, sonic perforation, magnet transfection, photoperforation, microfluidics, and gene gun.79 Compared with chemical nonviral systems, physical methods are primarily unsuitable for human body gene delivery in small animals such as mice. Here, we focus on chemical nonviral vectors. Nonviral delivery vectors based on nanotechnology mainly include liposomes, polymer nanoparticles, and inorganic nanoparticles (Table 1 and Figure 4).
| Nanocarrier types | Targeting ligands | Nanocarriers | Payloads | Target | Advantages | Reference |
|---|---|---|---|---|---|---|
| LNPs | LNPs | mRNA (CAR-T) | T cells | LNP-transfected CAR-T cells have a higher survival rate (>75%) than electroporated cells. | 80 | |
| Vitamin E | ssPalmE | DNA vaccines | DC | ssPalmE caused higher gene expression activity than ssPalm. | 81 | |
| Vitamin E, OVA | ssPalm | pDNA | E.G7-OVA tumor and B16-F10 melanoma metastasis. | 82 | ||
| KALA | ssPalmE-KALA | DC vaccines | DC | OVA-encoding mRNA inducted the OVA specific cytotoxic T-lymphocyte activity. | 83 | |
| Polymer nanoparticle | PBAE | pDNA | T cells | DNA-carrying nanoparticles can effectively deliver CAR genes into T cell nuclei. | 84 | |
| PEI, PBAE | Antigen-encoding mRNAs | Terpolymers showed several times higher transfection efficiency than PEI. | 85 | |||
| FA | DOTAP, MPEG-PCL-MPEG | pCKb11 | Tumor cells | The FPPPD-delivered pCKb11 was safe and well-tolerated in mice without systemic toxicity. | 86 | |
| PDSA | cationic lipid core, lipid-PEG shell | siRNA | Tumor cells | Polymer nanoparticle can effectively encapsulate siRNA and react with GSH in the cytoplasm. | 87 | |
| HA-CHO | PEI/DNA | Tumor cells | Two different in situ crosslinking routes enhance the stability of the nanoparticles. | 88 | ||
| pHEMA-g-pDMAEMA | T cells | Polymer has more than 50% transfection efficiency in the Jurkat human T-cell line. | 89 | |||
| mPEG-bPEI-PEBP | CAR | Jurkat cells | The polymer can promote DNA escape into the cytoplasm. | 90 | ||
| Inorganic nanoparticles | MSNs | Fe2+, Ru2+ | mtDNA | MRF target oxidative damage to mtDNA of tumor cells. | 91 | |
| POPC | MSN microrods | Antigen | APC | MSR-SLBs can promote the polyclonal and antigen-specific expansion. | 92 | |
| Fused porous silicon nanoparticles | siRNA | TAM | Fusion porous silicon nanoparticles have a strong gene silencing effect. | 93 | ||
| Other types | HA | HA-PCLA, levodopa | OVA, GM-CSF | ISHs effectively locate immune cells and control the delivery of immunomodulatory factors. | 94 | |
| ManNP | Nanohydrogel | siRNA | M2 | ManNP enhances the uptake of M2 polarized macrophages that express CD206. | 95 | |
| CpG | DNA hydrogel | DNA | DNA hydrogel stimulate immune cells to release CKs. | 96 | ||
| Exosomes | PD-L1 | The circulating exosomal PD-L1 is positively correlated with the IFN-γ. | 97 | |||
| Exosomal circUHRF1 | NK | Exosomal circUHRF1 inhibits the function of NK. | 98 |
- Abbreviations: APC, antigen-presenting cell; CpG, cytosine-guanine; DC, dendritic cell; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; FA, folic acid; HA, hyaluronic acid; LNP, lipid-based nanoparticle; NK,natural killer; pDNA, plasmid DNA; PBAE, Poly(β-amino ester); PD-L1, programmed death ligand 1; PDSA, poly(disulfide); PEI, polyethyleneimine; ssPalm, SS cleavable and pH-activated lipid materials; POPC,1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; siRNA, small interfering RNA; TAM, tumor-associated macrophage.
3.1 Lipids and lipid-based nanoparticles (LNPs)
Lipid-based delivery systems include micelles, liposomes, and LNPs. Micelles are composed of a single layer of lipid molecules. The term “liposome” was first coined in the 1960s, shortly as a dosage form for drug delivery. In 1987, the first cationic lipid DOTMA was prepared.99 Subsequently, many similar cationic lipids, such as DOTAP, DDAB, CTAB, and DOPE, were successively prepared and applied to gene delivery in different cells.100, 101 Early liposomes refer to closed vesicle structures formed by amphiphilic phospholipids and cholesterol to form a stable bilayer. There are three main types according to charge properties: cationic liposomes, anionic liposomes, and neutral liposomes.102 LNPs are usually composed of four components: ionizable cationic lipids promote self-assembly into virus-sized particles (~100 nm) and release plasmids to the cytoplasm; adipocyte-linked PEG can extend the half-life of the preparation; cholesterol, a kind of stability, changes the rigidity of the lipid membrane; naturally occurring phospholipids support the structure of lipid bilayers, such as phosphatidylcholine and phosphatidylethanolamine and other lipids.58, 103 Drugs are generally encapsulated in an ultramicroscopic spherical carrier preparation in the middle of a lipid bilayer (the hydrophilic part is exposed to the water phase, and the hydrophobic region is aggregated at the tail to form an ultra-microcapsule structure).104 The lipophilic lipid bilayer and the hydrophilic inner core can carry lipophilic drugs and hydrophilic drugs, respectively. LNPs differ from traditional liposomes mainly because they form a micellar particle core inside, which is a morphological structure whose formulation and synthesis parameters can be changed. The liposome drugs marketed all use LNPs as carriers.
