Ultrasound-assisted extraction of fucoxanthin from Sargassum angustifolium and Cystoseira indica brown algae
Abstract
The ultrasound-assisted extraction (UAE; 150 W, 100 amplitude) of fucoxanthin from Sargassum angustifolium and Cystoseira indica brown algae was investigated and compared with conventional extraction methods. Different solvents (methanol, acetone, and methanol/acetone) and sonication times (1, 5, 10, and 15 min) were applied. Among a variety of solvents, methanol extract of C. indica (0.77 ± 0.05 mg/g) and methanol/acetone extract of S. angustifolium (0.70 ± 0.02 mg/g) was more effective in fucoxanthin extraction. UAE had a significant effect on yield extraction and 15 min sonication had a high recovery yield about 0.79 ± 0.01 mg/g and 0.81 ± 0.01 (mg/g) from S. angustifolium and C. indica methanolic extracts, respectively. Moreover, the crude extract of C. indica exhibited inhibitory growth against Escherichia coli and Staphylococcus aureus, while S. angustifolium extract had weak antimicrobial activity. According to the gas chromatography results, γ-Linolenic acid and arachidonic acid were detected as main PUFA in S. angustifolium and C. indica, respectively.
Novelty impact statement
- Ultrasound-assisted extraction increased the recovery yield of fucoxanthin from the Persian Gulf brown seaweeds Sargassum angustifolium and Cystoseira indica.
- The best solvents for fucoxanthin extraction from C. indica and S. angustifolium were methanol and methanol/acetone, respectively.
- The antibacterial activity of C. indica extract was better than S. angustifolium extract.
1 INTRODUCTION
Macroalgae or seaweeds are the most abundant source of bioactive compounds and are commonly found in three major groups of marine algae, Chlorophyceae (green algae), Phaeophyceae (brown algae), and Rhodophyceae (red algae) which exhibit numerous promising biological activities (Mohamed et al., 2012). Brown seaweeds, the second-most abundant group of marine algae, include approximately 2000 species. Among them, Sargassum spp., Laminaria spp., Ascophyllum spp., Fucus spp., and Turbinaria spp. are considered as species, most commonly used at the industry level (Sivagnanam et al., 2015).
Fucoxanthin is a major carotenoid of edible brown seaweeds and is characterized by its unique structure, including an allenic bond and 5,6-monoepoxide and contributes more than 10% of the estimated total production of carotenoids in nature (Kim, Kang, et al., 2012). This xanthophyll is abundant in edible brown algae such as Undaria, Eisenia, Sargassum, and Laminaria (Kim, Kang, et al., 2012) and microalgae such as Bacillariophyceae (e.g., Odontella aurita, Chaetoceros sp., Phaeodactylum tricornutum, Cylindrotheca closterium), Prymnesiophyceae (e.g., Isochrysis galbana, Pavlova lutheri), Chrysophycea (e.g., Pelagococcus subviridis), Raphidophyceae (e.g., Psammamonas australis), and Dinophyceae (e.g., Kryptoperidinium foliaceum) (Foo et al., 2021). There are several studies showing the potential of fucoxanthin to improve the nutritional quality of food, with immune-boosting and health benefits (Foo et al., 2021). Thus, fucoxanthin has attracted much attention because of its therapeutic activities such as anti-cancer activity, antioxidant activity, hepato-protective, skin-protective, antiangiogenic, cerebrovascular, bone-protective, ocular-protective, cardiovascular-protective, anti-inflammatory, antimalarial properties, anti-diabetic, and anti-obesity effects (Oliyaei et al., 2020). According to our previous study, the consumption of fucoxanthin (400 mg/kg) with a purity of about 55% had an antidiabetic effect on type 2 diabetic mice (Oliyaei et al., 2021). Thus, this is becoming particularly evident as fucoxanthin is presently under the spotlight of many investigations.
