Salivary gland hypofunction in KK-Ay type 2 diabetic mice
KK-Ay 2型糖尿病小鼠唾液腺功能减退
Abstract
enBackground
Hypofunction of different organs in the body is associated with diabetes, including in the oral cavity. Diabetes is often associated with xerostomia, but the underlying mechanism is not well characterized. Thus, the mechanisms underlying diabetes-induced xerostomia were investigated in this study in KK-A y mice as an experimental model of type 2 diabetes.
Methods
The mechanisms involved in diabetes-induced xerostomia were investigated using the ex vivo glandular perfusion technique, histological analysis, and immunohistochemical and intracellular signaling analyses.
Results
Ex vivo submandibular gland secretions from KK-Ay mice decreased by 30% following stimulation with 0.3 μmol/L carbachol (CCh), a cholinergic agonist. Acinar cell weight was comparable between KK-Ay and control mice, whereas duct cell weight was significantly greater in KK-Ay mice. Concentrations of Na+ and Cl− in the secreted saliva decreased significantly in KK-Ay mice, supporting the finding of increased ductal tissue in KK-Ay mice. Immunohistochemistry revealed no significant differences between KK-Ay and control mice in terms of the expression of Cl− and water channels, Na+–K+–2Cl− cotransporters, and membrane proteins critical for fluid secretion. Cellular signaling analysis revealed that the increase in [Ca2+]i in response to 0.3 μmol/L CCh was reduced by 30% in KK-Ay mice, although there was no significant difference in the thapsigargin (1.0 μmol/L)-induced increase in store-depleted calcium between KK-Ay and control mice.
Conclusions
These results demonstrate that submandibular fluid secretion is diminished in KK-Ay mice because of a diminished increase in [Ca2+]i. Duct cell weight increased in KK-Ay mice, possibly leading to increased ion reabsorption and thus decreased Na+ and Cl− concentrations in the secreted saliva.
摘要
zh背景
体内不同器官出现功能减退都与糖尿病有关, 包括口腔中的器官。糖尿病经常与口腔干燥症有关, 但是其潜在的机制尚不清楚。因此, 本研究将KK-Ay小鼠作为2型糖尿病实验模型探究糖尿病诱导的口腔干燥症的潜在发病机制。
方法
使用离体腺体灌流技术、组织学分析以及免疫组织化学与细胞内信号分析来研究糖尿病诱导的口腔干燥症的相关发病机制。
结果
KK-Ay小鼠的离体下颌下腺经过0.3 μmol/L的氨甲酰胆碱(carbachol [CCh], 一种胆碱能受体激动剂)刺激后, 分泌物减少了30%。KK-Ay与对照组小鼠之间的腺泡细胞重量相似, 但是KK-Ay小鼠的导管细胞重量显著更大。在KK-Ay小鼠分泌的唾液中Na+与Cl-的浓度均显著下降, 支持KK-Ay小鼠导管组织增加的结果。免疫组织化学分析结果表明KK-Ay与对照组小鼠之间在Cl-与水通道、Na+-K+-2Cl-转运蛋白、分泌液体的关键性膜蛋白表达方面都没有显著性差异。细胞信号传导分析结果表明, KK-Ay小鼠使用0.3 μmol/L的CCh刺激后[Ca2+]i上升幅度减少了30%, 虽然在KK-Ay与对照组小鼠之间毒胡萝卜素(1.0 μmol/L)-诱导的存储耗尽后的钙离子浓度上升没有显著性差异。
结论
这些结果表明, 在KK-Ay小鼠中因[Ca2+]i上升幅度减少造成颌下腺分泌液减少。KK-Ay小鼠导管细胞重量增加, 这可能会导致离子重吸收增加, 因此在分泌的唾液中Na+与Cl-的浓度随之下降。
Introduction
Saliva is primarily produced by three pairs of major salivary glands, the parotid, submandibular, and sublingual glands, which, together, account for more than 90% of fluid production.1, 2 Submandibular saliva is the main component of total saliva in the human resting state,1 and parotid saliva is the main component of stimulated saliva in humans and mice.1, 3 The basic secretory mechanisms are almost identical among these glands. Salivary glands are innervated and controlled by both the sympathetic and parasympathetic nervous systems; in particular, activation of muscarinic cholinoceptors play a major role in fluid secretion.