Recently, LNPs have also been successfully applied for the delivery of genes in vitro and in vivo. For instance, Billingsley et al. designed a library of 24 ionizable LNPS for screening preparation that can efficiently transfect primary T cells with CAR mRNA. Results showed that LNP-transfected CAR-T cells have a higher survival rate (>75%) than electroporated cells (~30%).80 An attractive mRNA delivery system made of cationic liposomes (L), cationic polymers (P), and mRNA (R) could promote dendritic cell (DC) targeting and exert effective antitumor responses.105 LNPs composed of SS cleavable and pH-activated lipid materials (ssPalm) are used as delivery vehicles for DNA vaccines in vivo.81 Compared with LNPs composed of ssPalms with different hydrophobic scaffolds, ssPalmE-LNPs of LNPs containing ssPalm with vitamin E scaffolds (ssPalmE) caused higher gene expression activity. ssPalmE-LNPs loaded with ovalbumin (OVA, a model tumor antigen) plasmid DNA (pDNA) induced significant antitumor.81 In other studies, ssPalmE-LNPs loaded with pDNA induced an antitumor effect on E. G7-OVA tumor and B16-F10 melanoma metastasis.82 In addition, the combination of ssPalmE-LNP and α-helical cationic peptide “KALA” (ssPalmE-KALA) was developed as a lipoplex-type mRNA carrier for DC vaccines. Compared with the lipid complex prepared by ssPalm with a fatty acid scaffold (myristic acid), the transfection of ssPalmE-KALA loaded with mRNA promoted protein expression and proinflammatory CK production from bone marrow–derived dendritic cells (BMDCs).83
Compared with other systems, LNPs have many advantages as important nonviral gene carriers. First, LNPs are modifiable, which can improve the problems of poor targeting selectivity of nucleic acids and vectors, short blood circulation time, and poor stability in vivo. For example, negatively charged “helper polymers” can enhance the efficacy of LNPs, enabling efficient codelivery of siRNA and mRNA.106 LNPs coated with hydrophilic polymers have good pharmacokinetic properties in vivo, making them attractive carriers for gene delivery.107 Simple incorporation of monoacyl derivatives into PEG-lipids resulted in peptide-modified LNPs with improved properties.108 CL4H6 lipid-loaded siRNAs composed of pH-sensitive cationic lipids can be selectively and efficiently taken up and show gene silencing activity in TAMs.109 Second, LNPs can efficiently deliver RNA in vivo, protect it from nuclease degradation,110 and produce prolonged protein expression at the injection site.111, 112 Safe and effective novel amino-ionizable lipid LNPs have been reported for the delivery of Cas9 mRNA and sgRNA.113 At the same time, LNPs have good cell affinity and histocompatibility because of their similar structure to biofilms. The lipid-based liquid crystal nanoparticles constructed by Shufang He et al., called nanoconverters, can change their structure within cells in an acidic environment and induce endosomal membrane fusion to facilitate the release of siRNA.114 LNPs can also reduce drug toxicity. Compared with free drugs, drugs encapsulated by LNPs are less accumulated in the heart and kidneys. LNPs can improve the stability of drugs, and some drugs with unstable properties and functions in specific environments can be protected by LNPs-like bilayers. LNPs are mainly used as immune adjuvants and carriers of nucleic acids, antigen peptides, proteins, and other immunostimulatory molecules in tumor immunotherapy.107, 115, 116 LNPs have become one of the most attractive and commonly used gene delivery tools.117
As a delivery vehicle, LNPs have obvious advantages, but some areas need to be improved. The first is the stability of the LNPs. When the temperature and pH change, the LNPs are prone to aggregation, phospholipid oxidative hydrolysis, and drug leakage. Second, some nonnatural liposomes are inherently immunogenic. In addition, LNPs are also prone to interact with blood, which may lead to aggregation or instability.118
3.2 Polymer nanoparticles
“Polymer nanoparticles” is a term used to refer to polymer nanospheres and nanocapsules. The main polymers used for gene delivery are cationic polymers and amphiphilic polymers. Polymer nanoparticles include poly(cyanoacrylate) (PCA), poly(D,l-lactide-co-glycolide) (PLGA), poly(glutamic acid) (PGA), polyamidoamine (PAA), poly(ε-caprolactone) (PCL), polyethyleneimine (PEI), polyaminoester (PAE), poly(ethylene oxide) and poly(l-lysine) (PLL), poly dimethylaminoethylmethacrylate (PDMAEMA) and poly(D,l-lactide) (PLA).119-122 Polymer nanoparticles usually have a high density of amine groups that can be protonated at physiological pH when mixed with negatively charged nucleic acids to form stable complexes through electrostatic interactions and entropy changes.123, 124
Studies have shown that nanoparticles containing immunomodulators can efficiently deliver mRNA and siRNA. Poly(β-amino ester) (PBAE) has a half-life of 1 to 7 h in aqueous conditions; PBAE is the core material of targeted T-cell nanocarriers and can shield positive charges to reduce off-target binding. Tyrel T. Smith's team84 combined T-cell-targeting anti-CD3e f(ab′)2 fragments with biodegradable PBAE form nanocarriers, encapsulating two plasmids of the CAR transposon cassette and encoding PiggyBac (PB) transposase to stabilize CAR integration. DNA-carrying nanoparticles can effectively deliver CAR genes into T-cell nuclei. PLGA and PCL polymers are commonly used biodegradable polymers in the biomedical field due to their biodegradability and biocompatibility. PLGA is widely used to package tumor antigens and other immune adjuvants.125 Intravenous injection of PCL and PBAE terpolymers into mice showed several times higher transfection efficiency than PEI aggregated in the spleen, which makes the use of these vectors for intravenous delivery of antigen-encoding mRNAs possible.85 Gao Xiang's team developed a self-assembled nanoparticle delivery system based on DOTAP, MPEG-PCL-MPEG, and FA-PEG-PCL-PEG-FA to deliver plasmids and inhibit the infiltration of immunosuppressive cells in tumor tissues.86, 126 PLGA-PEG/PBAE nanoparticles are capable of high loading and sustained release of GFP plasmids for efficient and sustained gene delivery.127 A biodegradable and redox-responsive nanoparticle platform consisting of a solid poly(disulfide) (PDSA)/cationic lipid core and lipid-PEG shell can deliver siRNA to tumor cells. Polymer nanoparticles can effectively encapsulate siRNA in the extracellular environment and can also react with high concentrations of glutathione (GSH) in the cytoplasm, inducing the carrier to rapidly release siRNA in cells.87 Hyaluronic acid (HA)-containing aldehyde groups (HA-CHO) were modified on PEI/DNA composite particles to form double-crosslinked nanoparticles in situ. Polymer nanoparticles possess both electrostatic and chemical crosslinks between the outer layer and core, and two different in situ crosslinking routes enhance the stability of the nanoparticles. Targeting ligands on the HA layer also mediate the specific recognition of cancer cells.88 Polyamidoamine (PAMAM) dendrimers, a hyperbranched polycationic compound that binds to a negatively charged nucleic acid ribose-phosphate backbone via electrostatic interactions, are a novel gene delivery vehicle that can easily penetrate tumor blood vessels and deliver intratumorally accumulation. Positively charged siRNA/dendrimer delivery complexes form stable nanocomplexes with negatively charged fragments of targeting peptides through electrostatic interactions, avoiding siRNA degradation by nucleases and enabling RGDK-specific targeting of cancer cells.128 Brynn R. Olden demonstrated that the loop-branched pHEMA-g-pDMAEMA polymer has more than 50% transfection efficiency in the Jurkat human T-cell line and is less toxic (>90% survival rate).89 The same group studied the barriers associated with vector-based T-cell transfection, including reduced cell uptake and slower acidification of the endosome. Since pH is an indispensable condition for triggering in vivo release, the designs of polymer nanoparticles must consider alternative release mechanisms in vivo.129 With the improvement of hydrophobic balance and the regulation of positive surface charge, the polymer can provide effective and safe gene delivery. PAMAM-modified PEG can enhance the water dispersibility of PAMAM, thereby obtaining a complex with a water-soluble shell and a water-insoluble core and increasing the expression of IL-12.130 PEI is a class of cationic synthetic polymers rich in amine groups. At neutral pH, the protonation of the amino group on PEI produces a high positive charge density, which electrostatically interacts with the negatively charged nucleic acid to form a stable complex. Once endocytosis occurs, protonated PEI will induce an osmotic effect (“proton sponge effect”) to induce endosome rupture and subsequent release of the complex into the cytosol.131 In a recent study, an amphiphilic triblock copolymer, methoxy polyethylene glycol-branched polyethyleneimine-poly (2-ethylbutyl phospholane) (mPEG-bPEI-PEBP), delivered CAR and plasmids to Jurkat cells. The mPEG-bPEI-PEBP copolymer can promote the escape of DNA into the cytoplasm for transcription and expression through endocytosis swelling and endosome destruction.90 PEI is one of the most commonly used cationic polymers in gene delivery due to its high transfection efficiency, but the use of PEI is limited due to its nonbiodegradability and high toxicity.