In addition, the appropriate extraction method should be selected to increase the extraction yield and to contribute to the high biological activity of the carotenoid extract. Solvent extraction via maceration (soaking or direct organic-solvent extraction) and soxhlet extraction are the most common extraction techniques. The correct adjustment of parameters such as organic solvent, liquid to solid ratio, temperature, pH, and extraction time, greatly influences the yield and composition of the resulting carotenoid (Cheng et al., 2020). Moreover, the selection of the method to obtain the best yield from natural sources is mainly dependent on the nature of the compounds and extraction solvent. Among them, the extraction solvent can have the main influence on the quality and quantity of the target compound. The selection of the solvent is carried out based on the bioactive compound attribute such as chemical properties, polarity, or hydrophobicity (V Le et al., 2018). Up to now, several conventional extraction techniques have been reported for fucoxanthin extraction from macro and microalgae. Each of these methods subjected to improved optimization by varying parameters, such as solvent type, different algae, time and temperature, and extraction methods (Foo et al., 2015; Kanazawa et al., 2008; Kim, Jung, et al., 2012; Kim, Kang, et al., 2012; Noviendri et al., 2011; Piovan et al., 2013; Sudhakar et al., 2013; Xiao et al., 2012). However, the use of large amounts of organic solvents, the prolonged times, and temperatures during the conventional processes cause the deleterious of the biological activity of carotenoids, alternative extraction techniques are required to conserve their structure and overcome the disadvantages of conventional extractions. The increasing consciousness of health and environmental issues has led to the development of efficient and eco-friendly novel techniques, which can improve the performance of the extraction and purification process. Ultrasound-assisted extraction (UAE) is a novel developed method with high extraction efficiency and is appropriate for the industrial scale (Chuyen et al., 2018).
UAE is one of the sustainable approaches that increase the yield and the rate of mass transfer in several solid–liquid extraction processes by disrupting the cell walls. Conventionally, UAE is efficient in terms of extraction time, temperature, and reducing solvent consumption (Khan et al., 2010). Moreover, UAE diminishes the danger of thermal degradation, thus is appropriate for sensitive materials (Shirsath et al., 2012). The mechanical effect of ultrasound provides greater penetration of the solvent into cellular materials, via cavitation effects, and improves the release of cell contents into the bulk medium (Chemat et al., 2017). Indeed, cavitation generated using ultrasound is known to produce physical effects such as liquid circulation current and turbulence which increases mass transfer rates and facilitates solvent access to cell content (Chemat et al., 2017). On the other hand, swelling of cells or the breakdown of cell walls resulting in the cavitation and collapse of bubbles (Chuyen et al., 2018), thus high diffusion rates across the cell wall in the first case or a simple washing out of the cell contents in the second (Khan et al., 2010).
The Persian Gulf in Iran is the source of different types and species of seaweeds and three specific species of brown algae are found abundantly such as Padina, Sargassum, and Cystoseira (Kordjazi et al., 2019). It has been estimated that there are more than 150 species of algae from the Iranian coastal area of the Persian Gulf and Oman Sea (Yegdaneh et al., 2016). Member of genus Sargassum, such as Sargassum angustifoluim possess high nutritional value and contains 35% protein, 12.45% crude lipid, and 41.49% total carbohydrate (Kordjazi et al., 2019). Cystoseira indica is another tropical brown seaweed abundant in southwest Asia and distributed with high density and biomass in Iran's southeast coast (Fariman et al., 2016). Cystoseira is a widely distributed genus of brown algae with several biological attributes, such as antibacterial, antifungal, and cytotoxic activities (Yegdaneh et al., 2016).
To the best of our knowledge, there are not enough researches about the extraction of fucoxanthin from these two seaweeds. In the present study, the UAE method for fucoxanthin extraction from Sargassum angustifolium and C. indica was developed using different solvents and compared with conventional techniques. Furthermore, the fatty acid content of two seaweeds and antimicrobial activity of extracts was investigated.
