The therapeutic effects of berberine for gastrointestinal cancers
Abstract
Cancer is one of the most serious human health issues. Drug therapy is the major common way to treat cancer. There is a growing interest in using natural compounds to overcome drug resistance, adverse reactions, and target specificity of certain types of drugs that may affect several targets with fewer side effects and be beneficial against various types of cancer. In this regard, the use of herbal medicines alone or in combination with the main anticancer drugs is commonly available. Berberine (BBR), a nature-driven phytochemical component, is a well-known nutraceutical due to its wide variety of pharmacological activities, including antioxidant, anti-inflammatory, antibacterial, antifungal, antiparasitic, antidiabetic, antihypertensive, and hypolipidemic. In addition, BBR exerts anticancer activities. In present article, we summarized the information available on the therapeutic effects of BBR and its mechanisms on five types of the most prevalent gastrointestinal cancers, including esophageal, gastric, colorectal, hepatocarcinoma, and pancreatic cancers.
1 INTRODUCTION
Gastrointestinal (GI) cancers consist of malignancies originating from the small intestine, rectum, colon, pancreas, gallbladder, esophagus, stomach, bile ducts, and liver. These tumors are responsible for roughly 4.1 million new cases and 3 million fatalities per year throughout the world.1 The two most common GI cancers are gastric and colorectal cancers, which affect 952,000 and 1.4 million patients annually, respectively. Each year, about 700,000 people die from each type of GI cancers.2, 3 Liver cancer is also extremely prevalent, with 782,000 new cases diagnosed each year and a high fatality rate of 746,000 deaths annually.4 Although esophageal and pancreatic malignancies are less prevalent (456,000 and 338,000, respectively), their low overall survival rates put them among the major causes of cancer-related fatalities.5, 6 GI malignancies account for the most common type of tumors, with a tendency to affect both sexes. Five types of GI cancers (colorectal cancer, gastric cancer, esophageal cancer, pancreatic cancer, and hepatocarcinoma) are particularly important because of the high prevalence and high mortality and morbidity associated with these cancers. Anticancer drugs for cancer treatment are the first line of cancer treatment, but many studies have found that “routine” medicines are limited in their efficacy. To solve this problem, herbal medicines along with the main drugs have been tested for cancer treatment.7
Berberine (BBR) hydrochloride (BH), an isoquinoline alkaloid, is found in a variety of medicinal plants, including the Ranunculaceae Juss and Berberidaceae Juss.8 It possesses anti-inflammatory, antibacterial, and antipyretic properties, among other therapeutic effects. BH has been frequently utilized in clinical settings to treat diabetic nephropathy, mycotic infection, cardiovascular disorders, and lung disease.9 BBR via a variety of mechanisms, including cell autophagy, apoptosis, and cell-cycle progression, exerts its effects to suppress tumor cells.10 By activating autophagy, BBR decreases cell growth as well as reducing therapeutic resistance. On the other hand, BBR can maintain cellular homeostasis in adult adipocytes by suppressing autophagy.11 This review describes the potential of BBR in the treatment and reduction of complications in the five most important types of GI cancers.
2 PHARMACOLOGICAL EFFECTS AND SOURCES OF BERBERINE
A benzyl tetra isoquinoline alkaloid or 2,3-methylenedioxy-9,10-dimethoxyprotoberberine chloride (C20H18NO4+), known as BBR, has a molar mass of 336.36122 g/mol. BBR is a distinctive phytochemical obtained from the different plant roots, such as Berberis aristata, Berberis vulgaris L., Berberis aquifolium Pursh, Phellodendron chinense CK Schnei, Hydrastis canadensis L., Coptidis rhizoma, which is a dried rhizome of Coptis chinensis Franch, and Camellia japonica L.12, 13
Plants that contain BBR have had medical use as early as 3000 years ago, in ancient Egyptian, Chinese, Iranian, and Ayurvedic medicines. In ancient Chinese medicine, BBR was often applied for patients with disorders of the GI tract, particularly gastroenteritis.14 Recently, BBR has gained great interest due to its various pharmacological properties, low cost, and low toxicity. The pharmacological properties of BBR have been characterized as anti-inflammatory, antimicrobial, antidiabetic, antioxidant, sedative, lipid-regulatory, antinociceptive, anticholinergic, and antiemetic properties.15 Moreover, different investigations have demonstrated that BBR is applicable in the following conditions: cardiovascular diseases (due to antiarrhythmic, antiplatelet aggregation, and anti-heart failure effects), hypertension, neurological disorders, several types of cancers, and GI disorders.16, 17 The cellular and molecular mechanisms of BBR therapeutic features include antiapoptotic, anti-inflammatory, autophagy-promoting, and antioxidative activities. These mechanisms are involved in several signaling pathways, including mitogen-activated protein kinase (MAPK) signaling, phosphatidylinositol-3 kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR), the Janus Kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3), and nuclear factor erythroid 2-related factor 2/heme oxygenase-1 (Nrf2/HO-1) pathways.18-21
BBR shows poor oral bioavailability because its water solubility is low; orally, less than 5% of BBR is absorbed. Intestinal P-glycoprotein serves as an efflux pump to remove the alkaloid from luminal mucosal cells via an active process, accounting for its low absorption. It is suggested that the administration of P-glycoprotein inhibitors for improving the absorption of BBR may enhance BBR bioavailability. BBR bioavailability has also been shown to increase when administered as dihydroberberine or dhBBR. BBR is metabolized to dhBBR by reduction using nitroreductases of gut microbiota. However, in the intestine, dhBBR is transformed back to BBR through nonenzymatic oxidation. Thus, hypothetically, the coadministration of BBR with probiotics (for regulating gut microbiota) can improve BBR bioavailability.22-24
3 EFFECTS OF BERBERINE ON VARIOUS GASTROINTESTINAL CANCERS
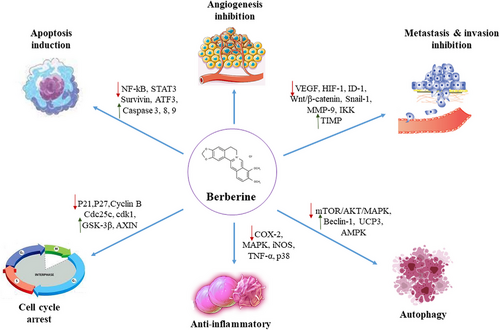
As previously mentioned, in different experimental and clinical levels, BBR plays crucial roles against numerous cancer types. In this section, we will review the anti-GI impacts of BBR with a special focus on its molecular pathways. In Figure 1, the anticancer effects of BBR have been illustrated.