Compared with other delivery vehicles, polymeric nanoparticles have a higher loading capacity for poorly water-soluble drugs. Acid-sensitive core-shell nanoscale coordination polymer particles (NCPs) loaded with carboplatin prodrug and PD-L1 siRNA (siPD-L1) in the core and digoxigenin on the shell for trimodality state cancer therapy.132 By modulating the composition, responsiveness, surface charge, polarity, degradability, and molecular weight of polymer nanoparticles, the gene delivery efficiency and release kinetics of polymers can be altered for therapeutics. For example, PEGylation can improve the stability of PBAE, further enhancing the therapeutic effect of tRNase gene therapy and improving the survival rate of animals.133 Some polymer molecules carry ester bonds that are biodegradable, and they can improve the biodegradability and cytotoxicity of the polymer. Studies have reported that esterase-hydrolyzable cationic polymers utilize high esterase levels in macrophages to rapidly release the entrained DNA to efficiently transfect cancer cells. Nasha Qiu's team designed an esterase-responsive polymer, poly[N-[2-(acryloxy)ethyl]-N-[p-acetoxybenzene PQDEA]-Diethylammonium chloride] (PQDEA)-modified DSPE-PEG, and introduced the ligand aminoethylanisamide (AEAA), which formed DSPE-PEG-AEAA/DOPC/PQDEA (APEG-LPs) polymer nanoparticles.134 The transfection efficiency of APEG-LPs/pDNA in the original 264.7 cells and KPC cells was more effective than that of the positive controls PEI and Lipo2000.135 Calcium phosphate (CaP) coatings can biodegrade under mildly acidic subcellular conditions (lysosomes). Some studies have modified the CaP coating on nanoparticles by biomineralization, which can avoid the premature release of siRNA from the carrier.136 Furthermore, the surface charge of polymer nanoparticles plays a key role in cellular targeted uptake and activation of immune responses.123 Some studies have modified PBAE–mRNA complexes with PGA functionalized with antibodies (anti-CD3 or anti-CD8) to form charge-neutral nanocarriers, which endow the carriers with lymphocyte targeting. Research has shown that polymer nanoparticles targeting CD3 carrying the mRNA encoding the immunogene can not only bind to cells but also stimulate receptor-mediated endocytosis.137 McKinlay et al. developed charge-switchable release transporter CARTs cationic polymers that deliver mRNA. After CART delivers the gene to the cell, it undergoes rearrangement from cationic amine to biodegradable neutral amide, which dissociates the mRNA complex and releases it into the cytoplasm for protein translation.138 The polymer can protect the content from the environment and provide a sustained and adjustable release rate. In addition, polymer nanoparticles can transfect many T cells at one time.139 Polymer nanoparticles can be used as both an adjuvant and a delivery system for immunostimulatory molecules.140 Polymer nanoparticles combined with cancer immunotherapy are a promising cancer treatment method that can activate immune cells, regulate the TME, and enhance antitumor immunity.
At present, most of the nano-drugs in the market are LNPs, and only a few polymer nanoparticles have entered clinical experiments, which are related to their properties. Some polymeric nanoparticles have inherent immunogenicity, particle aggregation, and cytotoxicity.141 PEG-modified polymer nanoparticles may also be immune cleared due to their inherent properties or may activate the immune system to cause hypersensitivity reactions. The in vivo environment of human and animal models is very different. Polymer nanoparticles are easily combined with molecules in the blood and are prone to cytotoxicity. At the same time, although positively charged cationic polymers are beneficial to the endocytosis of nanocomplexes, they are also prone to cytotoxicity, and the combination with negatively charged components in vivo will also affect the in vivo transport of the complexes. There is still a long way to go before the clinical translation of polymer nanoparticle drugs.
3.3 Inorganic nanoparticles
Inorganic nanoparticles can destroy the gaps between vascular endothelial cells, pass through the blood vessel wall and enter the tumor tissue by forming temporary pores.142 Inorganic nanoparticles include carbon nanotubes (CNTs), metal nanoparticles (MNPs), and silicon-based nanoparticles, which are widely used in immunotherapy.143 CNTs are more likely to cause an immune response than other nanoparticles. MNPs include mesoporous silica nanoparticles (MSNs), TiO2 nanoparticles, and gold nanoparticles. Compared with other nanoparticles, MNPs have a higher density and are easier to absorb, showing high reproducibility and high efficiency in cancer vaccination.144
There are several studies dedicated to the delivery of genes using inorganic nanoparticles. Shi Jianlin's team proposed a treatment strategy targeting tumor-specific mitochondrial DNA (mtDNA) oxidative damage and activating innate immunity.91 The team constructed MSNs loaded with Fe2+ and Ru2+, called MSN-Ru2+/Fe2+ (MRF), which can target oxidative damage to mtDNA of tumor cells. Alexander S Cheung reported the mesoporous silica micro-rods (MSR)-supported lipid bilayers (SLBs) of a natural antigen-presenting cell (APC) system formed by a liquid lipid bilayer of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and mesoporous silica microrods. Compared with commercial expansion beads (Dynabeads), APC mimic scaffolds (APC-ms) formed by MSR-SLBs can promote the polyclonal and antigen-specific expansion of primary mouse, human T cells, and CD19 CAR-T cells.92 Byungji Kim et al. demonstrated that fused porous silicon nanoparticles could deliver siRNA to targeted cells to induce gene silencing. Fusion porous silicon nanoparticles loaded with siRNA have a strong gene silencing effect in vitro (>90%) and in vivo (>80%), which can target TAMs, reprogram the immune system to enhance T-cell infiltration, and downregulate immunosuppression.93