2 MATERIALS AND METHODS
2.1 Materials
S. angustifolium and C. indica were harvested from the Persian Gulf coast of Iran. These seaweeds were supplied from Algae Resource Development Technology Company (Shiraz, Iran) and Persian Gulf Algae Development Technology (Bushehr, Iran), respectively. Acetone and ethanol (96% v/v) were obtained from Dr. Mojallali chemical laboratories (Tehran, Iran) and methanol and hexane were purchased from Merck (Germany). Silica gel was bought from the Nanochemia company (Iran). Standard fucoxanthin was prepared from J&K Scientific Ltd. (>95% purity established by HPLC, China).
2.2 Fucoxanthin extraction
2.2.1 Conventional extraction
The dried seaweeds were milled then fucoxanthin was extracted. Fucoxanthin extraction procedure using the method given by Indrawati et al. (2015) with a slight modification. The extractions were performed under different solvents. For conventional extraction, the powdered S. angustifolium and C. indica were soaked (1:30, w/v) with different solvents methanol, acetone, and methanol-acetone (7:3, v/v) and shaken (100 rpm) at 37°C for 24 hr in a dark place to limit the possibility of oxidation and then filtered. The extraction was performed three times until became colorless.
2.2.2 Ultrasound-assisted extraction
The UAE of fucoxanthin was carried out under 150 W (100 amplitude) for 1, 5, 10, and 15 min for each treatment (Ultrasonic Homogenizer, SONOPULS HD-4200, Bandelin). As described above, each extracted solution was filtrated for quantification. Fucoxanthin was detected at 450 nm (UV-VIS Spectrophotometer, Shimadzu, UV-1650PC) and the amount of fucoxanthin content in seaweeds from each solvent was quantified as mg/g dry weight.
2.3 Purification of fucoxanthin
The best condition of fucoxanthin extraction from each algae was selected and filtered. The filtrates were then concentrated at 37°C on a rotary evaporator. Fucoxanthin isolated from each seaweed was used in column chromatography with silica gel as stationary phase (particle size 100–200 mesh) for purification. Then n-hexane-acetone (6:4; v/v) was used as mobile phase to recover fucoxanthin. Finally, residual fucoxanthin was eluted by acetone. All of the extract solutions were together concentrated by rotary evaporation (Kim, Jung, et al., 2012).
2.4 HPLC analysis of fucoxanthin
Purification analysis of fucoxanthin was performed with the HPLC system (KNAUER, Germany) equipped with UV/Vis detector 2600 and C18 column (sphere-image, ODS-2, 300 × 4 mm; 5 µm). After filtering with a 0.45 μm membrane filter, the samples (20 μl) were injected into the HPLC system. The mobile phase of methanol/acetonitrile (50:50, v/v) was eluted at a 0.6 ml/min flow rate. Fucoxanthin was detected at 450 nm and the results were compared by the standard of fucoxanthin (Norra et al., 2017).
2.5 Antibacterial activity
The antimicrobial effect of crude extracts obtained from two brown seaweeds was determined by the disc diffusion method. One milliliter of the methanol/acetone seaweed extracts was applied per sterile filter paper square (1 × 1 cm). The prepared filter papers were placed on the surface of each plate count agar which was spread by bacterial stock cultures of Escherichia coli and Staphylococcus aureus. All of the plates were incubated at 37°C for 24 hr and the antimicrobial activity of extracts was indicated by the growth-free zone of inhibition around the respective filter paper (Saranya et al., 2014).
2.6 Fatty acid contents
The fatty acid analysis of two brown algae was performed using the method (Bligh & Dyer, 1959). The fatty acid contents of the samples were determined by the injection of fatty acid into a gas chromatography (GC) system (SP-3420 A, Beijing, China) coupled to flame ionization detector, and a BPX-70 fused silica capillary column (30 cm × 9 0.25 mm; 0.25 mm film thickness). Carrier gas was N2 with the injected volume of 1 µl and a split ratio of 1:10. The temperature of the injector and detector were 250 and 300°C, respectively. The oven temperature was planned from 140 to 200°C as follows: the initial oven temperature was kept at 140°C for 5 min. Afterward, the temperature was increased to 180°C at 20°C/min and retained for 9 min. Next increased to 200°C at a rate of 20°C/min with maintenance for 3 min at this temperature. The results were expressed as percentage of relative peak area.
