The association between sodium glucose cotransporter-2 inhibitors vs dipeptidyl peptidase-4 inhibitors and renal outcomes in people discharged from hospital with type 2 diabetes: A population-based cohort study
Abstract
Background
We investigated the association between post-hospital discharge use of sodium glucose cotransporter-2 inhibitors (SGLT-2is) compared to dipeptidyl peptidase-4 inhibitors (DPP-4is) and the incidence of hospitalization for acute renal failure (ARF) and chronic kidney disease (CKD) in people with type 2 diabetes.
Methods
We conducted a retrospective cohort study using linked hospital and prescription data. Our cohort included people aged ≥30 years with type 2 diabetes discharged from a hospital in Victoria, Australia, from December 2013 to June 2018. We compared new users of SGLT-2is with new users of DPP-4is following discharge. People were followed from first dispensing of a SGLT-2i or DPP-4i to a subsequent hospital admission for ARF or CKD. We used competing risk models with inverse probability of treatment weighting (IPTW) to estimate subhazard ratios.
Results
In total, 9620 people initiated SGLT-2is and 9962 initiated DPP-4is. The incidence rate of ARF was 12.3 per 1000 person-years (median years of follow-up [interquartile range [IQR] 1.4 [0.7–2.2]) among SGLT-2i initiators and 18.9 per 1000 person-years (median years of follow-up [IQR] 1.7 [0.8–2.6]) among DPP-4i initiators (adjusted subhazard ratio with IPTW 0.78; 95% confidence interval [CI] 0.70–0.86). The incidence rate of CKD was 6.0 per 1000 person-years (median years of follow-up [IQR] 1.4 [0.7–2.2]) among SGLT-2i initiators and 8.9 per 1000 person-years (median years of follow-up [IQR] 1.7 [0.8–2.6]) among DPP-4i initiators (adjusted subhazard ratio with IPTW 0.83; 95% CI 0.73–0.94).
Conclusions
Real-world data support using SGLT-2is over DPP-4is for preventing acute and chronic renal events in people with type 2 diabetes.
1 INTRODUCTION
Initiation of sodium-glucose cotransporter 2 inhibitors (SGLT-2is) has been linked to an initial decline in estimated glomerular filtration rate (eGFR).1-6 However, SGLT-2is have also been associated with reduced risk of acute renal failure (ARF) in clinical trials1-3, 7, 8 and have been shown to slow decline in eGFR in people with type 2 diabetes following an initial (and reversible) decline.4, 9-12 Several large clinical trials have shown SGLT-2is slow progression of chronic kidney disease (CKD).9, 10, 12-14
Replicating findings observed in clinical trials in a real-world population is important, as clinical trial samples are seldom representative of the broader population with diabetes.15-17 Indeed, most estimates of the effect of SGLT-2is on ARF are drawn from high-risk populations, and their generalizability to the broader population with type 2 diabetes is unclear. For example, the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial included individuals with demonstrated baseline CKD, a median age of 62, and no participants over the age of 74, with no stratification of older people with respect to incidence of adverse renal events.10
Other observational studies have reported on whether SGLT-2is are associated with reduced risk of ARF.5, 7, 18-21 There is a lack of consensus and limitations among these studies as some report no association and others show a reduction in ARF with SGLT-2i initiation.5, 7, 18-21 Some previous studies that reported no significant effect of SGLT-2is on ARF have used any oral glucose lowering drug,19 glucagon-like peptide 1 (GLP1) agonist,20 DPP-4i/GLP1 agonist/gliclazide,4 or no use as a comparator group.22 Other observational studies have reported 21%,5 21%,18 and 53%5, 18 reductions in rates of ARF with SGLT-2i initiation versus dipeptidyl peptidase-4 inhibitor (DPP-4i) initiation.
The objective of this study was to investigate the association between post-hospital discharge initiation of SGLT-2i compared to DPP-4i and the incidence of subsequent hospital admission for ARF and CKD in people with type 2 diabetes.