Inorganic nanoparticles have emerged as an attractive approach for therapeutic nucleic acid and drug delivery. Compared with other delivery vehicles, inorganic nanoparticles have the advantages of a longer storage period, higher physical stability, precise size control, tunable physical properties, and high surface area. However, inorganic nanoparticles are many insoluble and easily aggregated substances in biological media, limiting biomedical applications. Therefore, researchers have developed various encapsulation methods to improve the solubility of inorganic nanoparticles in aqueous solutions and prevent aggregation. For example, gold nanoparticles encapsulated in graphene oxide can reduce cytotoxicity. Other modifications of inorganic nanoparticles include delamination of charge reversal polyelectrolytes and lysine-based head group coatings.145 However, the shortcomings of inorganic nanoparticles are also obvious. Some inorganic nanomaterials are difficult to biodegrade in the body, resulting in a long residence time in the body, which leads to some adverse effects. Therefore, the research and development of biodegradable inorganic nanomaterials play an important role in promoting their safe applications in the biomedical field. Second, the low solubility of inorganic nanoparticles, low particle surface potential, easy agglomeration, and toxicity problems also limit their clinical applications.146, 147
3.4 Other types
Hydrogels are three-dimensional polymers formed by chemical or physical cross-linking of hydrophilic or amphiphilic macromolecular chains, in which the hydrophilic functional groups of –OH, –CONH–, –CONH2– and –SO3H in the polymer structure can swell but without dissolving and keep the structure intact when containing a large amount of water.148, 149 Hydrogels have strong performance adjustment capabilities and have great potential in nanomedicine, pharmaceuticals, and bio-nanotechnology.150 Due to the characteristics of swelling, stimulus response behavior, and flexibility, hydrogels protect encapsulated biomolecules from degradation and elimination in the body.151 Hydrogels can encapsulate more than one biologically active substance with different physical and functional properties, such as vaccines, CKs, and nucleic acids.59, 152, 153 Given their unique properties, several ongoing trials use hydrogels for gene delivery. Hydrogels are often used in DNA vaccine delivery platforms to regulate immune responses.153 Research reported that the hydrogel system could attract host DCs and T cells to the injection site. Some inoculated DCs migrate to the local lymph nodes, thereby providing a continuous process to activate the immune response.154, 155 The injectable smart hydrogel (ISH) is composed of levodopa and poly(ε-caprolactone-co-lactide) ester functionalized hyaluronic acid (HA-PCLA), loaded with immunomodulatory factor (OVA expression plasmid, POVA)-bearing nano-scale complex and GM-CSF. ISHs effectively locate immune cells and control the delivery of immunomodulatory factors by subcutaneous administration.94 This technology can bypass the need for transfection into isolated cells and direct the sustained release of a cancer vaccine for immune regulation. Leonard Kaps's team reported another example that nanohydrogel particle-modified mannose residues (ManNP) were more effective than nontarget NonNP nanogels to deliver siRNA to M2 polarized macrophages. ManNP prevents nonspecific uptake by nontarget cells and significantly enhances the uptake of M2 polarized macrophages that express CD206 (mannose receptor).95 At present, synergistic photothermal and immune therapy has become one of the most attractive cancer treatment strategies. The Tomoya Yata team designed a composite immunostimulatory DNA hydrogel composed of hexapod-like structured DNA (hexapodna) with cytosine-guanine (CpG) sequences and gold nanoparticles. Laser irradiation of the hydrogel results in the release of hexapods, which effectively stimulate immune cells to release proinflammatory CKs.96
Extracellular vesicles (EVs) are 30–150 nm particles naturally released by living cells. EVs classified as exosomes and ectosomes exist in the body fluids of various organisms, including saliva, serum, and urine, and are widely used for various diseases.156 EVs are regarded as carriers of molecular signaling and horizontal gene transfer between cells.157 EV-based cancer immunotherapy is mainly divided into immune cell-derived EVs and tumor-derived EVs. EVs are primarily specific proteins, transmembrane marks, esters, and peptides. The lipid and protein composition of exosomes will affect their pharmacokinetic properties, improve bioavailability and reduce adverse reactions. The shape and size of exosomes are approximately 30–80 nm, and they can be easily taken up by macrophages after transfection. Tumor cell-derived exosomes present neoantigens or MHC peptide complexes to activate T cells or directly activate natural killer (NK) cells and macrophages.158 Exosomes can also regulate immune responses by affecting gene expression and signaling pathways in recipient cells, mainly through miRNA transfer. Exosomes are designed to deliver therapeutic payloads such as short interfering RNA, antisense oligonucleotides, chemotherapeutics, and immunomodulators. The following section introduces several interesting preclinical immunogene studies with exosomes. Study demonstrated that metastatic melanoma released EVs in the form of exosomes carrying PD-L1. The level of circulating exosomal PD-L1 is positively correlated with the level of IFN-γ and is increased during anti-PD-1 treatment.97 In addition, Peng-Fei Zhang reported that exosomal circUHRF1 secreted by hepatocellular carcinoma tissues inhibits the function of NK cells by degrading miR-449c-5p and upregulating the expression of T-cell immunoglobulin and mucin domain 3.98
4 APPLICATION OF NONVIRAL VECTORS IN SOLID CANCERS
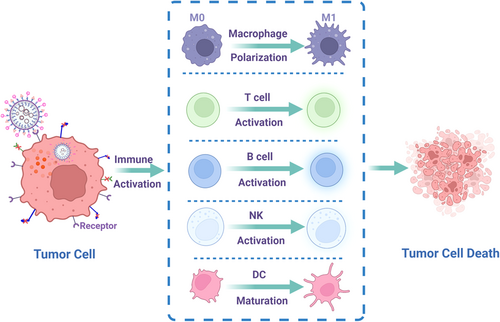
Nonviral vectors can deliver genes to corresponding cells in various ways, then activate different immune cells (e.g., lymphocytes, macrophages, and DC cells), and finally kill tumor cells (Figure 5). We summarize recent research results of different solid tumors treated with immunogen therapy via nonviral vectors (Table 2).