2.7 Statistical analysis
Statistical analysis was conducted using the ANOVA procedure using SAS software (SAS Institute, Cary, NC, USA). Duncan's test was used for the estimation of statistical significance (p < .05). The data were expressed as the means ± standard deviation (SD).
3 RESULTS AND DISCUSSION
3.1 Determination of fucoxanthin content
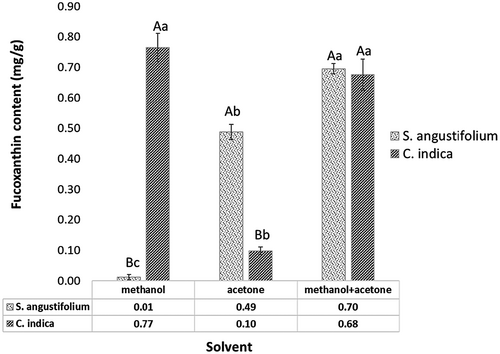
The extraction of fucoxanthin from two brown seaweeds through different solvents was investigated and is shown in Figure 1. To evaluate the efficiency of the solvents, methanol was considered as the best solvent for the extraction of fucoxanthin from C. indica compared to the other ones. There were significant differences in methanol and acetone fucoxanthin extraction from S. angustifolium and C. indica (p < .05). The combination of methanol/acetone had the same extraction yield for both seaweeds but was more effective for fucoxanthin isolation from S. angustifolium (0.70 ± 0.02 mg/g). However, methanol with the 0.77 ± 0.05 mg/g yielded the best fucoxanthin content from C. indica. Because of the toxicity of methanol (Kim, Jung, et al., 2012), the combination of methanol/acetone could be suitable for extraction of fucoxanthin from both S. angustifolium and C. indica. The results were in accordance with findings reported by Sudhakar et al. (2013) obtained the high extraction yield of fucoxanthin by 90% acetone extraction from Sargassum wightii, S. ilicifolium, S. longifolium, Padina gymnospora, and Turbinaria ornate compared with 100% acetone and 90% ethanol. On the contrary, Xia et al. (2013) reported the effective solvents for isolation of fucoxanthin from O. aurita were methanol (16.18 mg/g), followed by ethanol (15.38 mg/g) and acetone (13.93 mg/g). Whereas, ethanol exhibited better yield rather than acetone during fucoxanthin extraction from P. tricornutum (Kim, Jung, et al., 2012). Generally, the potential for a solvent to extract compounds from algae depends on the hydration and permeability of the microalgal cell wall and the solubility of the target compound (Kim, Jung, et al., 2012). It might be related to ethanol affects cell hydration, permeability, and integrity which is related to its ability to form hydrogen bonds. Moreover, Kim, Jung, et al. (2012) using different temperatures (30, 50, and 70°C) to improve extraction yield. They claimed that as the temperature could improve the extraction yield of fucoxanthin because of decomposition of fucoxanthin-Chl a-protein complexes and detach the bonds (Kim, Jung, et al., 2012). The other investigation showed that the temperature, light intensity, and photosynthetic activity of seaweeds have great influence on the fucoxanthin content of macroalgae (Nomura et al., 2013). In addition, the fucoxanthin content is varied among different species and seasons. Fariman et al. (2016) isolated fucoxanthin from C. indica and Nizamuddinia zanardinii by acetone/methanol and purified by column chromatography. They reported that the C. indica have higher fucoxanthin rather than N. zanardinii in all seasons. The highest value of fucoxanthin isolated from C. indica, was obtained in December (3.56 ± 0.20 mg/g DW). The seaweeds were used in our study, harvested in autumn.