3.1 Esophageal cancer
Elevated level of the expression of hypoxia-inducible factor 1 (HIF-1) was found to be correlated with resistance to radiotherapy and unfavorable prognosis in different cancer patients, including esophageal cancer.25, 26 Yang et al. demonstrated BBR treatment with different concentrations (0–150 μM) caused significant downregulation in the expression of vascular endothelial growth factor (VEGF) and HIF-1α, which led to reversed radiation resistance both in vivo and in vitro.27 Given their radiation-resistant features, the occurrence of esophageal squamous cell carcinoma (ESCC) in esophageal cancer patients results in poor prognosis.28 Hence, promoting radiation sensitivity in ESCC cancer patients can be a treatment strategy for enhancing prognosis in these patients. Liu et al. reported that the elevated expression of RAD51 in KYSE30, KYSE450, KYSE410, EC109, and TE-1 esophageal cancer cells was associated with radiation resistance, whereas BBR intervention significantly suppressed the expression of RAD51 and attenuated radioresistance in mentioned cells.29 Mammalian target of mTOR is a serine/threonine protein kinase that has crucial roles in the mediation of cell-related processes, including survival, proliferation, autophagy, and protein synthesis.30 Aberrant regulation of mTOR led to the overexpression of it in different types of cancer.31 Via suppressing the phosphorylation of Akt, mTOR, and promoting the phosphorylation of AMP-activated protein kinase (AMPK), BBR treatment in KYSE-70 and SKGT4 esophageal cancer cell lines caused inhibitory properties on the cell survival and cell proliferation of cancer cells. Besides, BBR accentuated the expression of p21 in tumor cell lines.32 Iizuka et al. evaluated the anti-esophageal cancer effects of BBR in six esophageal cancer cell lines. Cell proliferation of cancer cells was considerably decreased after BBR treatment.33 Müller et al. demonstrated the expression level of chemokine receptors CXCR4 and CCR7 were upregulated in human breast cancer cells, leading to cancer metastasis and invasiveness.34 By inducing downregulatory effects on the expression of CXCR4 and CCR7, BBR treatment decreased migration and metastasis features of KYSE-30 esophageal cancer cells.35 Wnt/β-catenin signaling pathway has been implicated in various human cancer types, and this pathway was shown to be overexpressed in cancers.36 Ren et al. demonstrated that after the administration of BBR, the expression levels of Wnt3a and β-catenin were significantly decreased both in vivo and in vitro.37 Mechanistic summary of the efficacy of BBR against esophageal cancer are shown in Table 1.
| Cell line | Route | Effects |
|---|---|---|
| ECA109 and TE13 | In vitro (0–150 μM) and in vivo (n = 6, 5 mg/kg) | Treatment decreased the HIF-1α and VEGF and consequently, promoted radiosensitivity in vivo and in vitro27 |
| KYSE30, KYSE450, KYSE410, EC109, and TE-1 | In vitro (0–200 μM for 24, 48, and 72 h) | RAD51 was attenuated after treatment with berberine29 |
| KYSE-70 and SKGT4 | In vitro (0, 20, 40, 60, and 80 μmol/L for 12, 24, and 48 h) | Upregulation of p21 was observed32 |
| YES-1 to YES-6 (6 cell lines) | In vitro (.035–5.6 μg/ml for 72 h) | Proliferation inhibitory effects were observed in all of the cell lines after berberine treatment33 |
| KYSE-30 | In vitro (0, 1, 2, 4, 8, 16, 32, 64, 128, and 256 μM for 24, 48, and 72 h) | Berberine attenuated cell proliferation35 |
| Eca9706, TE-1, and EC109 | In vitro (0–200 μM) and in vivo (n = 6, 20 mg/kg) | The expressions of Wnt3a and β-catenin decreased after berberine treatment37 |
- Abbreviations: HIF-1α, hypoxia-inducible factor 1-alpha; VEGF, vascular endothelial growth factor.
3.2 Hepatocarcinoma
Activator protein 1 (AP-1) is a transcription factor that possess a pivotal role in the induction of tumor initiation and progression.38 A concentration of 10 μM BBR caused a remarkable inhibitory effect on the expression of AP-1 in KIM-1 human hepatoma cells resulting in decreased cancer growth rate.39 Yip et al. demonstrated that BBR treatment (0, 5, 10, and 20 μM) in Huh7 cancer cells caused considerable reduction in the expression of Bcl-2 and Akt, whereas the expression level of Bax, BH3 interacting domain death agonist (Bid), cell death activator A (CIDEA), harakiri (HRK), and p21 were promoted after treatment.40 Sorafenib is a multikinase inhibitor and is used in hepatic cancer patients.41 Yet, in most patients, chemoresistance to sorafenib is a challenge for the management the hepatic cancer.42 By upregulating the expression of PARP and cleaved caspase-3 and downregulating the expression level of Bcl-2 and VEGF, BBR has been shown to cause chemosensitivity to sorafenib in SMMC-7721 and HepG2 hepatocellular cancer cell lines.43 Dysregulation of nuclear factor κB (NF-κB) and its downstream signaling pathways have been implicated in numerous human disorders, especially in cancer patients.44 Therefore, the application of NF-κB inhibitors along with classic therapeutic agents can be an optimal treatment strategy in cancer patients.45 Li et al. showed that BBR treatment (10, 50, and 100 μM for 24, 48, and 72 h) reduced the NF-κB p65 expression levels in HepG2 liver cancer cells, causing NF-κB-mediated apoptosis.46 SLC1A5 is a Na+-dependent glutamine transporter implicated in different types of human cancer, including hepatocellular