2 METHODS
2.1 Data sources
We conducted a retrospective cohort study using linked hospital and prescription data from the state of Victoria, Australia. Victoria is the second most populous state in Australia with a population of 6.68 million. We included all people with type 2 diabetes discharged from a public or private hospital in Victoria from January 12, 2013 to June 30, 2018. We used data from the Victorian Admitted Episodes Dataset (VAED), which contains data on administrative and demographic information, as well as medical diagnoses and procedures during hospital admission. The VAED was linked to data from the Pharmaceutical Benefits Scheme (PBS) for information on medication dispensing and the National Death Index (NDI) for dates of death. The PBS is an Australian government scheme that provides medicines at a subsidized price for Australian citizens and people from countries with reciprocal agreements. Most medications dispensed from community pharmacies or upon discharge from hospital in Australia are subsidized under the PBS, this figure being 93.5% in the 2019–2020 financial year.23, 24 The NDI contains dates and causes of death for deaths occurring within Australia.
This study was approved by the Australian Institute of Health and Welfare Ethics Committee (approved amendment to EO2018/4/468) and Monash University Human Research Ethics Committee (Project ID 14339).
2.2 Study population and exposures
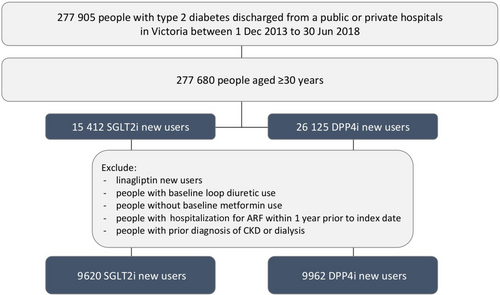
The study cohort was defined as people aged ≥30 years and discharged from a Victorian public or private hospital with an International Classification of Diseases, Tenth Revision, Australian Modification (ICD-10-AM) code indicative of type 2 diabetes (E11) between January 12, 2013 and June 30, 2018.
The index admission was defined as the first hospital admission during the study period among individuals with a diagnosis code for type 2 diabetes (see Table A1 for cause of index hospitalization). The index date was defined as the date when either an SGLT-2i or DPP-4i was initiated on or after the discharge date (Figure 1). Users who were dispensed the DPP-4i linagliptin were excluded as this is the preferred DPP-4i in reduced renal function. PBS data were available from 2006. This allowed the date of first supply to be ascertained because neither SGLT-2i nor DPP-4i were available in Australia prior to 2006. Anatomical Therapeutic Chemical codes were used to identify first SGLT-2i and DPP-4i dispensings (see Table A2). Only new users of SGLT-2i or DPP-4i were considered. This meant that people were included in the study only if they had no dispensing record of SGLT-2i and DPP-4i prior to the index date. People receiving both an SGLT-2i and a DPP-4i for the first time on the same day were excluded. DPP-4is were chosen as the comparator to reduce confounding by disease severity as both SGLT-2i and DPP-4i are second-line agents for type 2 diabetes in Australia.25
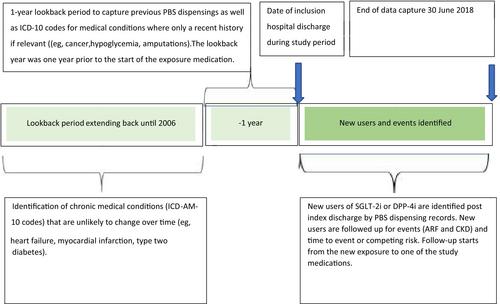
The cohort was restricted to people using metformin within 1 year prior to the index date and excluded people with use of loop diuretics within 1 year prior to the index date, with hospitalization for ARF within 1 year prior to index date, or a prior diagnosis of CKD or dialysis since 2006 to index date. Our rationale for this was to minimize channeling bias as at the time of the study SGLT-2i were contraindicated in severe renal impairment. Figure 2 shows the flow chart for selection of patients.
2.3 Outcomes
Outcomes included first hospital admission with a primary diagnosis code for ARF (ICD-10-AM code N17) or CKD (ICD-10-AM code N18). People were followed up from the index date until the first outcome, death, or the end of data period (June 30, 2018).