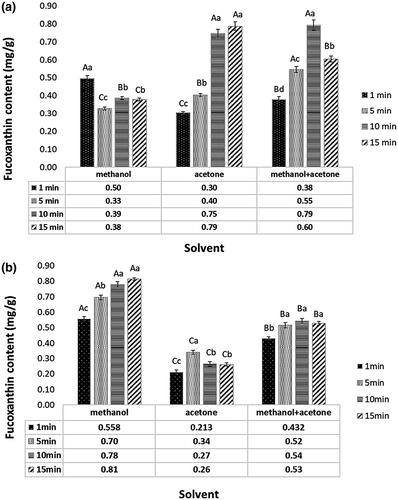
To further investigate the effect of UAE, experiments were conducted at different sonication times (1, 5, 10, and 15 min). According to Figure 2, results exhibited that increase of sonication time from 1 to 15 min enhanced the extraction yield. In comparison with conventional extraction methods, UAE (Figure 2a) caused improving the fucoxanthin yield from S. angustifolium (p < .05). The highest fucoxanthin content of S. angustifolium extract was obtained from acetone at 15 min sonication time (0.79 ± 0.03 mg/g), however, there was not significant difference with methanol/acetone at 10 min sonication (p > .05). As indicated in Figure 2b, the extraction yield of fucoxanthin from C. indica also improved to 0.81 ± 0.01 (mg/g) with methanol extraction for 15 min sonication. In both seaweeds, UAE improved the fucoxanthin yield and greatly reduced the extraction time. Moreover, the effect of time appears to be the crucial factor for fucoxanthin extraction. Although ultrasound has a positive influence on yield, sonication time should be optimized because of reverse effect on sensitive materials (Shirsath et al., 2012). Also, the reduction of fucoxanthin content with 15 min sonication was observed. This reduction might be due to the partial destruction of fucoxanthin during sonication because of increasing the temperature. These results were opposite of Pasquet et al. (2011) and Kim, Jung, et al. (2012) who reported that the UAE did not improve the fucoxanthin extraction yield from Dunaliella tertiolecta and P. tricornutum, respectively. Nie et al. (2021) also found that ethyl lactate showed the high fucoxanthin extraction yield from Sargassum fusiforme during UAE with extraction time, 27 min; extraction temperature 75°C. They reported that the extraction rate of fucoxanthin increased by increasing the temperature and reach the maximum value of 656.88 µg/g at 75°C and suggested the combination of ultrasound and green solvents for fucoxanthin extraction. Other studies focused on the UAE of lipid and bioactive components from microalgae (Adam et al., 2012; Dey & Rathod, 2013; Kim et al., 2013). UAE provides favorable extraction conditions such as a decrease in temperature and pressure which makes it suitable for thermosensitive biological molecules such as carotenoids that are vulnerable to decomposition or alteration in their molecular structures when exposed to heat (Cheng et al., 2020). However, it should be mentioned that there are some factors impacting on the sonication extraction, such as time, temperature, solid/solvent ratio, solvent type (Dey & Rathod, 2013). The UAE improves the mass transfer of the liquid-liquid extraction process by generating cavitation within the material. Indeed, some microbubbles are created during sonication which is growing fast and finally collapsed. When the cavitation bubbles are produced and collapsed, the cell walls of the material will be destructed and the release of the solutes is promoted (Chuyen et al., 2018). During the bubble collapse, the inertia of the surrounding water causes high pressure and high temperature. If these collapses occurred near the solid surface, induce micro jets and shock waves that generate erosion, fragmentation, and disruption of cellular surface (Adam et al., 2012; Chemat et al., 2017). Moreover, the implosion of cavitation bubbles in a liquid medium leads to macro-turbulences and to a micromixing (Chemat et al., 2017). However, it should be considered that the increasing the mass transfer by UAE method resulting in increasing the all soluble constituents into the solvents (Shirsath et al., 2012). Thus, not only fucoxanthin but also other components were extracted. This explained why the fucoxanthin yield obtained from 15 min sonication had not great differences compared with the 10 min treatment.