carcinoma.47-49 Overexpression of this plasma membrane transporter in cancer is associated with a greater glutamine metabolism and, subsequently, further proliferation of tumor.49 In Hep3B and BEL-7404 hepatic cancer cells, and in hepatic cancer-bearing mice, by inhibiting c-Myc, BBR treatment caused suppression in SLC1A5 expression, lading to lower glutamine metabolism rate.50 Numerous investigations have demonstrated that an overexpression of arachidonic acid and its downstream cascades, including COX and lipoxygenase (LOX), may be a potential reason for stimulating tumor initiation, growth, and proliferation.51, 52 Zhao et al. reported that BBR treatment in HepG2 hepatocellular cancer cell line, attenuated LOX-5 expression, and therefore, decreased the production of leukotriene B4 as one of the most important end products of arachidonic acid metabolic pathway.53 FoxOs transcriptional factors are a group of O class mammalian forkhead-box family, which have important roles in the inhibition of tumor initiation and tumor growth by modulating various cellular events and molecular pathways like downregulation via the PI3K/Akt.54, 55 In HepG2cell line, BBR treatment significantly upregulated the expression of FoxO1, FoxO3a, tumor suppressor gene PTEN, Bim protein, proapoptotic Bax, the ratio of Bax/Bcl-2, and caspase activation. However, PI3K/Akt axis was negatively regulated after BBR treatment.56 Dysregulation of inhibitor of differentiation/DNA binding-1 (ID-1) as a transcriptional agent has crucial roles in different cancer-related events, including tumor initiation, progression, metastasis, invasiveness, and angiogenesis in different cancer types.57 Tsang et al. demonstrated that BBR supplementation (10 mg/kg/2 days) in hepatocellular lung metastasis tumor-bearing mice reduced the proliferative features and invasiveness of cancer cells via suppressing ID-1 expression and subsequent inactivation of HIF-1α/VEGF signaling pathway.57 Wang et al. noted that BBR treatment in HepG2, Hep3B hepatocellular cancer cells significantly upregulated miR-23a in a p53-dependent manner, which is significantly downregulated in hepatic cancer tissue.58 Recently, Mustafa et al. examined the biochemical/immunohistochemical effects of BBR in mice liver. Immunohistochemical analysis proposed a significant reduction in the quantitative expression of the key oncogene cyclin D1 in both low dose (60 mg/kg) and high dose (120 mg/kg) of BBR.59 These findings show the amelioration of hepatocarcinoma by BBR more prominently in mice, by suppressing the expression of cyclin-dependent kinase activator (CD1), reducing LFTs as well as serum AFP (alpha-fetoprotein). Therefore, and based on these new findings, BBR may help control the CD1 perturbation associated with aggressive forms of hepatocellular carcinoma.59 Other studies on the therapeutic benefits of BBR in the treatment of hepatic cancer are summarized in Table 2.
| Component (cell line) | Route | Effects |
|---|---|---|
| Berberine (HepG2 and MHCC97-L) | In vitro (50, 100, 200, and 400 μM for 12 h) | Upregulated Bax expression, release of cytochrome c, activation of caspase-3 and -9, Beclin-1, and P38 MAPK signaling, whereas decreased the expression of Akt and mTOR-signaling pathway60 |
| Berberine (SMMC-7721) | In vitro (.015 μmol/ml) | Upregulated the expression of TNF-α, and tumor growth61 |
| Berberine (Hep3B and HepG2) | In vitro (50 μM for 24 h) | Promoted AMPK activation, and subsequently suppressed β-catenin and mTOR signaling pathways62 |
| Berberine (HepG2, Hep3B, and SNU-182) | In vitro (10, 20, 50, and 100 μM for 24 and 72 h) | Upregulated the expression of KLF6, ATF3, and p21. On the other hand, decreased the expression of E2F1 and PTTG163 |
| Berberine (nonalcoholic steatohepatitis-derived hepatocellular carcinoma) | In vivo (250 mg/kg for 12 weeks, n = 10) | Decreased the activity of COX-2, and expression level of phosphorylated p38 MAPK and ERK64 |
| Berberine (MHCC97L) | In vitro (0, 7.8125, 15.625, 31.25, 62.5, 125, 250, 500, and 1000 μM for 24 h) | Repressed GPT1 and, subsequently, inhibited ATP production in cancerous cells65 |
| 9-O-benzoyl-substituted berberine (HepG2) | In vitro (15 μM for 24 h) | Caused a considerable upregulation in the expression of AMPK signaling pathway66 |
| Berberine (HepG2, Bel-7402, and SMMC-7721) | In vitro (3.125, 6.25, 12.5, 25, and 50 μM)and in vivo (70 mg/kg for 14 days) | Decreased the expression of cyclin D1, cyclin E, cdc2, β-catenin, and phosphorylation of Akt, mTOR, and ERK67 |
| Berberine (HepG2) | In vitro (10, 20, 30, and 40 μM for 24 h) | Downregulated the expression of metalloproteinases-9 and suppressed the activity of PI3K-Akt and ERK pathways68 |
| Berberine (SMMC7721 and HepG2) | In vitro (6.25 μM for 24 h) | Repressed the phosphorylation of mTOR69 |
| Berberine and 9-/13-lipophilic substituted berberine derivatives (HepG2) | In vitro (different concentrations for 24 h) | Caused remarkable apoptotic and cytotoxic properties against liver cancer cells70 |
| Berberine (HepG2) | In vitro (different concentrations for 24 h) | Caused GRP78-mediated autophagy cell death71 |
| Berberine (HepG2) | In vitro (.1, .2, .5, 1, 2, 5, 10, 20, 50, and 100 μM for 24 h) | Mediated CAR signaling pathway72 |
| Berberine (HepG2) | In vitro (0, 1, 10, 30, 50, and 100 for 72 h) | Caused activation of p38 MAPK signaling pathway, upregulation of p38, Bax, caspase-3, and ROSs generation in cancer cell line73 |