Muscarinic cholinoceptors are present in the basolateral membrane of acinar cells,4 secretory end pieces. Following cholinoceptor activation, there is an increase in [Ca2+]i mediated via the inositol 1,4,5-trisphosphate (IP3) pathway and calcium-activated calcium influx mechanisms.5 This increase in [Ca2+]i then activates anion channels located in the luminal membrane, namely calcium-activated chloride channel transmembrane member 16A (TMEM16A), also known as anoctamin-1, with subsequent anion release into the lumen triggering fluid secretion.6, 7 Anion accumulation induces cation movement into the lumen, resulting in an osmotic gap between inside the cell and the lumen. Consequently, osmotic pressure induces water movement into the lumen through the water channel aquaporin-5 (AQP5) and tight junctions.8 Acinar cells are polarized cells; Na+–K+–2Cl− cotransporters (NKCC1) in the basolateral membrane stimulate the accumulation of Cl− (up to 60 mmol/L) in acinar cells.9 In contrast, duct cells reabsorb large quantities of Na+ and Cl− from the primary saliva produced by acinar cells. The final saliva in the oral cavity then becomes hypotonic relative to plasma.10-12
Reduced salivary gland function is observed in Sjögren's syndrome, as well as after radiation therapy for head and neck cancerous lesions, where inflammation is observed in acinar cells.13, 14 Reduced salivary gland function is also associated with metabolic diseases, such as diabetes mellitus. Some of the effects of diabetes on salivary glands include diabetes-induced reductions in sympathetic activity to the salivary gland,15 a possible role for autonomic neuropathy in the abnormal response of the parotid gland to parasympathetic nerve stimulation in diabetic rats,16 and reduced parasympathetic vasodilatation in the submandibular gland (SMG) in diabetic rats, which may result in salivary dysfunction.17 Furthermore, diabetes increases the expression of sodium–glucose cotransporter 1 (SGLT1), which works as a water transporter, in salivary duct cells, which may be one of the causes of diabetes-induced salivary dysfunction.18, 19 However, direct effects of diabetes on the salivary glands are not well characterized. In the present study, we analyzed ex vivo glandular fluid secretion, histology, and cellular signaling in diabetic KK-Ay mice to determine how diabetes induces xerostomia.
Methods
Materials and animals
Unless stated otherwise, reagents were purchased from Sigma Japan (Tokyo, Japan). Diabetic KK-Ay and control C57BL/6J mice, 4–8 weeks of age, were purchased from CLEA Japan (Tokyo, Japan). Mice were maintained under a 12-h light/dark cycle and were fed ad libitum. All experiments were approved by the Animal Committee of Kyushu Dental University (No. 14-001 and No. 15-011).
Blood glucose measurements
Venous blood was drawn from the tail vein of KK-Ay and control mice aged 4, 6 and 8 weeks. Blood glucose levels were measured using the glucose dehydrogenase flavin adenine dinucleotide method and a FreeStyle Freedom Lite glucose monitor (Abbott Japan, Chiba, Japan).
Ex vivo perfusion of mouse SMG
The details of the surgical procedures used in the present study have been described previously.20 Briefly, mice were anesthetized with chloral hydrate (400 mg/kg, i.p.), after which the SMGs were removed, along with the common carotid artery and duct. The carotid artery was immediately cannulated and perfused at a rate of 1 mL/min with physiological saline solution (PSS; composition [in mmol/L]: NaCl 120; KCl 4.3; NaHCO3 25; MgCl2 1.0; CaCl2 1.0; glucose 5; HEPES 10) equilibrated with 95% O2 and 5% CO2. Saliva secreted in response to the muscarinic agonist carbachol (CCh; 0.3 μmol/L) was collected over a 10-min stimulation period into a glass capillary tube. The collected saliva was stored in 500-μL tubes at −80°C until analysis. Salivary Na+ and Cl− concentrations were determined with electrodes using the Dri-Chem 7000 system (Fuji Film Medical, Tokyo, Japan).