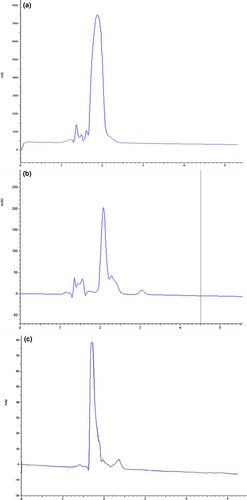
3.2 HPLC analysis
To confirm the presence of fucoxanthin in the extracted sample and quantification of purification, HPLC analysis was conducted. Figure 3. represents the results of HPLC analysis for fucoxanthin extracted with the best solvent condition (methanolic extracts of both seaweeds with 15 min sonication) from S. angustifolium and C. indica as compared to standard fucoxanthin. As could be inferred from the HPLC chromatograms, the major highest peak was obtained from two brown seaweeds with the retention time of about 2 min.
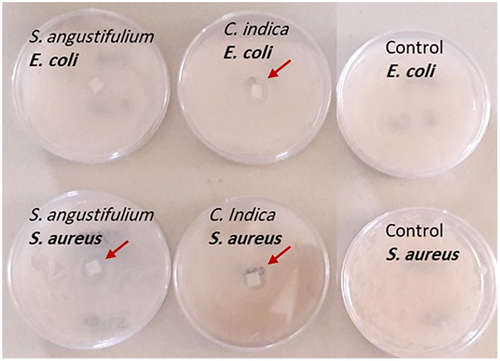
3.3 Antibacterial effect
The antimicrobial activity S. angustifolium and C. indica extracts were investigated for Gram-negative bacteria, E. coli and Gram-positive bacteria, S. aureus. For both seaweeds, the methanol/acetone extract was selected. According to Figure 4, the extracts of C. indica extract (equivalent 0.68 mg/g fucoxanthin) strongly inhibited the growth of E. coli and S. aureus, while, the S. angustifolium extract (equivalent 0.70 mg/g fucoxanthin) showed low antimicrobial activity against the S. aureus and no inhibitory effect on E. coli as Patra et al. (2008) reported the strong antimicrobial activity of Sargassum sp. methanol extract (4,000 µg/100 µl) against both microorganisms. However, it seems that high dose or large concentrations of seaweed extracts was needed. The antibacterial activity of fucoxanthin might be related to its anti-inflammatory effect and its inhibitory activity to produce the pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) by suppressing the NF-κB activation and the MAPK phosphorylation. As well as inhibition of cyclooxygenase-2 and inducible nitric oxide synthase proteins expression. Gram-negative bacteria contain lipopolysaccharide, an endotoxin, which affects the inflammatory response and caused infection (Karpiński & Adamczak, 2019). Furthermore, the antibacterial potential of S. wightii and Turbinaria ornata extract against a wide range of bacteria was reported (Vijayabaskar & Shiyamala, 2011). Moreover, it is appeared that the antimicrobial activities of seaweed extracts are influenced by different parameters such as location, seaweed species, and types of solvent as there were several reports that showed methanol extract had a good antimicrobial effect compared with hexane and ethyl acetate while some studies introduced the chloroform as better solvent (Tuney et al., 2007). For instance, Ozdemir et al. (2006) observed that the hexane extract of D. membranacea and C. barbata had higher antimicrobial activity against S. aureus, S. typhimurium, and C. albicans rather than methanol, dichloromethane, and chloroform extracts. On contrary, Demirel et al. (2009) observed that dichloromethane extract showed higher antimicrobial activity rather than methanol. Demirel et al. (2011) evaluated the crude extracts antimicrobial effect of Laurencia obtusa and Laurencia obtusa var. pyramidata against Gram-positive and Gram-negative bacterium and stated that the L. obtuse essential oil had higher inhibitory activity. In addition, the environmental factors and seawater contamination has an impact on antibacterial activities as Tuney et al. (2007) reported that the seaweed growth in red-tide regions has better antibacterial activity. The same observation was reported by Rajauria et al. (2013). They revealed the effective antimicrobial effect of 60% methanolic extract of Himanthalia elongata against L. monocytogenes, E. faecalis, P. aeruginosa, and S. abony. They obtained high amount of total phenol and flavonoid. The antimicrobial effect of seaweed extracts may be attributed to their several bioactive compounds with antimicrobial activity. The inhibition effect of extracts is related to their constitution and concentration (Vijayabaskar & Shiyamala, 2011). The same observation was obtained by Karkhaneh Yousefi et al. (2020). They compared antimicrobial activity of fucoxanthin isolated from four brown algae (Dictyota indica, Padina tenuis, Colpomenia