2.4 Covariates
As covariates we considered baseline medication use, comorbidities, socioeconomic disadvantage, type 2 diabetes severity, and year of initiation of exposure medication. Baseline medication use was identified using PBS dispensing records within 1 year prior to the index date (inclusive) (Table A2). Comorbidities were identified from the VAED using ICD-10-AM codes from hospitalizations from 2006 until the index date (Table A3).26 Socioeconomic disadvantage was measured using the Socio-Economic Indexes for Areas: Index of Relative Socioeconomic Disadvantage (IRSD).27 An IRSD quintile score of 1 to 5 was generated for each patient based on their postcode when they had the initial exposure medication commenced, the lower the score, the higher level of relative socioeconomic disadvantage.28 A modified version of the Diabetes Complication Severity Index (DCSI) was used to assess type 2 diabetes severity. The DCSI uses ICD-10 codes from hospital admissions data over a 1-year lookback period from the index date to quantify the effect of diabetes on multiple organ systems.12 The higher the score, the more severe the type 2 diabetes complications.25
2.5 Statistical analysis
Baseline characteristics of SGLT-2i and DPP-4i initiators are presented as frequencies and percentages or median and interquartile range (IQR). We also present the number and proportion of people with the outcomes and median time (with IQR) of follow-up. Competing risk analyses with inverse probability of treatment weights (IPTWs) were conducted to estimate the risk of hospital admission with ARF or CKD using separate models. IPTWs were used to balance the baseline characteristics of the exposure and comparator groups thus minimizing the effects of prescriber bias.29 The propensity score estimation model included age, sex, calendar year, other medication use (refer to Table A2 for a complete list), comorbidities (refer to Table A3 for a complete list), socioeconomic disadvantage, and diabetes severity. All variables presented in Tables A2 and A3 were included in the models. We conducted competing risk analyses using weighted Fine and Gray's subdistribution hazard models to estimate the subhazard ratios (sHRs) and 95% confidence intervals (CI) for ARF and CKD. All-cause death was considered as the competing event and censoring occurred at the end of the study period for those with event-free survival. We conducted all analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3 RESULTS
3.1 Patient characteristics
In total, there were 9620 new users of SGLT-2is and 9962 new users of DPP-4is who met the inclusion criteria of baseline use of metformin and nil history of CKD or ARF (out of 15 412 SGLT-2i initiators and 26 125 DPP-4i initiators). The proportion of females was 41.4% in the SGLT-2i group and 42.2% in the DPP-4i group. A higher proportion of those dispensed SGLT-2is compared to DPP-4is were aged 30–59 years (Table 1). Overall, 2.2% of people dispensed SGLT-2is and 6.7% of people dispensed DPP-4is were 80 years or older. The proportions of SGLT-2i and DPP-4i initiations over time were similar, with the number of initiations increasing over time in both groups. A small number of initiators (1.1% in the SGLT-2i group and 0.9% in the DPP-4i group) had an index discharge in 2018. Aldosterone antagonist use was lower in new users of SGLT-2is compared to DPP-4is (2.0% and 5.6% respectively). Prevalence of comorbidities were similar in new users of SGLT-2i compared to DPP-4i. Median time (IQR) from hospital discharge until start of treatment was slightly longer in new users of SGLT-2i (1.5 years [0.8–2.4]) compared to DPP-4i (1.3 years [0.6–2.2]) (Table 2).