| Cancer | Nanocarrier types | Ligands | Nanocarriers | Payloads | Target | Reference |
|---|---|---|---|---|---|---|
| Lung cancer | Polymer nanoparticle | CpG | Lungs | 159 | ||
| LNP | EpCAM | LPP-P4-Ep | siCD47, siPD-L1 | EpCAM | 160 | |
| Polymer nanoparticle | Nanovesicle system | TUSC2, anti-PD-1 | Cancer | 161 | ||
| Polymer nanoparticle | PEG-3 | IL-12 | IL | 162 | ||
| Colon cancer | Polymer nanoparticle | PBAE | 4-1BBL, IL-12 | tAPCs | 163 | |
| Polymer nanoparticle | MPEG-PLA | IL-15 | IL | 164, 165 | ||
| LNP | CPL | MP, TNFSF14 | 166 | |||
| LNP | Protamine | Liposome | CXCL12, PD-L1 | 167 | ||
| Polymer micelle | NVP-BEZ 235, CSF-1R-siRNA | TAM | 168 | |||
| LNP | Protamine | Liposome-protamine | Oxaliplatin, PD-L1 | 169 | ||
| Breast cancer | Polymer micelle | FA | MPEG-PLA | pMIP-3β | 170 | |
| Polymer micelle | HA-SH | PMet | DOX, IL-12 | 171, 172 | ||
| Polymer nanoparticle | CPT | CPT-Arginine (CR) | pSpam1, pshPD-L1 | ECM | 173 | |
| Polymer nanoparticle | PCL | A2AR siRNA | 174 | |||
| Polymer nanoparticle | PLEGP | VEGF siRNA | 175 | |||
| Polymer nanoparticle | Mannose | LCP | mRNA vaccine encoding MUC1 | DCs | 176 | |
| Polymer nanoparticle | POEG-st-Pmor | IL-36γ, DOX | 177 | |||
| Prostate cancer | Polymer nanoparticle | OVA, palmitic acid | Lipid-PEG | TLR7/8 agonist R848, mRNA vaccine | MHC-1 APCs, T cells | 178 |
| Polymer nanoparticle | G0-C14, PLGA lipid-PEG | PTEN mRNA | suppressor genes | 179 | ||
| Polymer nanoparticle | Nanoparticle | CAR/TCR mRNA | CAR, TCR | 180 | ||
| Polymer nanoparticle | NP-MN | DNA vaccine | 181 | |||
| Polymer nanoparticle | SCM | DOX, PD-L1 siRNA | 182 | |||
| Liver cancer | Polymer nanoparticle | INO, PEI (LA-PegPI) | pDNA | 183 | ||
| Polymer nanoparticle | AEAA | LCP | RLN plasmid | aHSC | 184 | |
| Polymer nanoparticle | Thymine dendrimer | TT-LDCP | PD-L1 siRNA, pIL-12 | STING | 185 | |
| Polymer nanoparticle | LPD | RNA vaccine | DC | 186 | ||
| Polymer nanoparticle | LCP | PD-L1 and CXCL12 trap | Hepatocytes | 187 | ||
| Polymer nanoparticle | FA | Chitosan nanoparticle | mIP-10, vaccine | DC | 188 | |
| Polymer nanoparticle | PBAE | cDNA of TRAIL | TRAIL | 189 | ||
| Glioma cancer | Polymer nanoparticle | iRGD, HSA | SPNP | STAT3i | MHC Ⅱ | 190 |
| LNP | DOTAP | mRNA | DC | 191 | ||
| Polymer nanoparticle | PBAE | IRF5, IKKβ | TAM | 192 | ||
| LNP | DOTMA, DOPE | Antigens mRNA160 | APCs | 193 | ||
| LNP | Lipids, phospholipids, cholesterol, PEG-lipid | Tumor antigens gp100 and TRP2 | 194 | |||
| Glioma cancer | SLN | iRGD | SLN | siEGFR, siPD-L1 | 195 | |
| LNP | miR-124 | STAT3 | 196 | |||
| Polymer nanoparticle | Lipid-protamine | Wnt5a | 197 | |||
| Polymer nanoparticle | CLPEI, DS | siPD-L1 | 198 | |||
| Polymer nanoparticle | PBAE | CpG | DC, T cells | 199 | ||
| Polymer nanoparticle | PBAE | CDN | STING | 200 | ||
| Polymer nanoparticle | CH2R4H2C | PEG-PCL | NF-κB siRNA | NK | 201, 202 | |
| LNP | DOTAP | mRNA | 203 |
- Abbreviations: APC, antigen-presenting cell; CDN, cyclic dinucleotide; CpG, cytosine-guanine; DC, dendritic cell; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; ECM, extracellular matrix; EpCAM, epithelial cell adhesion molecule; FA, folic acid; IL-12, interleukin-12; MHC, major histocompatibility complex; MPEG-PLA, methoxy poly(ethylene glycol)-poly(lactide); PD-L1, programmed death ligand 1; pDNA, plasmid DNA; siRNA, small interfering RNA; TAM, tumor-associated macrophages; TCR, T-cell receptor, TNFSF14, TNF superfamily member 14; VEGF, vascular endothelial growth factor.
4.1 Lung cancer
Lung cancer has the highest morbidity and mortality globally, and its new drug research and marketing are the most common among cancers.4 Histologically, lung cancer can be divided into small cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC). Nearly 85% of lung cancer cases are NSCLC, of which the most common histological subtypes are squamous cell carcinoma and adenocarcinoma. Surgery is still the current treatment for NSCLC because only radical surgery can hope for a cure. Immunotherapy is an emerging treatment for advanced NSCLC that aims to reactivate the adaptive immune system against cancer. In 2015, the Food and Drug Administration (FDA) approved two immunotherapy drugs, nivolumab and pembrolizumab, for lung cancer, which are milestones and paradigm shifts in lung cancer treatment.
Currently, new therapies based on nanotechnology are being developed to improve the patient's response. Nanotechnology-based gene delivery has been widely used in lung cancer, such as Jillian L. Perry's team used particle replication in nonwetting template (PRINT) nanoparticles to deliver CpG. Nanocarriers prolong the retention time of CpG in the lungs and prolong the rise time of antitumor CKs.159 Another study reported targeting epithelial cell adhesion molecule (EpCAM) cationic liposomes (LPP-P4-Ep) to deliver si-CD47 and si-PD-L1. LPP-P4-Ep showed high EpCAM tumor targeting and low toxicity and enhanced the immunotherapy effect.160 Finally, some clinical trials showed promising results in lung cancer. For example, the tumor suppressor gene TUSC2 delivered by the nanovesicle system mediates tumor regression in clinical trials. The selective endocytosis of nanovesicular lipid preparations and the enhanced permeability and retention properties of the tumor vasculature make TUSC2 nanovesicles more likely to be selected by cancer cells.161
4.2 Colon cancer
Colon cancer is a common malignant tumor of the digestive tract, and approximately 1.2 million patients worldwide are diagnosed with colorectal cancer each year.204 The current standard for adjuvant treatment of colon cancer is the combination of fluoropyrimidine and oxaliplatin (OXA).205 However, combination therapy is only helpful for 15%–25% of patients receiving treatment, which means that more than 70% of patients receive chemotherapy without benefit but with toxicity.206 With the development of technology, the treatment of colon cancer has also developed from surgery, chemotherapy, and radiotherapy to the current biological targeted therapy and immunotherapy. The Food and Drug Administration (FDA) has approved PD-1/PD-L1 inhibitors for second-line treatment.207
Combining immunotherapy with some nanoplatforms has been proposed as a strategy to increase overall treatment efficacy. For instance, Stephany Y Tzeng's team found that nanoparticles based on PBAEs loaded with genes can reprogram cancer cells and the microenvironment so that cancer cells can act as tumor-associated antigen-presenting cells (tAPCs) by inducing the expression of co-costimulatory molecules (4-1BBL) and IL-12.163 In addition, a nanoparticle complex DMA based on DOTAP and MPEG-PLA was constructed to deliver IL-15 (DMA-pIL15). The DMA-pIL15 complex significantly inhibited tumor growth by inhibiting angiogenesis, promoting cell apoptosis, and activating the host immune system.164 In another study, this nanocomposite for the delivery of IL-12 also significantly inhibited tumor growth.165 Thus, immunogene therapy has good application prospects for colon cancer.