sinuosa, and Iyengaria stellate) from Qeshm Island, Persian Gulf. They claimed that they had an inhibitory effect against E. coli and S. aureus. However, they claimed that there are other bioactive compounds in the extract that could be responsible for antibacterial results (Karkhaneh Yousefi et al., 2020). For instance, phlorotannins are the main polyphenol group in brown seaweeds and have the antibacterial action potentials. The interactions between bacterial proteins and phlorotannins considered as the main mechanism of phlorotannins antimicrobial activity (Eom et al., 2012). In the present study, the fucoxanthin enriched extract contained polyphenolic compounds such as phlorotannins, which can exhibit the antibacterial activity.
3.4 Fatty acid contents
The fatty acid compositions of each brown algae are presented in Table 1. According to the results, high content of saturated fatty acid (63.79%) was quantified in S. angustifolium. The main saturated fatty acids in S. angustifolium were palmitic acid, myristic acid, and lauric acid with a small amount of arachidic acid, behenic acid, and stearic acid. Palmitic acid was the major fatty acid in S. angustifolium was in agreement with different Sargassum species including S. fusiforme, S. pallidum, S. horneri, and S. thunbergii (Chen et al., 2016). S. angustifolium also was rich in unsaturated fatty acids. Oleic acid and γ-linolenic acid were quantified as the major monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) in its fatty acid profile. In addition, some fatty acids found in the least amount such as palmitoleic acid, eicosenoic acid, and arachidonic acid were detected in S. angustifolium. Furthermore, erucic acid was only detected in S. angustifolium. The total MUFA and PUFA was 21.57% and 14.60%, respectively. The findings of this study are close to the results of Noviendri et al., 2011 who reported a similar fatty acids profile and revealed that the palmitic acid was the abundant fatty acid in both S. duplicatum and S.binderi. In addition, the analysis of the GC profile of C. indica showed the similar results. Additionally, C. indica was rich in oleic acid, arachidonic acid, linoelnic acid, and γ-linolenic acid. The trace of some fatty acids such as stearic acid, eicosapentaenoicacid, and eicosadienoic acid was detected in C. indica. Moreover, the total saturated fatty acid, MUFA, and PUFA were 57.43%, 27.99%, and 14.58%, respectively. The results were in agreement with Ivanova et al. (2012) who obtained the same fatty acid profile in C. barbata. In addition, Vizetto-Duarte et al. (2015) study revealed that palmitic acid was the main saturated fatty acid in C. compressa, C. nodicaulis, and C. barbata. Panayotova et al. (2017) also reported 70.63% saturated fatty acid and 16.29% PUFA for C. barbata. However, it should be considered that the fatty acid profile of seaweeds is influenced by species, habit, light, salinity, pollution and environmental conditions (Ivanova et al., 2012). The variation in fatty acid composition between seaweeds is attributed to the growth location and season. It seems that the PUFA content of seaweeds in winter is higher than the spring which is related to the lower temperature (Nomura et al., 2013). The same results were reported by Fariman et al. (2016) who investigate the fatty acid composition of Iranian C. indica. The lipid and fatty acid content directly affected by temperature and increased in the winter. The cold weather causes enhancement in PUFA and reduction in saturated fatty acids (Fariman et al., 2016). Moreover, Kordjazi et al. (2019) evaluated fatty acids in extracts of S. ilicifolium and S. angustifolium were collected from the Persian Gulf. They identified the myristic acid, palmitic acid, stearic acid, and arachidic acid as the main saturated fatty acids and palmitoleic acid, oleic acid, αlinoleic acid, and α-linolenic acid as unsaturated fatty acids of S. ilicifolium and S. angustifolium. Thus, sargassum genus is rich in MUFA and PUFA, which possess important health benefits.