| Total | SGLT-2i initiators (n = 9620) | DPP-4i initiators (n = 9962) |
|---|---|---|
| Baseline characteristics | ||
| Age, years, n (%) | ||
| 30–59 | 4276 (44.5) | 3786 (38.0) |
| 60–69 | 3603 (37.5) | 3296 (33.1) |
| 70–79 | 1531 (15.9) | 2215 (22.2) |
| 80+ | 210 (2.2) | 665 (6.7) |
| Sex, n (%) | ||
| Female | 3987 (41.4) | 4206 (42.2) |
| Year of index discharge, n (%) | ||
| 2013 | 395 (4.1) | 554 (5.6) |
| 2014 | 4017 (41.8) | 4679 (47.0) |
| 2015 | 2736 (28.4) | 2621 (26.3) |
| 2016 | 1639 (17.0) | 1447 (14.5) |
| 2017 | 727 (7.6) | 574 (5.8) |
| 2018 (6 months of data available) | 106 (1.1) | 87 (0.9) |
| Year of SGLT-2i or DPP-4i initiation, n (%) | ||
| 2013/2014a | 178 (1.8) | 948 (9.5) |
| 2015 | 1677 (17.4) | 1953 (19.6) |
| 2016 | 2737 (28.5) | 2844 (28.6) |
| 2017 | 3451 (35.9) | 2799 (28.1) |
| 2018 (6 months of data available) | 1577 (16.4) | 1418 (14.2) |
| Median time from hospital discharge date until start of treatment in years, median (IQR) | 1.5 (0.8–2.4) | 1.3 (0.6–2.2) |
| Diabetes complications severity indexb, n (%) | ||
| 0 | 8821 (91.7) | 9113 (91.5) |
| 1 | 558 (5.8) | 543 (5.5) |
| ≥2 | 241 (2.5) | 306 (3.1) |
| Medication use up to 1 year prior to SGLT-2i or DPP-4i initiation, n (%) | ||
| ACE inhibitors/ARB | 4309 (44.8) | 4360 (48.6) |
| Beta-blockers | 1477 (15.4) | 1516 (24.5) |
| Calcium channel blockers | 933 (9.7) | 957 (15.3) |
| Lipid-lowering medications | 4557 (47.4) | 4735 (52.3) |
| Aldosterone antagonists | 191 (2.0) | 131 (5.6) |
| Digoxin | 80 (0.8) | 133 (3.7) |
| Oral anticoagulant | 120 (1.3) | 172 (1.7) |
| Antiplatelet | 1007 (10.5) | 1052 (10.6) |
| Antipsychotics | 284 (3.0) | 333 (3.3) |
| Anticholinergic | 14 (0.2) | 42 (0.4) |
| Medical conditions prior to index date, n (%) | ||
| Unstable angina | 506 (5.3) | 400 (4.0) |
| Angina pectoris | 540 (5.6) | 451 (4.5) |
| Peripheral vascular disease | 248 (2.6) | 276 (2.8) |
| Myocardial infarction | 682 (7.1) | 573 (5.8) |
| Heart failure | 152 (1.6) | 162 (1.6) |
| Hypertension | 2889 (30.0) | 2630 (26.4) |
| Atrial fibrillation | 509 (5.3) | 582 (5.8) |
| Stroke | 267 (2.8) | 347 (3.5) |
| Chronic obstructive pulmonary disease | 342 (3.6) | 373 (3.7) |
| Cancerc | 322 (3.4) | 479 (4.8) |
| Severe hypoglycemia | 15 (0.2) | 19 (0.2) |
| Diabetes polyneuropathy | 639 (6.6) | 526 (5.3) |
| Diabetic eye disease | 1591 (16.5) | 1633 (16.4) |
| Diabetic foot | 429 (4.5) | 345 (3.5) |
| Other diabetic complications | 4835 (50.3) | 4066 (40.8) |
| Amputation | 30 (0.3) | 24 (0.2) |
| Acidosisc | 32 (0.3) | 34 (0.3) |
| IRSD quintiled, n (%) | ||
| 1 (highest level of relative socioeconomic disadvantage) | 2249 (23.4) | 2338 (23.5) |
| 2 | 1841 (19.2) | 1991 (20.0) |
| 3 | 2142 (22.3) | 2209 (22.2) |
| 4 | 2006 (20.9) | 1999 (20.1) |
| 5 (lowest level of relative socioeconomic disadvantage) | 1369 (14.3) | 1415 (14.2) |
| Missing | 13 (<0.1) | 10 (<0.1) |
- Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin 2 receptor blocker; DPP-4i, dipeptidyl peptidase-4 inhibitor; IQR, interquartile range; SGLT-2i, sodium glucose cotransporter-2 inhibitor.
- a The 2 years were combined to avoid cells with small numbers (less than 6) as per the Australian Institute of Health and Welfare requirements.
- b A modified version of the Diabetes Complications Severity Index (DCSI) was used to assess diabetes severity.23
- c Medical conditions from a 1-year lookback (all other medical conditions were taken from the total lookback since 2006).