Histological analysis
After SMGs had been dissected from mice, they were fixed in 4% paraformaldehyde phosphate buffer solution for 24 h before being embedded in paraffin. Sections were deparaffinized before hematoxylin–eosin (HE) staining and were subsequently examined under a digital microscope (VHX-5000; Keyence, Osaka, Japan). The area of acinar and duct cells in the SMG was calculated from HE-stained images using the automatic area measurement function of the digital microscope.
Detection of NKCC1, AQP5, and TMEM16A in mouse SMGs
Mouse SMGs were processed for immunohistochemistry as described previously.6 Prepared sections were incubated overnight with an anti-peptide antibody against TMEM16A (Abcam, Tokyo, Japan), AQP5 (EMD Millipore, Darmstadt, Germany), or NKCC1 (Santa Cruz Biotechnology, Santa Cruz, TX, USA) and immunostaining was developed as described previously.6
Measurement of [Ca2+]i
In salivary acinar cells, [Ca2+]i is an important intracellular signal for water secretion.12, 21 Submandibular glands were surgically isolated from mice that had been anesthetized with chloral hydrate (400 mg/kg, i.p.). Glands were minced using fine scissors and then digested with 520 U/mL collagenase L (Nitta Gelatin, Osaka, Japan) for 15 min at 37°C. The digested tissue was washed three times with Eagle's minimum essential medium (MEM; Life Technologies, Carlsbad, CA, USA) and dispersed in 5 mL MEM; the cells were then incubated for 10 min at 37°C with 2 μmol/L Fura-2-acetoxymethyl ester (Fura-2AM). The [Ca2+]i in Fura-2AM-loaded acinar cells was measured in the presence of external Ca2+, using the aforementioned PSS, after the addition of 0.3 μmol/L CCh or 1 μmol/L thapsigargin. To investigate Ca2+ mobilization in the absence external Ca2+, the PSS was replaced with calcium-free solution, in which the CaCl2 in the PSS was replaced with NaCl. Fluorescence was detected under a microscope equipped with a fluorescence analysis system (Aquacosmos; Hamamatsu Photonics, Hamamatsu, Japan) at an excitation wavelength of 340 or 380 nm and an emission wavelength of 510 nm. The [Ca2+]i is expressed as a ratio of fluorescence at 340/380 nm. The increase in [Ca2+]i was calculated from the integral value of [Ca2+]i for the CCh or thapsigargin stimulation periods as the area under the curve.
Statistical analysis
Data are presented as the mean ± SEM. The significance of differences between control and KK-Ay mice was evaluated using unpaired Student's t-test. P < 0.05 was considered significant. All experiments were performed using at least three different mice for each condition, “n” referring to the number of experiments performed.
Results
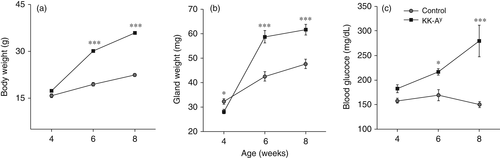
Body weight, gland weight, and blood glucose concentrations
Body weight increased in both KK-Ay and control mice with age (Fig. 1a). Body weight was comparable between 4-week-old KK-Ay and control mice, but was significantly greater in 6- and 8-week-old KK-Ay than control mice (P < 0.001). The weight of SMGs was significantly lower in 4-week-old KK-Ay than control mice (P < 0.05), but at 6 and 8 weeks of age was significantly greater in KK-Ay mice (P < 0.001; Fig. 1b). Blood glucose concentrations in KK-Ay and control mice are shown in Fig. 1c. Blood glucose concentrations were stable in control mice at all ages, but increased with age in KK-Ay mice. Although blood glucose concentrations were comparable between KK-Ay and control mice at 4 weeks of age, they were significantly higher in KK-Ay mice at 6 and 8 weeks of age (Fig. 1c).