| Berberine (HepG2) | In vitro (0, 5, 10, and 15 μM for 24, 48, and 72 h) | Downregulated the expression of VEGF as an oncogenic agent74 |
| Berberine (HepG2) | In vitro (1–50 μM for 3 days) | Caused cell-cycle arrest and growth inhibition in a dose-dependent manner75 |
| Berberine (HepG2) | In vitro (5, 25, 50, 100, and 200 μM for 12, 24, and 48 h) | Promoted the expression level of MicroRNA-22-3p, whereas attenuated the expression of SP1, CCND1, and BCL276 |
| Berberine (HepG2) | In vitro (10, 50, and 100 μM for 24 h) | Activated AMPK signaling and subsequently suppressed mTOR complex 177 |
| Berberine (HepG2, SMMC-7721, and Bel-7402) | In vitro (12.5, 25, and 50 μM for 24 h) | Increased the level of phosphorylated AMPK, phosphorylated Akt, the ratio of Bax/Bcl-2, caspase-3, and -978 |
| Berberine (HepG2 cells and HEK293T) | In vitro (40 μM for 2 and 4 h) | Upregulated the expression of miR-21-3p79 |
| Berberine (HepG2) | In vitro (30, 60, and 120 μM for 24 h) | Suppressed the expression of Skp2 and promoted the expression of FoxO3a80 |
| Berberine (HepG2) | In vitro (50 and 100 μM for 18 and 24 h) | Modulated PHLPP2-Akt-MST1 kinase signaling pathway81 |
| Berberine (HepG2 and Bel-7404) | In vitro (12.5, 25, 50, and 100 μM for 24, 48, and 72 h) | Inhibited the expression of cPLA2 and COX-2 and suppressed the arachidonic acid metabolic pathway82 |
| Berberine (HepG2 and SMMC7721) | In vitro (31.25, 62.5, 125, 250, or 500 μM for 24 h | Downregulated the expression of CD14783 |
| Berberine (Huh7 and HepG2) | In vitro (10, 20, and 40 μM) for 24 h)and in vivo (n = 6, 5 mg/kg/day) | Repressed Nrf2 signaling pathway, and radiosensitizes hepatic cancer cells84 |
| Berberine (HepG2 and H22) | In vitro (5 μg/ml for 4 days) | Inhibited the caspase-3-iPLA2-COX-2 signaling pathway85 |
| Berberine and berberine nanosuspension (Huh7 and HepG2) | In vitro (0–100 μg/ml for 72 h) and in vivo (n = 10, 100 mg/kg) | Berberine showed inhibitory effects on the cancer cells86 |
| Berberine (NA) | In vivo (8 mg/kg body weight pretreatment and 2, 4, 8, and 12 mg/kg body weight posttreatment) | Upregulation of PP2A and downregulation of Akt and JNK were observed after 30 days87 |
| Berberine and berberine-bile acid analog (B4) (SMMC-7721) | In vitro (.8–50 μM for 24, 48, and 72 h) | B4 upregulated the release of cytochrome c, PARP, and caspase-3 expressions88 |
| Berberine and berberine-solid lipid nanoparticles (H22) | In vitro (0, .1, 1, 10, and 100 μg/ml) and in vivo (100 mg/kg for 21 days) | Berberine reduced the proliferation of H22 cancer cells in vivo and in vitro89 |
- Abbreviations: AMPK, AMP-activated protein kinase; CAR, constitutive androstane receptor; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol-3 kinase; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor.
3.3 Pancreatic cancer
Citrate synthase is an important enzyme with a prominent role in the production of citrate via catalyzing the reaction between oxaloacetic acid and acetyl coenzyme A.90 By increasing glucose to lipid conversion, the activity of this enzyme can lead to the development of pancreatic cancer.91 Hence, the aberrant activity of citrate synthase represents a possible mechanism for the accumulation of lipids as important substrates for developing pancreatic cancer cells.92 BBR treatment (10 and 50 μM for 1 and 48 h) in MIA PaCa-2 cells caused cell-cycle arrest in G1 phase, senescence and induction of autophagy, and upregulation of caspase-3 activity. Furthermore, in view of the reduced activity of citrate synthase, a significant mitochondrial dysfunction was observed upon treatment with BBR.93 Liu et al. demonstrated that after BBR treatment (2.5, 3.75, 5, 10 μM) in Panc-1 (CRL-1469) cancer cells, citrate metabolism and mitochondrial function of tumoral cells were considerably reduced, which led to the decreased biosynthesis of fatty acids by regulating ACLY, ACO1, and SLC25A1.94
As mentioned earlier, due to their important functions in cancer development, an inhibition of CSCs may represent efficacious anticancer medicines.95 Park et al. reported that BBR treatment in pancreatic CSCs of PANC-1 cell line reduced side-population cancer cells proportion and significantly downregulated the expression of POU5F1, CSCs-associated SOX2, and NANOG cells.96 In another study conducted by Park et al., BBR treatment in PANC-1 and MIA-PaCa2 cell lines caused intracellular ROSs generation and significantly promoted the activity of caspase-3/-7. Therefore, BBR led to cell-cycle arrest in the G1 phase in mentioned cell lines.97
BBR treatment (10–200 μM for 24 h) was shown to cause a significant increase in the p53-mediated apoptotic pathway in BxPC-3 pancreatic ductal adenocarcinoma cells, compared with normal epithelial cells of pancreas. In contrast, the expression levels of caspase-3 and -7 were not significantly affected after BBR treatment. Furthermore, all the changes in this study caused G1/S and G2/M phases’ cell-cycle arrest.98 Ming et al. showed that .3–6 μM of BBR in PANC-1, MiaPaCa-2 cancer cells led to cell-cycle arrest in G1 phase, suppression of DNA biosynthesis, and cell proliferation. Further, BBR downregulated the expression of intracellular ATP levels, mTORC1, and ERK activation, whereas upregulated the activation of AMPK.99 Table 3 illustrates the effects of BBR on different pancreatic cancers.