- d Socioeconomic disadvantage was measured using the Socio-Economic Index for Areas: Index of Relative Socioeconomic Disadvantage (IRSD).27
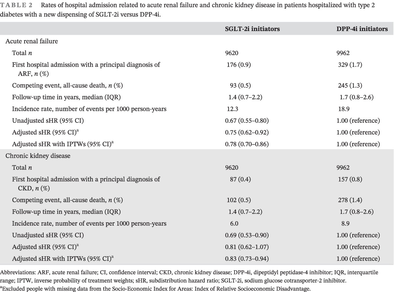
| SGLT-2i initiators | DPP-4i initiators | |
|---|---|---|
| Acute renal failure | ||
| Total n | 9620 | 9962 |
| First hospital admission with a principal diagnosis of ARF, n (%) | 176 (0.9) | 329 (1.7) |
| Competing event, all-cause death, n (%) | 93 (0.5) | 245 (1.3) |
| Follow-up time in years, median (IQR) | 1.4 (0.7–2.2) | 1.7 (0.8–2.6) |
| Incidence rate, number of events per 1000 person-years | 12.3 | 18.9 |
| Unadjusted sHR (95% CI) | 0.67 (0.55–0.80) | 1.00 (reference) |
| Adjusted sHR (95% CI)a | 0.75 (0.62–0.92) | 1.00 (reference) |
| Adjusted sHR with IPTWs (95% CI)a | 0.78 (0.70–0.86) | 1.00 (reference) |
| Chronic kidney disease | ||
| Total n | 9620 | 9962 |
| First hospital admission with a principal diagnosis of CKD, n (%) | 87 (0.4) | 157 (0.8) |
| Competing event, all-cause death, n (%) | 102 (0.5) | 278 (1.4) |
| Follow-up time in years, median (IQR) | 1.4 (0.7–2.2) | 1.7 (0.8–2.6) |
| Incidence rate, number of events per 1000 person-years | 6.0 | 8.9 |
| Unadjusted sHR (95% CI) | 0.69 (0.53–0.90) | 1.00 (reference) |
| Adjusted sHR (95% CI)a | 0.81 (0.62–1.07) | 1.00 (reference) |
| Adjusted sHR with IPTWs (95% CI)a | 0.83 (0.73–0.94) | 1.00 (reference) |
- Abbreviations: ARF, acute renal failure; CI, confidence interval; CKD, chronic kidney disease; DPP-4i, dipeptidyl peptidase-4 inhibitor; IQR, interquartile range; IPTW, inverse probability of treatment weights; sHR, subdistribution hazard ratio; SGLT-2i, sodium glucose cotransporter-2 inhibitor.
- a Excluded people with missing data from the Socio-Economic Index for Areas: Index of Relative Socioeconomic Disadvantage.
A lower proportion of the SGLT-2i initiators (0.5%) died from any cause compared to the DPP-4i initiators (1.3%) in the cohort where ARF was reported. A similar trend was seen in the cohort where CKD was reported with 0.5% of the SGLT-2i initiators dying compared to 1.4% of DPP-4i initiators (Table 2).
3.2 Acute renal failure and chronic kidney disease
The incidence rate of first hospitalization for ARF during the follow-up period was 12.3 events per 1000 person-years among SGLT-2i initiators and 18.9 events per 1000 person-years among DPP-4i initiators. In the adjusted model, SGLT-2i initiators had a 22% lower rate of first hospitalization for ARF compared with DPP-4i initiators (adjusted sHR with IPTW 0.78; 95% CI 0.70–0.86) (Table 2). The incidence rate of first hospitalization for CKD was 6.0 events per 1000 person-years among SGLT-2i initiators and 8.9 events per 1000 person-years among DPP-4i initiators. In the adjusted model, SGLT-2i initiators had a 17% lower rate of first hospitalization for CKD compared with DPP-4i initiators (adjusted sHR with IPTW 0.83; 95% CI 0.73–0.94) (Table 2).
4 DISCUSSION
We demonstrated that initiation of SGLT-2is versus DPP-4is at hospital discharge was associated with a 22% reduction in the rate of readmission for ARF and 17% reduction in rate of CKD admissions in people who initiated SGLT-2is versus DPP-4is. These findings from real-world data enhance what we know from randomized controlled trials (RCTs)1-3, 7, 8 and other observational studies5, 18 and suggest that SGLT2i do not cause ARF or have acute detriments on kidney function in people without severely reduced eGFR.
This was the first Australian study to demonstrate the real-world benefits of SGLT-2is versus DPP-4is for reducing ARF and CKD. There are numerous reasons that explain how our real-world data enhances what we know from RCTs. First, we were able to adjust for a range of clinically important covariates to minimize confounding by comorbidity and channeling bias by excluding people with prior medication use and comorbidities that predispose to ARF or CKD.30 Many prior studies did not exclude this prior medication use and comorbidities. Second, the study analyzed data for a large state-wide cohort over a 6-year period (July 2012 to June 2018). All patients who were hospitalized were included (there was no opt-out option), which gave an accurate representation of the Victorian population.