Salivary secretion by ex vivo mouse SMG
Salivary flow rates in KK-Ay and control mice are shown in Fig. 2a,b. Perfusion of SMGs with the muscarinic agonist CCh (0.3 μmol/L) induced stable salivary flow in both KK-Ay and control mice. The total volume of saliva secreted by SMGs from KK-Ay and control mice during the 10-min stimulation period is shown in Fig. 2c. At all ages examined, SMGs from KK-Ay mice secreted significantly less saliva than SMGs from control mice, with the difference between KK-Ay and control mice increasing with age.
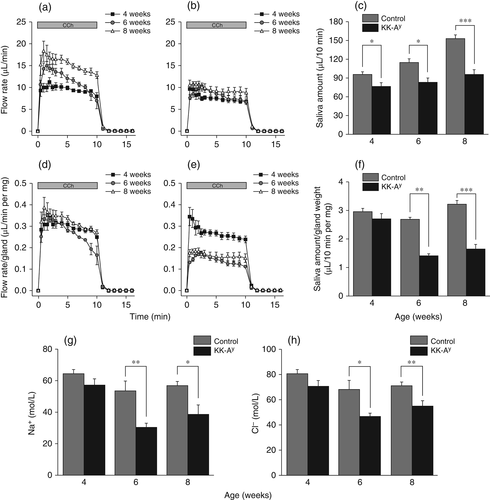
In 6- and 8-week-old KK-Ay mice, the volume of saliva secreted during the 10-min stimulation period normalized against gland weight was significantly lower than in control mice (Fig. 2f). Similarly, concentrations of both Na+ and Cl− in the saliva were significantly lower in 6- and 8-week-old KK-Ay than control mice (Fig. 2g,h).
Acinar and duct cells in mouse SMG
To investigate the mechanism underlying the reduced salivary function in KK-Ay mice, morphological analyses were conducted. As indicated in HE-stained images of SMGs from KK-Ay and control mice (Fig. 3a,b), there was a decrease in the area of acinar cells in KK-Ay compared with control mice, whereas the duct cell area was greater in KK-Ay mice. As indicated in Fig. 1b, the weight of SMGs from KK-Ay mice was greater than that of SMGs from control mice. Therefore, we calculated acinar and duct cell weight on the basis of gland weight (Fig. 1b) and acinar and duct cell area (Fig. 3c). Acinar cell weight was comparable between KK-Ay and control mice (Fig. 3d), whereas duct cell weight was significantly greater in KK-Ay mice (Fig. 3e), indicating that decreased salivary secretion in KK-Ay mice was not caused by a reduction in acinar cells.
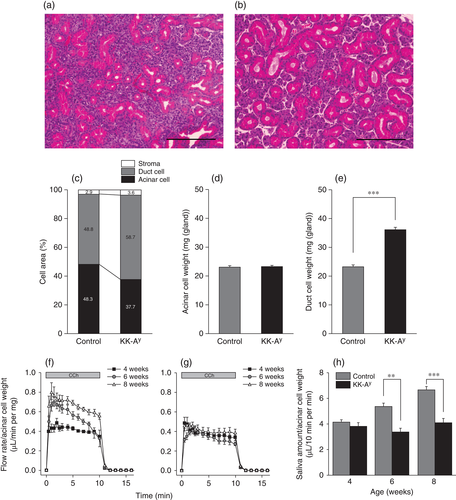
To confirm the secretary function of acinar cells in KK-Ay and control mice, salivary flow rate (Fig. 3f,g) and the total volume of saliva secreted during the 10-min stimulation period (Fig. 3h) were normalized against acinar cell weight. As indicated in Fig. 3h, the total volume of saliva secreted, normalized against acinar cell weight, was comparable between KK-Ay and control mice at 4 weeks of age, but significantly lower in KK-Ay than control mice at 6 and 8 weeks of age.