4.3 Pancreatic cancer
According to statistics, in 2021, new cases of pancreatic cancer will be ranked 10th among men and 8th among women, and the fatality rate of pancreatic cancer will be 4th.4 Pancreatic cancer of “The King of Cancer” has some issues of fewer effective treatments, poor therapeutic effects, and difficulty in diagnosis. Currently, pancreatic cancer is still treated with surgery as the primary method, combined with chemotherapy, radiotherapy, targeted therapy, and other adjuvant treatments.89, 208 However, the surgical resection rate of pancreatic cancer is only approximately 20%, the 5-year survival rate after surgery is 15%–20%, and the 10-year survival rate is 3.9%. Chemotherapy not only has poor efficacy and side effects but is also prone to drug resistance. Early clinical and basic research showed that pancreatic cancer is sensitive to immune stimulation, which can prolong the survival time of patients and improve the quality of life of patients.209 At present, the practical clinical application of immunotherapy for pancreatic tumors is still in a relatively simple mode focusing on enhancing the immune state of the body system, including T-cell checkpoint inhibitors, oncolytic viruses, and chimeric antigen receptor-engineered T cells. Pancreatic cancer has abundant immune cells in the matrix; most belong to the immunosuppressive subgroup, such as Treg cells, Th cells, TAMs, and MDSCs.210, 211 Compared with other solid tumors, intratumorally effector T cells are rare and express high levels of immune checkpoint receptors, such as PD-1. Therefore, pancreatic cancer needs to undergo microenvironment remodeling to survive.
Nanotechnology combined with immunotherapy offers some new therapeutic strategies for pancreatic cancer patients. For instance, studies have been conducted on the phosphorylated low-toxicity natural product α-mangostin phosphate (MP), a plasmid that can express the immune-enhancing cytokine TNF superfamily member 14 (TNFSF14), and CaP liposomes for complexation and coprecipitation to obtain a “nano-engineer.” The combined treatment of MP and TNFSF14 could downregulate the matrix components within the tumor and restore the function and structure of blood vessels, creating a TME that is conducive to cytotoxic T lymphocyte (CTL) infiltration.166 Liposome-protamine-DNA nanoparticles (LPDs) loaded with plasmids encoding CXCL12 and PD-L1 effectively modulate the immunosuppressive TME and promote T-cell infiltration, resulting in long-term persistence of antitumor effects and prolonging the survival rate. On the other hand, nanoparticles loaded with trap plasmids accumulated in the tumor area and were quickly removed in the blood and spleen, which reduced the autoimmunity induced by immune checkpoint inhibitors or the systemic inflammation caused by CK.167 RMan Li et al. developed M2 TAM-targeted nanomicelles to deliver the PI3K-γ inhibitor NVP-BEZ 235 and CSF-1R-siRNA for specific TAM reprogramming antitumor immune response activation. Nanomicelles improved the targeting efficiency of M2 TAMs both in vitro and in vivo and improved the antipancreatic tumor effect.168
4.4 Breast cancer
According to the latest cancer statistics published by the International Agency for Research on Cancer of the World Health Organization, female breast cancer surpassed lung cancer for the first time, becoming the most common cancer in the world, accounting for approximately 11.7% of new cancer cases.3 Traditional treatment modes, such as surgery, radiotherapy, chemotherapy, endocrine therapy, and targeted therapy, have difficulty significantly improving the survival prognosis of breast cancer patients, especially advanced patients, and immunotherapy as a new treatment is expected to further improve the survival rate of breast cancer patients.212 Roche's PD-L1 inhibitor Tecentriq® and Merck's PD-1 inhibitor Keytruda® have been approved to treat metastatic triple-negative breast cancer,213 and both therapies are antitumor treatments that improve the immune system. At present, there are an increasing number of studies on immune genes in the treatment of breast cancer.
There are several studies dedicated to the delivery of gene immunotherapy using nonviral vectors. Yihong He et al. designed the nanomicelle FDMCA-pMIP-3β. FDMCA-pMIP-3β could enhance the activity of immune cells and significantly inhibit the growth of tumors by promoting the maturation of DC cells, the M1 type of macrophages, and the activation of lymphocytes.170 Zhen Zhao et al. developed a new biocompatible linear copolymer poly[bis(ε-Lys-PEI) Glut-PEG] (PLEGP) loaded with vascular endothelial growth factor siRNA for the treatment of triple-negative breast cancer. The siRNA/PLEGP1800 nanocomposite had almost no cytotoxicity and had a good cell uptake rate, transfection efficiency, biocompatibility, and tumor permeability.175 Moreover, Lina Liu's team constructed mannose-modified lipid calcium phosphate (LCP) nanoparticles and delivered an mRNA vaccine encoding tumor antigen MUC1 to DCs in lymph nodes, which could activate and expand tumor-specific T cells, thereby greatly enhancing the T-cell immune response and antitumor response.176
4.5 Prostate cancer
Prostate cancer is now the second most common tumor disease threatening men's health. According to cancer statistics, in 2021, there will be 248,530 new cases of prostate cancer, accounting for 26% of all male malignancies in America.4 The treatment strategy for prostate cancer includes monitoring, local treatment, and systemic treatment. Systemic treatment includes hormone therapy, chemotherapy, and immunotherapy. However, 40% of patients undergoing radical prostatectomy and 50% of patients undergoing radiotherapy will have relapses. Due to the continuous progress in research on cancer signaling pathways, mutations, and drug resistance mechanisms, the treatment of advanced prostate cancer is rapidly changing. In recent years, many experimental data have shown that immunotherapy can significantly improve survival and quality of life.214, 215 At present, there are three main types of immunotherapy, including Sipuleucel-T (Provenge) vaccines, Prostva, and Ipilimumab for prostate cancer.215-217 The FDA has approved the cancer vaccine Provenge (currently the only approved DC vaccine) for prostate cancer.216 Recently, immunotherapy has received increasing attention.