| Component (cell line) | Route | Effects |
|---|---|---|
| Berberine (MIA-PaCa-2) | In vitro .4, 2, 10, and 50 μM | Berberine induced cell-cycle arrest at G1 phase and caspase-3 activation93 |
| Berberine (Panc-1 (CRL-1469)) | In vitro 2.5, 3.75, 5, and 10 μM for 72 h | Decreased the citrate metabolism, mitochondrial function, and fatty acids production94 |
| Berberine (PANC-1 and MIA-PaCa-2) | In vitro 10 and 15 μM for 72 h | The expressions of SOX2, POU5F1, and NANOG genes were downregulated after treatment96 |
| Berberine (BxPC-3) | In vitro 10, 50, 100, 150, and 200 μM for 24, 48, and 72 h | Treatment with berberine upregulated the expressions of p53 and BRCA1-mediated DNA damage response98 |
| Berberine (PANC-1 and MIA-PaCa-2) | In vitro (.3–6 μM) and in vivo (n = 10, 5 mg/kg) | Cell-cycle arrest at G1 was observed in vitro. Inhibited cell proliferation in vivo99 |
| Berberine and phenyl-substituted berberine triazolyls (SW-1990) | In vitro 7.8 μM | Cytotoxicity effects of berberine were more powerful than most of its derivatives100 |
| Berberine and modified berberine compounds (MIA-PaCa-2, MDA-PANC-28 (CVCL_3917), and AsPC-1) | In vitro 250–2000 nM of each compound | Inhibition of tumor growth and colony formation were observed101 |
| Berberine, NAX038, NAX060, and metformin (MIA-PaCa-2, MDA-PANC-28 (CVCL_3917), AsPC-1, and BxPC-3) | In vitro, 100 nM of berberine and its derivatives | Berberine and its modified compounds showed their synergistic AMPK-mediated anti-pancreatic cancer effects when combined via metformin102 |
- Abbreviation: AMPK, AMP-activated protein kinase.
3.4 Gastric cancer
As the principal family of zinc-dependent enzymes, matrix metalloproteinases (MMPs) have been reported to be one of the most important agents in the process of extracellular matrix (ECM) digestion.103 Various investigations have shown that MMPs play crucial roles in different cancer-related events, including apoptosis, cell differentiation and proliferation, migration, and invasion.104 MMP-2 has been reported to be more overexpressed in various human neoplasms compared with other MMPs, which are involved in cancer development. To that end, the expression level and activity of MMP-2 have a remarkable direct association with tumor development, invasiveness, and migration.105 Emerging evidence have indicated that MMP-2 plays a pivotal role in the tumor cells–mediated degradation of ECM, and subsequent epithelial–mesenchymal transition occurrence.106 In gastric cancer cells, an overexpression of MMP-2 has also been associated with increased invasiveness and metastasis.106 Lin et al. evaluated the effects of BBR treatment (25, 50, 75, and 100 μM for 6, 12, and 24 h) in SNU-5 gastric cancer cells, showing that BBR treatment prominently increased the production of ROSs, downregulated NF-κB p65, MMP-1, and MMP-2 gene expression, whereas upregulated MMP-9 gene expression. In addition, BBR significantly suppressed tumor invasiveness in a concentration-dependent manner.107
Signal transducer and activator of transcription are a group of crucial proteins that have been reported to be involved in the mediation of signal transduction from the ECM into the nucleus.108, 109 STAT proteins are recognized as one of the most important transcription factors.110-112 Currently, seven members of STAT proteins family have been recognized. Among them, STAT3 has pivotal functions in the initiation and progression of cancer and malignancy, by affecting numerous target genes and signaling pathways.113 Survivin is recognized as a crucial apoptosis inhibitor agent, which has unique important properties for preventing the initiation of molecular responses related to apoptosis and promoting cell-cycle progression.114, 115 Aberrant expression of survivin has been shown to be dependent on the expression of various factors, and among them, STAT3 plays crucial roles in the expression of survivin.116, 117 Therefore, due to its oncogenic role, ectopic expression and activation of STAT3 have been demonstrated to be an initial event in gastric cancer induction.118 Pandey et al. demonstrated that BBR (5, 10, 25, and 50 μM) caused a significant dose-dependent inhibition on the viability of cancer cells in AGS gastric adenocarcinoma cell line. Furthermore, BBR downregulated the expression of survivin and decreased pSTAT3 levels.119 Fatty acid–binding proteins (FABPs) belong to a superfamily of lipid-binding proteins, which have been known as important enzymes in the lipid metabolism.120, 121 By regulating fatty acid uptake and consequent intracellular transport, FABPs play a role in the metabolism of lipids.122 FABPs have been reported to be strongly associated with cancer initiation and development in specific types of cancer, such as hepatocellular carcinoma.123 Li et al. evaluated the effects of BBR (0, 10, 20, and 40 μM) on the MGC803 gastric cancer cells and MGC803 xenograft mouse models in vivo. They found that BBR has significant inhibitory properties on the cancer cell survival and proliferation in a concentration- and time-dependent manner. Protein expression of FABPs (FABP4 and FABP5) as potential tumor markers of gastric cancer was decreased after BBR treatment. In addition, intervention with BBR resulted in a suppressed expression of peroxisome proliferator-activated receptor alpha, downregulated expression of Bcl-2, increased expression of cleaved caspase-3 and Bax in MGC803 xenograft tumors.124
Dysregulation of JNK/p38 MAPK signaling has been demonstrated to be an important event in the induction of malignancies, and aberrant activation of this pathway plays pivotal functions in the initiation and progression of different types of cancer.125 BBR treatment (6.25, 12.5, 25, 50, 100, and 200 μM) in SNU-1 gastric cancer cells caused significant changes in the morphology of SNU-1 cells and remarkable inhibition on the viability of tumoral cells. By suppressing the expression of NF-κB, and promoting the expression levels of caspase-3, -8, and -9, BBR induced noteworthy proapoptotic properties in gastric cancer cells. Finally, results showed BBR suppressed JNK/p38 MAPK signaling pathway in a considerable manner and decreased the migration and invasiveness abilities of cancer cells.126
Zhang et al. investigated the impacts of BBR treatment (14–108 μM for 6, 12, 24, 36, and 48 h) on the cell survival-, proliferation-, and autophagy-related signaling pathways and markers of BGC-823 cell line and xenograft models. Results showed that BBR degraded p62, whereas upregulated LC3-II, Beclin-1, and p-ULK1 as important autophagy-related biomarkers. Via suppressing mTOR, Akt, and MAPK, BBR increased cytostatic autophagy occurrence and subsequently reversed cell survival and proliferation in in vitro cancerous cells. Furthermore, BBR remarkably attenuated the levels of p-mTOR, p-p70S6K, p-Akt, p-ERK, p-JNK, and p-p38 in xenografts.127
BBR effectively inhibited the growth of xenograft tumors in vivo when administered during intermittent fasting (hypoglycemic conditions), and it was well tolerated by nude mice with no adverse effects. According to these findings, the BBR/low-glucose combination can inhibit the growth of gastric cancer via the PP2A/GSK3/MCL-1 signaling pathway. As a result, this combination of drugs and lifestyle could pave the way for a new type of safe and effective anticancer therapy. The effects of BBR on gastric cancer and underlying signaling pathways are summarized in Table 4.