Our study results are in alignment with previous RCTs and several other observational studies. An observational study in Canada including 39 094 people compared the 90 day risk of ARF in new initiators of SGLT-2is and DPP-4is, finding a 21% reduced risk of ARF for the SGLT-2i group compared to the DPP-4i group (risk ratio 0.79 (95% CI 0.64–0.98).13 Similarly, Cahn et al reported a 53% (95% CI 20–73) reduced risk of ARF in SGLT-2i versus DPP-4i users in their overall population.5 Some previous studies have reported no significant effect of SGLT-2is on ARF and have used any oral glucose lowering drug,19 GLP1 agonist,20 DPP-4i/GLP1 agonist/gliclazide,4 or no use as a comparator group.22 Different comparators may explain some of the differences between our results and the findings from these studies. Previous RCTs have shown that SGLT-2is are associated with reduced rates of progression of CKD incidence compared to DPP-4is.9, 10, 12-14, 29
To summarize major trials in this space, the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial compared people with type 2 diabetes and CKD to receive canagliflozin or placebo. The incidence of end-stage kidney disease was lowered by 34% in the canagliflozin group compared to the placebo group (HR 0.66 (95% CI 0.54–0.86).31 In an RCT by Pasternak et al13 and by Pollock et al,9 the annual decline in eGFR slowed significantly after SGLT-2i therapy was initiated.9, 13 Similarly, DAPA-CKD10 showed among patients with CKD, the risk of a combined end point of at least 50% sustained decline in eGFR, end-stage kidney disease, or death from renal causes being 0.56 (95% CI, 0.45–0.68).10
Our study had several limitations. First, the cohort included only people discharged from hospital, which limits generalizability. People who are hospitalized are usually sicker and have more comorbidities than the general population of people with type 2 diabetes. If the outcomes (AKI or CKD) were identified in the community they would have been missed as we are using only hospitalization data. However, this should be consistent across both groups and not specific to one medication class. Second, we did not have access to data on type 2 diabetes duration. However, we used DCSI as a surrogate for type 2 diabetes severity, which usually increases with duration. Third, during the study period, SGLT-2is were contraindicated in people with impaired renal function (an eGFR <45 mL/min/1.73 m2). As the study had no access to eGFR, to address this we excluded people who had taken linagliptin, the preferred DPP-4i for use in renal impairment. It is also possible that there have been evolutions in SGLT-2i prescribing practices since 2018 and that clinicians may be more likely to prescribe SGLT-2i in patients at risk of renal failure now than in 2013–2018. Fourth, it is unclear if people took the medications as directed. Adherence to the use of SGLT-2 inhibitors and DPP-4 inhibitors was not measured; however, we are looking at protective effects, hence using an intention to treat analysis (not following up for adherence) is conservative. Fifth, the study was unable to ascertain whether people were dispensed SGLT-2is or DPP-4is outside of the PBS reimbursement system. However, non-PBS use is likely to have been rare due to higher costs to the patient. Finally, initiation of SGLT-2i or DPP-4is while in hospital for the index admission was not captured, and the date of first use could have been before the date of discharge. This could be a source of bias, in that people initiated while in hospital who have an acute kidney event while still in hospital, might have that medication withdrawn. Thus, the population of those who are discharged on the medication are enriched for people not at risk of an early acute kidney event.
The lack of specific clinical data, such as baseline renal function being not available, is a major limitation in analyzing the risk of kidney outcomes associated with medication usage. However, this is common for most studies using administrative data. Administrative data can still provide useful results without specific laboratory results. Perhaps future studies using electronic hospital records could address this.
There is a potential of under-reporting of comorbidities in our data, because hospital admission data captures only severe forms of the disease, or that these patients have different treatment approaches depending on their comorbidities. Using our two-pronged approach (both hospital coded diagnoses and medication dispensing) would be expected to be superior to using either one by itself.