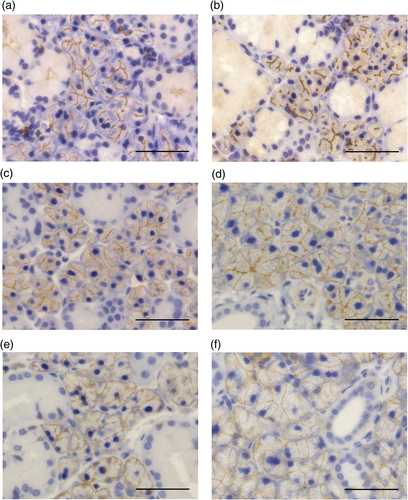
Expression of TMEM16A, AQP5, and NKCC1 in SMGs from KK-Ay and control mice
To investigate difference in acinar cell function between KK-Ay and control mice, immunohistochemistry was used to detect three major membrane proteins in acinar cells, namely TMEM16A, AQP5, and NKCC1. In control mice, TMEM16A was expressed at the acinar apical membrane and intercalated duct cells (Fig. 4a), whereas in KK-Ay mice it was expressed at only the acinar apical membrane (Fig. 4b). In contrast, AQP5 was expressed at the apical membrane of the acinar cells in both control (Fig. 4c) and KK-Ay mice (Fig. 4d) and NKCC1 was highly expressed at the basolateral membrane of acinar cells and faintly expressed in duct cells (Fig. 4e,f).
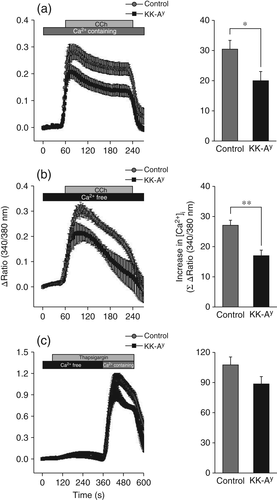
Analysis of [Ca2+]i signaling
In the present study, [Ca2+]i signaling in response to 0.3 μmol/L CCh in the presence of extracellular Ca2+ was investigated. In these experiments, CCh induced an increase in [Ca2+]i in SMGs from both KK-Ay and control mice (Fig. 5a). The increase in [Ca2+]i was calculated as the area under the curve during CCh stimulation. Acinar cells from KK-Ay mice exhibited a significantly smaller increase in [Ca2+]i than acinar cells from control mice, indicating that reduced [Ca2+]i may be responsible for the salivary dysfunction in KK-Ay mice. The increase in [Ca2+]i was made up of two stages: (i) Ca2+ release from the endoplasmic reticulum (ER); and (ii) store-operated Ca2+ entry (SOCE). Which of these stages was impaired in KK-Ay mice was determined by performing experiments in the absence of extracellular Ca2+ or in the presence of 1.0 μmol/L thapsigargin, respectively. In the absence of extracellular Ca2+, the increase in [Ca2+]i in acinar cells was lower in both the KK-Ay and control groups (Fig. 5b). In the presence of thapsigargin, which releases Ca2+ by inhibiting the Ca2+-ATPase in the ER, the thapsigargin-induced increase in [Ca2+]i in acinar cells was comparable between the KK-Ay and control groups, suggesting that SOCE was not diminished by diabetes (Fig. 5c).