| Component (cell line) | Route | Effects |
|---|---|---|
| Berberine (BGC-823 and SGC7901) | In vitro (0–100 μM for 48 h) and in vivo (n = 10, 10 mg/kg) | Cell proliferation was inhibited in vivo. Akt/mTOR/p70S6K/S6 pathway was inhibited in vitro128 |
| Berberine (AGS and SGC7901) | In vitro (10–80 μM for 24, 48, and 72 h) and in vivo (n = 3, 100 mg/kg/day) | HNF4a, WNT5A, and cytoplasmic β-catenin levels were attenuated after treatment129 |
| Berberine (SNU-5) | In vitro (25–200 μmol/L) | Cell-cycle arrest at G2/M phase and increase in p53 expression was observed130 |
| Berberine (MKN45, BGC823, and SGC7901) | In vitro (15–90 μM for 24, 48, and 72 h) and in vivo (156 mg/kg/day for 90 days) | Treatment with berberine attenuated the STAT3, Akt, ERK, NF-κB, Bcl-xL, and cyclinD1131 |
| Berberine and its admixture with cisplatin (SGC-7901 and BGC-823) | In vitro | Berberine enhanced the effects of cisplatin against gastric cancer cell line by upregulation of miR-203132 |
| Berberine (SGC-7901) | In vitro (0–30 μmol/L for 24 and 48 h) | Apoptosis and cell-cycle arrest at G1 phase was observed133 |
| Berberine hydrochloride (MGC-803) | In vitro (0–60 μM for 24 and 48 h) and in vivo (n = 6, 15 mg/kg for 23 days) | P38 MAPK, ERK1/2, and JNK phosphorylation significantly attenuated in vitro and in vivo134 |
| Berberine (MGC-803) | In vitro (20 μM for 24 and 48 h) | Upregulation of caspase-3 and downregulation of Bcl-2 was discovered135 |
| Berberine (AGS) | In vitro (15, 30, and 60 μM) | Berberine inhibited AGS cells adhesive ability to HUVECs, downregulated VCAM-1, and upregulated ICAM-1136 |
- Abbreviations: MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κB.
3.5 Colorectal cancer
Insulin-like growth factor 2 (IGF2) mRNA-binding protein 3 (IGF2BP3) has been recognized as an important member of oncofetal RNA-binding proteins (RBPs).137 The 5′-Untranslated region of IGF2 mRNA is the specific binding site of IGF2BP3. Binding to the 5′-untranslated region of IGF2 mRNA leads to the activation of its translation and consequent activation of its target PI3K/Akt signaling pathway.138, 139 IGF2BP3 was shown to be expressed in various cancer types, including colorectal cancer and renal cell carcinoma.140, 141 IGF2BP3 can be used a prognostic and diagnostic biomarker for colorectal cancer patients.142 Furthermore, in vitro and in vivo studies reported that an overexpression of IGF2BP3 is considerably associated with the promotion of aggressive phenotypes of colorectal cancer cells.143 Zhang et al. demonstrated the effects of BBR (0, 25, 50, and 100 μM for 24 h) on the cell proliferation and survival of HCT116 and SW480 colorectal carcinoma cells and xenograft models. Results showed the colony formation of cancer cells was inhibited in a dose-dependent manner. Tumor weight and volume were decreased after BBR intervention in colorectal cancer tumor–bearing mice. Upregulation of Bax, caspase-3, and cleaved caspase-3 and downregulation of Bcl-xLin in response to BBR promoted apoptosis in HCT116 and SW480 cells. Further, by downregulating IGF2BP3, BBR strongly suppressed PI3K/Akt signaling pathway. Inhibition of PI3K/Akt signaling led to significant suppression in cell proliferation and cycle transition.144
As a group of cholesterol sensors that are located in the endoplasmic reticulum, sterol-regulatory element binding proteins (SREBPs) have pivotal functions in the regulation of intracellular cholesterol via mediating Insig–SREBP–SCAP pathway.145, 146 Gao et al. demonstrated that by upregulating the expression of MMP-7 and activation of NF-κB signaling pathway, SREBP1 enhanced the invasiveness of colorectal cancer cells.147 By triggering G0/G1 phase cell-cycle arrest, BBR treatment (6.25, 12.5, 25, and 50 μM for 24 and 48 h) in DLD-1 and Caco-2 colon cancer cells suppressed the proliferation and invasion. BBR also diminished Wnt3α and β-catenin, whereas increased the expression level of glycogen synthase kinase 3β (GSK-3β) and AXIN. Furthermore, by decreasing SREBP cleavage-activating protein (SCAP)/Insig protein levels, BBR significantly inhibited the activation of SREBP-1 and its target genes, including FASN, ACC, and ACL in vitro. As two important lipogenic enzymes, the inhibition of these molecules led to a significant decrease in lipogenesis of xenografts and colon cancer cells, and consequent suppression of colon cancer progression.148 Wang et al demonstrated BBR on IMCE and HT-29 human colorectal adenocarcinoma cell lines and nude mice, and APC min/+ mice. They found BBR has anti-proliferation effects, downregulates EGFR and cyclin B1/B3/D1, induces apoptosis and cell-cycle arrest, and activates Cbl, in vitro and in vivo.149 Other studies on cancer cell lines and mice colon cancer model also indicated anti-inflammation and anti-proliferation effects of BBR, and its role in the induction of apoptosis and inhibition of EGFR-ERK signaling and the JNK/STAT3 and β-Catenin pathways. In Table 5, we have summarized in vitro and in vivo studies that evaluated the anticancer effects of BBR in colorectal cancer.