Currently, nanoparticles combined with immunogene therapy are the new therapeutic strategies for prostate cancer. Recently, an adjuvant pulsed mRNA vaccine nanoparticle was constructed by OVA-encoded mRNA, palmitic acid-modified TLR7/8 agonist R848 (C16-R848), and lipid-polyethylene glycol (lipid-PEG). The mRNA vaccine nanoparticles enhanced the antitumor immune response by increasing the expansion of OVA-specific CD8+ T cells and the infiltration of CD8+ T cells in the tumor site.178 N N Parayath reported an injectable nanocarrier to deliver in vitro-transcribed (IVT) CAR or T-cell receptor (TCR) mRNA, which could transiently reprogram circulating T cells to recognize disease-related antigens, similar to injecting engineered lymphocytes into the body.180 The deletion or mutation of tumor suppressor genes such as the coding gene phosphatase and tensin homolog (PTEN) on chromosome 10 may be related to the response or resistance of the immunosuppressive TME and immune checkpoint therapy.218, 219 Mohammad Ariful Islam reported that PTEN mRNA nanoparticles self-assembled from cationic lipid compounds G0-C14, PLGA polymer coated with a lipid-PEG shell, and PTEN mRNA were used to deliver PTEN mRNA to prostate cancer tumors. PTEN protein functions and inhibits the growth of tumor cells.220 In addition, the Yao-Xin Lin team developed a polymer nanoparticle platform for the in vivo delivery of PTEN mRNA to prostate cancer cells with PTEN deletion and melanoma cells with PTEN mutations. PTEN mRNA nanoparticles restored the sensitivity of tumor cells to immune checkpoint blockade (ICB) and induced the release of damage-associated molecular patterns (DAMPs) and the activation of autophagy to trigger an immune response and reversed the TME.179
4.6 Liver cancer
Liver cancer is one of the most common causes of cancer death globally, ranking fifth in the United States.221 The survival rate of liver cancer in the third stage is only 39.3%, and the prognosis of liver cancer is poor, with only 5%–15% of patients eligible for surgical resection.204 The treatment of the advanced stage is arterial chemoembolization (TACE), and the 2-year survival rate increases by 23%. Advanced patients will also choose oral sorafenib, a kinase inhibitor. Nevertheless, less than one-third of patients benefit from the treatment, and apparent drug resistance appears within 6 months of starting treatment. Neither current ablation therapy nor chemotherapy can effectively improve the outcome of this devastating disease. Further research is needed to find a better way to treat liver cancer. Immunotherapy can be combined with drugs for liver cancer to produce a synergistic effect.222
An increasing number of studies are on the combination of immunogene therapy and nonviral carriers, such as microspheres, liposomes, and nanoparticles.223 For instance, LCP nanoparticles modified with AEAA were used to deliver a relaxin (RLN) plasmid (pRLN LCP). pRLN LCP nanoparticles were targeted to metastatic tumor cells, activated hepatic stellate cells (aHSCs) in metastatic lesions, and transformed into an in situ RLN library. The expression of RLN reactivates the immune environment at the site of metastatic disease.184 Kuan-Wei Huang et al. designed tumor-targeting lipid-dendrimer-calcium phosphate nanoparticles (TT-LDCP NPs) with thymine-functionalized dendrimers. The enhanced gene delivery capability over time and later after STING treatment exhibited adjuvant immune properties. TT-LDCP NPs loaded with PD-L1 siRNA and immunostimulatory IL-2-encoding DNA reprogrammed the TME, increased tumor invasion and CD8+ T-cell activation, and enhanced the efficacy of vaccine immunotherapy.185 Moreover, the RNA vaccine can effectively activate APCs and lymphocytes, produce a strong systemic immune response, and inhibit tumor growth. Yake Zhang's team constructed an LPD with a small size, high RNA encapsulation efficiency, and high serum stability, which could deliver tumor-derived RNA to the tumor site. In vitro, the RNA vaccine targeted DCs, promoted DC maturation, and induced T lymphocytes to kill Hepa1-6 cells. In vivo, RNA vaccines could effectively prevent and inhibit the growth of hepatocellular carcinoma.186
4.7 Glioma
Glioma is the most common and malignant tumor of the central nervous system (CNS). Glioblastoma (GBM) is the most advanced form of glioma and the deadliest glioma.224 Currently, standard glioma treatment includes maximum safe surgical resection and combined radiotherapy and chemotherapy.225 The characteristics of malignant gliomas are strong tumor invasiveness, high heterogeneity, complex carcinogenic pathways, and inherent resistance to cell death.226 At present, the discovery of gene or protein drugs that are critical for gliomas has reached a deadlock. In contrast, immunotherapy with ICB, vaccines, CAR-T cells, and oncolytic viruses for gliomas has achieved some promising results.227 Immunogene therapy is expected to open a new era of precision treatment for glioma.
Over the past years, substantial efforts have been devoted to developing different nanoparticle-based formulations to improve the antitumor effectiveness of gene immunotherapy in glioma. How genes cross the blood–brain barrier (BBB) is a complex problem in the treatment of glioma. Jason V. Gregory et al. constructed a new type of synthetic protein nanoparticle (SPNP) based on iRGD peptides with cell penetration ability and polymerized human serum albumin (HSA). STAT3i SPNPs combined with radiotherapy successfully penetrated the blood–brain barrier to reach the tumor, induced the activation of DCs, and inhibited tumor growth by enhancing the expression of MHC Ⅱ involved in antigen presentation.190 At present, the development of safe and effective cancer vaccine preparations is the main focus of cancer immunotherapy. Elias J Sayour's team proved that DOTAP could effectively deliver mRNA to APCs. When administered intravenously, RNA-NPs mediate the systemic activation of APCs in reticuloendothelial organs (such as the spleen, liver, and bone marrow), increasing the level of MHC class I/II and B7 costimulatory molecules and the expression of maturation markers on APCs and triggering the effective expansion of antigen-specific T cells.191 In addition, the researcher described a targeted nanocarrier that used the electrostatic interaction between cationic PBAE polymer and anionic mRNA to self-assemble into nanocomposites. The nanocomposite of mRNA encoding the M1 polarized transcription factor could reprogram TAMs through IVT without systemic toxicity. The infusion of nanoparticles encoding interferon regulatory factor 5 (IRF5) combined with IKKβ (a kinase that phosphorylates and activates IRF537) reversed TAM and inhibited tumor growth in ovarian cancer, melanoma, and GBM models.192
4.8 Melanoma
Melanoma is a malignant tumor with high mortality, a high metastasis rate, and difficult treatment. The latest data from the World Health Organization (WHO) predict that by 2025, the number of melanoma-related deaths will increase by 20%, and by 2040, it will rise to 74%.228 Nano immunotherapeutic drugs can precisely target tumor-specific immunosuppressive cells (bxTAM, tolerant DCs, Tregs, MDSCs, etc.) and induce a strong tumor immune response.229 In 2014, the FDA approved two immune checkpoint inhibitors, nivolumab (anti-PD1) and ipilimumab (anti-CTLA4), for the treatment of metastatic skin melanin tumors, which can improve the overall survival of inoperable metastatic melanoma.230, 231 Researchers have never stopped exploring immunotherapy for melanoma.