One discrepancy is that heart failure prevalence is the same between groups; however, the proportions given heart failure drugs (such as aldosterone antagonist and digoxin) are different between groups. This could be due to the proportion that have heart failure with reduced ejection fraction compared to those with heart failure with preserved ejection fraction, where the treatment options are dramatically different. A limitation of the dataset is the specific type of heart failure is unavailable.
4.1 Implications for clinicians
This study found that in adults >30 years of age with type 2 diabetes, SGLT-2is, compared to DPP-4is, are associated with a reduced risk of being rehospitalized with ARF and CKD. This study contributes information toward understanding SGLT-2i renal outcomes and this result adds to the mounting evidence that is likely to assuage clinicians' concerns that SGLT-2i initiation is linked to ARF.
AUTHOR CONTRIBUTIONS
Kate E. D. Ziser, Stephen Wood, George S. Q. Tan, Jedidiah I. Morton, Jonathan E. Shaw, J. Simon Bell, and Jenni Ilomaki made substantial contributions to study conception and design, acquisition of data or analysis and interpretation of the data. J. Simon Bell and Jenni Ilomaki obtained grant funding. All authors drafted (Kate E. D. Ziser) or revised (Stephen Wood, George S. Q. Tan, Jedidiah I. Morton, Jonathan E. Shaw, J. Simon Bell, and Jenni Ilomaki) the article for important intellectual content. Kate E. D. Ziser, Stephen Wood, George S. Q. Tan, Jedidiah I. Morton, Jonathan E. Shaw, J. Simon Bell, and Jenni Ilomaki approved the final version to be published.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Victorian Department of Health as the source of Victorian Admitted Episodes Dataset data for this study, and the Centre for Victorian Data linkage (Victorian Department of Health) and the Australian Institute of Health and Welfare for the provision of data linkage. Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
The authors gratefully acknowledge funding provided by the Dementia Australia Research Foundation – Yulgilbar Innovation Grant.
DISCLOSURE
J. Simon Bell has received grant funding or consulting funds from the National Health and Medical Research Council, Medical Research Future Fund, Victorian Government Department of Health and Human Services, Dementia Australia Research Foundation, Yulgilbar Foundation, Aged Care Quality and Safety Commission, Dementia Centre for Research Collaboration, Pharmaceutical Society of Australia, Society of Hospital Pharmacists of Australia, GlaxoSmithKline Supported Studies Programme, Amgen, and several aged care provider organizations unrelated to this work. All grants and consulting funds were paid to the employing institution. Jonathan E. Shaw has received honoraria for lectures and for advisory boards from: Astra Zeneca; Sanofi; Novo Nordisk; MSD; Eli Lilly; Pfizer; Roche; Mylan; Boehringer Ingelheim; Zuellig; Roche. Kate E. D. Ziser, George S. Q. Tan, Jedidiah I. Morton, and Stephen Wood have no relationships or activities to disclose. Jenni Ilomaki has received funding or consulting funds from the National Health and Medical Research Council, Medical Research Future Fund, Victorian Government Department of Health and Human Services, Dementia Australia Research Foundation, Yulgilbar Foundation, National Breast Cancer Foundation, Amgen, and AstraZeneca. All grants and consulting funds were paid to the employing institution.
APPENDIX A
| Cause of index hospitalization | Frequency SGLT-2i n (%) | Frequency DPP-4i n (%) |
|---|---|---|
| Diabetes complications | 392 (4.1) | 320 (3.2) |
| Chest pain | 441 (4.6) | 302 (3.0) |
| Myocardial infarction | 244 (2.5) | 213 (2.1) |
| Cancer | 299 (3.1) | 419 (4.2) |
| Cataract | 213 (2.2) | 310 (3.1) |
| Angina | 221 (2.3) | 184 (1.8) |
| Obstructive sleep apnea syndrome | 181 (1.9) | 109 (1.3) |
| Atherosclerotic heart disease | 155 (1.6) | 128 (1.5) |
| Carpal tunnel syndrome | 144 (1.5) | 119 (1.4) |
| Screening for malignant neoplasm of intestine | 143 (1.5) | 132 (1.6) |
| Knee osteoarthritis | 142 (1.5) | 150 (1.8) |
| Gastrointestinal hemorrhage | 130 (1.4) | 109 (1.3) |
| Abdominal pain | 130 (1.4) | 143 (1.7) |
| GORD with esophagitis | 107 (1.1) | 84 (1.0) |
| Stroke | 73 (0.8) | 105 (1.1) |
| Others* | 6605 (68.7) | 7135 (71.6) |
| Total | 9620 | 9962 |
- Abbreviations: DPP-4i, dipeptidyl peptidase-4 inhibitor; SGLT-2i, sodium glucose cotransporter-2 inhibitor.