| Component (cell line) | Route | Effects |
|---|---|---|
| Berberine (HCA-7) | In vitro (10, 30, 50, and 100 μM for 48 h) | The expressions of ANAPC2, CCNA2, BMP7, PTP4A1, CaMKII, and ITGA5 was attenuated150 |
| Berberine (SW620 and LOVO) | In vitro (0, 5, 10, 20, 40, and 80 μM for 24, 48, and 72 h) and in vivo (n = 6, 50, 100, and 200 mg/kg/d in three group of berberine treatment) | Downregulation of JAK2/STAT3 signaling pathways was occurred151 |
| Berberine (SW480) | In vitro (0, 20, 50, 100, 200, and 300 μM for 24 h) | Berberine downregulated the expressions of Bax, Bcl-2, and c-Myc152 |
| Berberine (HT29 and HCT116) | In vitro (0, 10, 20, 40, 60, 80, and 100 μM for 12, 24, 48, and 72 h) | LncRNA CASC2 was upregulated and Bcl-2 was downregulated after treatment153 |
| Berberine (HCT116, SW480, and LOVO) | In vitro (0–100 μmol/L for 24, 48, and 72 h) and in vivo (n = 15, 40 mg/kg) | Berberine inhibited the NF-κB activity, cyclin D1, surviving and increased the p53 and caspase-3 expressions154 |
| Berberine (SW480, HCT-116, and HT-29) | In vitro (1, 10, and 50 μM for 1, 2, and 4 days) | NAG-1 and ATF3 induced by berberine155 |
| Berberine (HCT116) | In vivo (n = 6, 6.25, 12.5, and 25 mg/kg for 3 weeks) | ODC, C-MYC, and HIF-1α were attenuated, and OAZ1 and SSAT were upregulated156 |
| Berberine (NA) | In vitro (4, 8, and 16 μM for 72 h) | MiR-429, E-cadherin, and Par3 were decreased after treatment157 |
| Berberine (DLD-1) | In vitro (.01, .1, 1, and 10 μM for 24, 48, 72, and 96 h) | Inhibited COX-2 transcriptional activity158 |
| Berberine (HT-29, HCT116, SW480, SW620, and LoVo) | In vitro (20, 40, 50, 60, and 80 μM) |
Promoted lncRNA CASC2, while interacted with EZH2 and Bcl-2 Upregulated caspase-3 and -9 expression levels159 |
| Berberine (N = 10 mice in each group; LoVo, SW480, HT-29, and HCT116 cell lines) | In vivo (10, 30, or 50 mg/kg by oral gavage for 10 days) and in vitro (1.25, 2.5, 5, 10, 20, 40, 80, or 160 μM for 24–72 h) | Cyclin B1, cdc2, and cdc25c proteins were deceased after berberine treatment160 |
| Berberine (SW480, SW620, HT-29, and HCT116) | In vitro (1, 2, 5, 10, 20, 50, and 100 μM for 24 h) | Activated AMPK and consequently caused AMPK-mediated reduction in the phosphorylation and protein levels of integrin β1161 |
| Berberine (HCT 116) | In vitro (2.5, 5, and 10 μM for 24 and 48 h) | Via suppressing NF-κB, c-IAP1, c-IAP2, survivin, and Bcl-xL, reversed the chemoresistance to irinotecan162 |
| Berberine (N = 12; KM12C, KM12SM, and KM12L4A cell lines) | In vivo (.33 g/L dissolved in drinking water) and in vitro (25, 50, and 100 μM) | Suppressed the activity of Wnt signaling and downregulated the expression of cyclin D1 and c-Myc163 |
| Berberine (N = 5 mice in each group; HT-29 cells) | In vivo (100 mg/kg for 3 days) and in vitro (25, 50 μM for 1, 2, 6, 18, and 24 h) | Promoted the activation of ubiquitin ligase Cbl, expression of p21 and p15. Decreased total EGFR, cyclin D1, cyclin B1, cyclin B3, CDK4, and cdc25C149 |
| Berberine (IMCE) | In vitro (12.5, 25, 50, and 100 μM for 1, 3, 6, 18, and 24 h) | Stimulated AIF release, cathepsin B release, and PARP activation164 |
| Berberine (HCT-15 and HT-29) | In vitro (10 μM for and 24 h) | Decreased survivin and CDK4 expression level. Triggered microRNA-296-5p-mediated inhibition of suppression of Pin1/β-catenin/cyclin D1 signaling pathway165 |
| Berberine (Colo 205) | In vitro (.8–1600 μM for 12, 24, 36, 48, 60, and 72 h) | Suppressed the activity of N-acetyltransferase166 |
| Berberine, NAX053, NAX056, NAX057, NAX080, and NAX081 (HCT116 and SW613-B3) | In vitro (.5, 1, 2.5, 5, 7.5, and 10 μM for 72 h) | Berberine and its derivatives promoted the expression of caspase-3 and cell death167 |
| Berberine (SW480) | In vitro (12.5, 25, 50, 75, 100, and 200 μM for 24, 48, and 72 h) | Promoted Bcl-2 family proteins, caspases, and p21 expression levels and release of cytochrome c.168 Suppressed caspase-8-mediated angiogenesis, NF-κB, and Cox2 |
| Berberine, NAX012, NAX014, and NAX018 (HCT116 and SW613-B3) | In vitro (.5, 1, 5, and 10 μM for 24 and 48 h) | Immunofluorescent cellular staining of p53 and p21 were increased in HCT116 cells169 |
| Berberine (HCT-116 and DLD1) | In vitro (20, 40, 80, 100, 120, 140, and 160 μM for 24 h) | Elevated the expression of GRP78, also increased the expression of LC3 and Beclin-1, whereas reduced p62 expression as autophagy markers71 |
| Berberine (SW620) | In vitro (5, 10, 25, and 50 μM for 3, 6, 12, and 24 h) | Increased activation of caspase-3 and -8, cleavage of PARP, and cytochrome c release. Decreased expression of BID, c-IAP1, Bcl-2, and Bcl-XL21 |
| Berberine (HCT-8) | In vitro (.03, .06, .12, .24, and .47 mmol/L for 12, 24, 48, and 72 h) | Upregulated Fas, FasL, TNF-α, caspase-3, Bax, and p53 expression levels. Downregulated pro-caspase-3 and Bcl-2 expressions170 |
| Berberine (N = 10; HCT116 cells) | In vivo (120 and 240 mg/kg five times a week for 20 weeks) and in vitro (5, 10, 20, 40, and 80 μM for 24, 48, and 72 h) | Diminished colorectal cancer cell growth and proliferation via affecting Wnt/β-catenin signaling pathway171 |
| Berberine (N = 16 in each group; IMCE cells) | In vivo (4 weeks) and in vitro (25 μM for 12 h) | Inhibited EGFR-ERK signaling pathway and expression levels of IL-6 and TNF-α172 |
| Berberine (HCT116 and KM12C) | In vitro (0–100 μM for 15 and 24 h) | Suppressed HIF-1α, glucose uptake, GLUT1, LDHA, and HK2173 |
| Berberine (N = 10 in each group) | In vivo (75 mg/kg daily for 10 weeks) |
Downregulated MPO, MAPK, and COX-2. Downregulated apoptotic markers caspase-3 and P53 expression levels174 |
- Abbreviations: HIF-1α, hypoxia inducible factor 1-alpha; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κB.