Immunogene therapy could be modulated through nanotechnology to achieve a better antitumor effect. Cationic lipids DOTMA and DOTAP, zwitterionic lipid DOPE, and anionic mRNA160 self-assembled to form a nanocomplex RNA-LPX. In an ongoing phase I dose-escalation trial, low-dose RNA-LPX induced IFN-α-stimulated immune responses and strong antigen-specific T-cell responses in three melanoma patients.193, 232 Lipid nanoparticle formulations composed of ionizable lipids, phospholipids, cholesterol, and PEG-lipid conjugates are designed to deliver mRNA vaccines to induce CTL responses. In addition, nanoparticles encapsulating mRNA encoding tumor antigens gp100 (PMEL) and TRP2 (DCT) reduced the overall tumor size and prolonged survival.194 More recently, Yuan Qian's team constructed dual-targeting M2-like TAM core-shell fluorescent lipid nanoparticles (M2NP) by tightly binding amphiphilic α-peptides, α-M2pep, phospholipids, and nuclear stacking (near infrared) NiR dyes. M2NP-based anti-colony stimulating factor 1 receptor (anti-CSF-1R) siRNA delivery could reshape the immune microenvironment and restore the immune regulatory function of T cells.233, 234
5 DISCUSSION AND PERSPECTIVES
Particle size and surface charge of nanoparticles play an important role in the distribution of genes and drugs in vivo.235 The unique physiological characteristics of the lung (including large alveolar surface area, abundant blood flow, and low enzyme protein activity) make the nanomedicine of lung cancer have a good development prospect.236, 237 The existence of BBB limits the delivery of drugs/genes, thereby affecting the treatment of glioma. Delivery technology based on nanocarriers has become the new hope for drugs/genes delivery across BBB without damaging its structure or function.238 Pancreatic tumors are difficult to drugs/genes delivery due to their excessive fibrous tissue and dense stroma, which result a high mortality rate.239 Comedy-based treatment of pancreatic cancer remains a major challenge due to the difficulty of in-situ modeling and short patient survival. At present, various nanoscale materials and biomaterials have shown excellent effectiveness in experimental studies to combat tumor diseases. However, we still need to select appropriate carriers according to the actual situation.
Nonviral vectors can protect therapeutic agents from clearance from the surrounding biological environment, increase their half-life, minimize systemic toxicity, and facilitate drug delivery to the tumor site.18, 19 Nonviral vectors can also realize the codelivery of multiple drugs or contrast agents to achieve the purpose of integrating diagnosis and treatment. Nano-delivery vehicles would improve drug retention, permeability, and pharmacokinetic characteristics, thereby reducing side effects.101 Nonviral vectors also have controlled drug release properties, allowing the drug to be released in accordance with the dose within a certain period of time, reducing drug resistance. LNPs are the closest to clinical translation among nonviral vectors. Onpattro, the first siRNA drug launched in 2018, utilizes LNPs to deliver chemically modified siRNA to achieve cellular targeting, uptake, and endosomal escape.240, 241 The 2019 FDA-approved vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-COV-2), Moderna mRNA-1273, and Pfizer BNT162b2 both rely on LNPs to ensure safe and effective delivery,242-245 which brings hope for the clinical application of nonviral vectors in tumor gene therapy.
Although nonviral vector system-based immunogene therapy can activate the immune system to effectively fight cancer, most of the related research is in preclinical studies, and few strategies are approved clinically. An enormous challenge in the broad implementation of immunogene therapies for cancer remains safety, such as immunogenicity, off-target effects, and DNA damage toxicity.246 The conversion rate of nonviral vectors in clinical practice is low; as of April 2022, according to ClinicalTrials. gov (https://www.clinicaltrials.gov/) statistics, there are currently only 27 clinical trials on “nanoparticles” and “cancer” in Phase 3/4 studies. Problems such as low transfection efficiency, short duration of gene expression, safety, and difficulty in reaching target cells after in vivo administration hinder the clinical practice of nonviral vectors.247 Delivery of nanomedicine requires overcoming several biological barriers, including conditioning and uptake of the mononuclear phagocytic system, nonspecific distribution, hemorheological/vascular flow limitation, cellular internalization, endosomal and lysosomal escape, and drug efflux pumps.248-250 Differences in the composition of human and animal models in vivo and heterogeneity between patients can also limit the clinical translation of nanomaterials. Therefore, we need to continue to study the interaction process of nonviral vectors in the human body. To overcome the challenges inherent in nonviral vectors, their stability and efficacy still need to be improved. The hydrophobicity or hydrophilicity, charge density, fluidity, and size of the nonviral vectors will affect the carrier transfer efficiency.56, 68, 251, 252 The tumor microenvironment, including the high permeability of the blood vessel wall, lack of a lymphatic network, acidic and hypoxic microenvironment, increased interstitial pressure, and overexpression of various receptors and enzymes in cells are unique features.253-255 Based on the above unique properties, nonviral vectors were modified to achieve passive, active, and physicochemical targeting of drugs.29, 32, 256 First, the neutral or slightly negative charge on the surface of the nanoparticles can reduce the adsorption of blood proteins (such as opsonin) and avoid phagocytosis. For example, the surface of neutralized charged nanoparticles is coated with hydrophilic polymers such as PEG.257 In addition, therapeutic vector-targeted modifications can effectively target tumor cells,258-260 increasing efficiency and reducing side effects, such as HA, RGD, angiocp-2, DNA aptamer, and folic acid. The modifiability of nonviral vectors makes them an excellent tool for the delivery of genes. The safety of nonviral vectors and genes also needs to be further improved. The toxicity of nonviral vectors depends on many physicochemical parameters affecting the gene delivery process, such as the particle size, morphology, and zeta potential of the complexes.261, 262 To summarize the currently approved nano-drugs, the carriers are mostly simple in structure and composition. We can pay more attention to the development of some natural sources of nonviral vectors, such as exosomes derived from self-secretion with extremely low immunogenicity, low liver clearance, and functional modification.16 The genome-editing enzyme itself requires optimization, such as its editing efficiency, specificity, and immunogenicity challenges. Long-lasting effects of enhanced transgene expression focus primarily on modification of the delivered genetic material rather than modifications to nonviral vector formulations that are components of the genetic material. Differences in size and physical properties between nucleic acids can also affect carrier binding; for example, cotherapy of smaller nucleic acids with larger nucleic acid molecules can enhance the delivery and efficacy of smaller molecules, even if they act on different targets.260
These emerging immunogene therapies provide unparalleled opportunities for the treatment of cancer, which is considered incurable.36-38 However, the development of safe and efficient delivery technologies is still in its nascent stages, thereby hindering the clinical translation of immunogene therapy. Compared with viral vectors, nonviral vector systems have better biocompatibility, targeting efficiency, loading capacity, immunogenicity, proinflammatory effects, economic efficiency, degradation rates, clearance, and medical safety of these materials, straightforward ones, and scalable production.263 Currently, nonviral vectors have been applied to all aspects of tumor immunotherapy, such as drug conjugates, lipid nanocarriers, and polymer nanocarriers.142 The combination of nanoparticles and cancer immunotherapy is a promising way to treat cancer patients. Therefore, with the development of novel therapies with curative potential, nonviral delivery is expected to become predominant shortly for gene immunotherapy applications that will further transform the biomedical field.
AUTHOR CONTRIBUTIONS
Xiang Gao offered the main direction and significant guidance of this manuscript. Wen Nie, Jing Chen, and Bilan Wang drafted the manuscript. All listed authors have agreed to the final submitted version.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 82103635 and No. 82172630). This study was supported by the 135 Project for Disciplines of Excellence (No. ZYJC21022).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