- * Other causes of index hospitalization with frequency of <2% in study cohort.
| Drug class | ATC code |
|---|---|
| Exposure medications | |
| SGLT-2is | A10BK, A10BD07–08, A10BD15–16, A10BD19–21, A10BD23–25, A10BX11 |
| DPP-4is | A10BH (excluding A10BH05), A10BD10, A10BD12–13, A10BD18, A10BD21–22, A10BD24–25. |
| Baseline medicationsa | |
| ACEI/ARB | C09A, C09B, C09C, C09D (exclude C09DX04) |
| Beta-blockers | C07 |
| Calcium channel blockers | C08C |
| Lipid-lowering medications | C10AA, C10BA, C10BX, C10AB, C10AC, C10AD, C10AX |
| Aldosterone antagonists | C03DA |
| Digoxin | C01AA05 |
| Oral anticoagulant | B01AA |
| Antipsychotics | N05A |
| Antiplatelets | B01AC A10A |
| Anticholinergics | N06D |
- Abbreviations: ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin 2 receptor blocker; DPP-4i, dipeptidyl peptidase-4 inhibitor; SGLT-2i, sodium glucose cotransporter-2 inhibitor.
- a Baseline medications are identified using a fixed 1-year period prior to first dispensing date of exposure medication.
| Medical condition | ICD-10-AM | Time frame |
|---|---|---|
| Acidosis | E10.1, E11.1, E12.1, E13.1, E14.1, E87.2 | 2006 until first dispensing of exposure/comparator |
| Angina | I20.0, I20.1, I20.8, I20.9 | 2006 until first dispensing of exposure/comparator |
| Atrial fibrillation | I48 | 2006 until first dispensing of exposure/ comparator |
| Cancer | C00-C99 | Within 1 year prior to first dispensing of exposure/comparator (inclusive). |
| COPD and asthma | J44-46 | 2006 until first dispensing of exposure/comparator |
| Diabetic eye complications | H28.0, H35.8, H36.0, E10.3, E11.3, E12.3, E13.3, E14.3 | 2006 until first dispensing of exposure/comparator |
| Diabetic foot/peripheral angiopathy | E11.6B, M14.2, M14.6, M90.8, L98.4, E10.5, E11.5, E12.5, E13.5, E14.5 | 2006 until first dispensing of exposure/comparator |
| Diabetic mono/polyneuropathy | G99.0, G59.0, G63.2, E10.4, E11.4, E12.4, E13.4, E14.4 | 2006 until first dispensing of exposure/comparator |
| Diabetes with several/unspecified complications | E11.6, E10.6, E13.6, E14.6, E10.7, E11.7, E12.7, E13.7, E14.7, E10.8, E11.8, E12.0, E12.8, E13.8, E14.8 | 2006 until first dispensing of exposure/comparator |
| Heart failure | I50 | 2006 until first dispensing of exposure/comparator |
| Hypertension | I10-I15 | 2006 until first dispensing of exposure/comparator |
| Keto−/lactate acidosis | E10.1, E11.1, E12.1, E13.1, E14.1, E87.2 | Within 1 year prior to first dispensing of exposure/comparator (inclusive). |
| Lower limb amputations | Z89 | Within 1 year prior to first dispensing of exposure/comparator (inclusive). |
| Peripheral artery disease | I70-I73 | 2006 until first dispensing of exposure/comparator |
| Severe hypoglycemia | E10.0, E11.0, E12.0, E13.0, E14.0, E11.6A, E16.0–2 | Within 1 year prior to first dispensing of exposure/comparator (inclusive). |
| Stroke | I60-I64 | 2006 until first dispensing of exposure/comparator |
- Abbreviations: COPD, chronic obstructive pulmonary disease; ICD-10-AM, International Classification of Diseases, Tenth Revision, Australian Modification.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Australian Institute of Health and Welfare and the Centre for Victorian Data Linkage upon reasonable request.