4 CLINICAL TRIALS USING BERBERINE IN GI CANCER TREATMENT
Several in vivo and preclinical studies have been launched to assess the efficacy and safety of BBR. In-depth research into the efficacy of BBR and its derivatives is ongoing, and an increasing number of studies have focused on the potential antitumor role of BBR, possibly through immune system regulation.175
According to the observation of anticancer effects of BBR, it was clinically evaluated in cases of gastric cancer, gastric ulcer, and colorectal adenoma.176 In one study, the anticancer activity of BBR was investigated in order to evaluate the reduction of the effects of radiation therapy in patients with cervical cancer and lymphoma.177 BBR protected lung cells from damage caused by ionizing radiation in non-small cell lung cancer patients receiving radiation therapy. It selectively sensitized tumor cells to ionizing radiation in glioma patients, whereas healthy cells remained at the same level of sensitivity. BBR's ability to increase chemical sensitivity and reduce the side effects of chemical sensitizers has also been emphasized.177 In a randomized, pilot phase I trial study (NCT02365480) launched to determine the safety of BBR chloride in treating patients with ulcerative colitis who are in remission.178 These patients are in high risk for colorectal cancer. The use of BBR may reduce the risk of colorectal cancer in patients with ulcerative colitis. BBR is also currently being evaluated as a prophylactic agent in patients with colorectal adenoma with a history of colorectal cancer treated with gefitinib. Chen et al. in a double-blinded, randomized controlled study (NCT02226185) assessed the safety and clinical potential of BBR for the prevention of colorectal adenoma recurrence for 2 years.178 Participants received BBR (0·3 g twice daily) or placebo tablets via block randomization. According to results, 36% of participants in the BBR group and 47% in the placebo group had recurrent adenoma during follow-up. No colorectal cancers and no serious adverse events were detected during follow-up. These researchers stated that BBR .3 g twice daily was safe and effective in reducing the risk of colorectal adenoma recurrence and could be an option for chemoprevention after polypectomy.178 It is well known that colorectal adenomas are the precursor of colorectal cancer and that removing them will prevent colorectal cancer. In this light, a randomized, double-blind, phase II/III clinical trial (NCT03281096) was designed to investigate whether BH could reduce the occurrence of new colorectal adenomas among patients with previous Colorectal Cancer history of colorectal cancer.179
5 LIMITATIONS OF THE CLINICAL USE OF BERBERINE
BBR has the potential to prevent the onset and progression of many cancers. However, poor oral bioavailability, low water solubility, and biodistribution of BBR may decrease its anticancer activities. Preclinical studies have revealed that BBR has a very low oral bioavailability (5% in plasma), owing to its poor aqueous solubility, low-GI absorption, and rapid metabolism.179, 180 Furthermore, intramuscular and intravenous administration of BBR may cause anaphylactic reactions.180, 181 Low methodological quality and drug–drug interactions are other challenges for the application of BBR in clinical trials. As a result, numerous strategies for increasing BBR bioavailability and anticancer activity have been developed.182 These techniques primarily include various novel drug delivery systems. In this context, new dosage forms of BBR were developed using self-microemulsion, intestinal absorption enhancer, and solid lipid nanoparticles to increase its bioavailability and overcome poor water solubility, which led to an increase in cytotoxicity against cancer cells and prevent the rapid clearance of the drug from the blood.179, 181, 184
According to recent research, BBR affects body weight by upregulating the expression of AMPK and UCP3 to control energy expenditure. Hence, the toxic side effects of BBR cannot be objectively and accurately assessed by changing body weight.71 Several studies have shown that BBR is clinically safe and well tolerated by humans.186 In BBR consumption, just a few adverse reactions such as constipation and nausea reported, and no negative effects on participants’ diets are observed. For instance, a phase I clinical trial demonstrated that BBR was safe at high doses. Another study discovered that BBR was not toxic to healthy cells.175
The development of new formulations and derivatives, standard dosage, route of administration, duration, and adverse reaction of BBR is currently the main trend in BBR research to increase efficacy and safety and overcome its limitations in clinical use.
6 CONCLUSION
Due to the rapid increase in the incidence of GI cancers and the numerous inherent limitations to treatments of these types of cancers, the world health community has encountered a great challenge. Hence, the potential development of novel pharmaceutical components as treatment methods in the management of GI cancers has garnered great attention. BBR as an antitumoral phytochemical agent has crucial roles in the suppression of different cancers. The efficacy of BBR in treating GI cancers along with its safety and low cost has attracted the attention of many investigators and clinicians around the world. In this paper, we reviewed the latest evidence evaluating the efficacy of BBR against GI cancers, addressing its mechanistic modes of action. Strong evidence favors powerful anti-proliferative and antitumoral activities of BBR in the management of GI cancers. To date, however, studies of BBR's antitumor effects have primarily been conducted in vitro and in a few in vivo models. Therefore, in vivo studies, particularly human studies, are required to further elucidate and confirm BBR's therapeutic effects. Moreover, further in-depth experimental, clinical, and observational investigations are needed to better identify the potential of BBR, adjust its structure to more promising derivatives with stronger antitumor effects, or synergize with other chemotherapy drugs, to serve as adjuvant treatment in a plethora of cancers. In addition, innovative approaches, including CRISPR-CAS-9 gene editing, genomics, proteomics, and metabolomics can be used to investigate the mechanism of action of BBR in cancer therapy.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