| Fatty acid | Common name | S. angustifulum | C. indica |
|---|---|---|---|
| (%) | (%) | ||
| Saturated fatty acids | |||
| 12:0 | Lauric acid | 4.00 | 6.22 |
| 14:0 | Myristic acid | 8.52 | 8.61 |
| 16:0 | Palmitic acid | 47.10 | 37.97 |
| 18:0 | Stearic acid | 0.75 | 0.92 |
| 20:0 | Arachidic acid | 2.58 | 2.00 |
| 22:0 | Behenic acid | 0.84 | 1.71 |
| ∑ Saturated | 63.79 | 57.43 | |
| MUFA | |||
| 16:1 | Palmitoleic acid | 3.16 | 7.59 |
| 18:1 | Oleic acid | 15.99 | 15.43 |
| 20:1 | Eicosenoic acid | 2.42 | 4.97 |
| ∑ MUFA | 21.57 | 27.99 | |
| PUFA | |||
| 18:2 | Linoleic acid | 4.09 | 5.20 |
| 18:3 | γ-Linolenic acid | 4.78 | 2.60 |
| 20:2 | Eicosadienoic acid | 0.79 | 0.29 |
| 20:4 | Arachidonic acid | 3.91 | 5.73 |
| 20:5 | Eicosapentaenoic acid | 0.28 | 0.76 |
| 22:1 | Erucic acid | 0.75 | ND |
| ∑ PUFA | 14.60 | 14.58 | |
4 CONCLUSION
The investigated solvent type and UAE significantly influenced fucoxanthin extraction yield from S. angustifolium and C. indica. The extraction using methanol and methanol/acetone showed a higher content of fucoxanthin from C. indica and S. angustifolium, respectively. However, as the toxicity of methanol, the mixture of methanol/acetone is suggested for isolation of fucoxanthin from two seaweeds. While the solvent type had a strong influence on isolation yield, the sonication time exhibited the great effects on fucoxanthin extraction. Moreover, UAE reduced the extraction time compared with conventional methods. Further, UAE resulting in improving the fucoxanthin yield in methanol and acetone extracts may be due to greater cell wall disruption of seaweeds when the longer time was applied. The results of UAE showed that 10 min sonication among methanol/acetone solvent improved the extraction rate of fucoxanthin from S. angustifolium. Moreover, based on the results, it should be considered that S. angustifolium and C. indica contained a similar fatty acid profile with a high amount of palmitic acid and oleic acid as major saturated fatty acid and MUFA in both seaweeds. Moreover, γ-Linolenic acid and arachidonic acid were detected as PUFA in S. angustifolium and C. indica, respectively. Meanwhile, S. angustifolium extract had no antibacterial activity against S. aureus, C. indica exhibited the antibacterial effect on E. coli and S. aureus. Overall, UAE is recommended as a rapid extraction method and improving the yield of seaweeds heat-labile compounds.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the project supported by the Department of the Seafood Processing Research Group, Shiraz University (Grant number: 96GCU5M1984). Moreover, authors also thankful of Dr. Asma Behzadnia for assistance in antimicrobial assay.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
AUTHOR CONTRIBUTIONS
Najme Oliyaei: Data curation; Investigation; Methodology; Project administration; Software; Writing-original draft. Marzieh Moosavi-Nasab: Conceptualization; Funding acquisition; Resources; Supervision; Validation; Writing-review & editing.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