Discussion
In the present study, we used KK-Ay mice to analyze the mechanism underlying diabetes-induced xerostomia. The KK-Ay mouse, a model of type 2 diabetes (T2D), was established by Nishimura.22 To create KK-Ay mice, the Ay mutation at the agouti locus was introduced into the KK strain, which was developed by Kondo et al.23 Although KK mice are also a model of T2D, KK-Ay mice develop diabetes more stably because of the introduction of the Ay mutation.24, 25 In the present study, we were able to confirm that KK-Ay mice developed diabetes symptoms of obesity and hyperglycemia because their body weight and blood glucose concentrations were higher than in control (C57BL/6J) mice. Because in our experiments in vivo stimulation (i.e. intraperitoneal injection) with a muscarinic agonist kills KK-Ay mice, in the present study we used an ex vivo perfusion technique. We speculate that the death of KK-Ay mice following intraperitoneal injection of muscarinic agonists is associated with coronary artery disease (CAD), because T2D increases the risk of CAD. It has been reported muscarinic agonists induce coronary artery spasm in patients who have vascular endothelial cell abnormalities.26, 27
According to previous reports, diabetic patients have reduced salivary secretion.28, 29 As expected, in the present study the salivary secretion of KK-Ay mice was significantly decreased. Therefore, we hypothesized that organic changes in the salivary glands are the cause of reduced salivary secretion, and tested this using histological analyses. There was no significant difference in acinar cell weight between KK-Ay and control mice. Furthermore, we examined the expression of three important membrane proteins for salivation in KK-Ay and control mice and found no obvious differences between the two groups except for the disappearance of TMEM16A expression in intercalated ducts in KK-Ay mice. It has been reported that TMEM16A is expressed in both acinar cells and intercalated duct cells in the SMG, although the function of TMEM16A in duct cells is unknown.7 Further studies are needed to determine why TMEM16A disappeared from duct cells in KK-Ay mice.
Controlled temporal and spatial regulation of [Ca2+]i is necessary for fluid secretion.21 In the present study changes in [Ca2+]i were measured in response to the muscarinic agonist CCh (0.3 μmol/L) under different conditions (i.e. in the presence and absence of external Ca2+; Fig. 5a–c). Regardless of the presence or absence of extracellular Ca2+, both the initial response of [Ca2+]i and the sustained phase were significantly decreased in KK-Ay mice (Fig. 5a,b), suggesting that the muscarinic response is decreased in KK-Ay mice, presumably due to the release of Ca2+ stores, such as from the ER, because this is the one of the most apparent effects of diabetes.30 However, SOCE may not be affected by diabetes stress in KK-Ay mice because thapsigargin, a store pump inhibitor, had no effect on [Ca2+]i (Fig. 5c). Conversely, several studies have demonstrated that the increase in [Ca2+]i is associated with AQP5 translocation into the acinar luminal membrane, and impaired AQP5 translocation is induced by diabetes.31, 32 Thus, the diminished increase in [Ca2+]i in KK-Ay mice found in the present study may affect AQP5 translocation, which, in turn, may result in salivary dysfunction.
The relationship between diabetes and salivary glands is often discussed with regard to oxidative stress.33-35 Secretory glands are affected in diabetes, but, with regard to fluid secretion, it remains contentious as to whether diabetes directly reduces salivary flow.36 In the present study we demonstrated that diabetes mellitus significantly reduced muscarinic agonist-stimulated fluid secretion by approximately 30%, even though the acinar cell weight was identical between KK-Ay and control mice. One possible explanation for the reduced flow in KK-Ay mice is the increase in the ductal cell population. Water-permeable ductal cells are thought to be present in some tissues.37 Although we did not find AQP5 water channels in duct cells in mouse SMGs, SGLT1, which is located at the luminal membrane in salivary duct cells, may work as a water pump38 and induce water reabsorption by duct cells. Furthermore, we speculate that the increase in the duct cell population reduced Na+ and Cl− concentrations in the secreted saliva because of epithelial sodium channels and/or cystic fibrosis transmembrane conductance regulator Cl− channels located in the apical membranes of duct cells.39 Despite some limitations, this animal study improves our understanding of the effect of T2D in the oral environment.
Conclusion
The results of the present study demonstrate that submandibular salivation decreases in KK-Ay mice due to reductions in Ca2+ release from the ER, potentially caused by hyperglycemia-induced ER stress. Duct cell weight increases in KK-Ay mice, possibly leading to an increase in ion reabsorption and decreased concentrations of Na+ and Cl− in the saliva. Therefore, ion compositions may become useful diagnostic tools in the prediction of diabetes. Well-controlled human studies are necessary in the future to improve our understanding of the effect of T2D on saliva secretion.
Acknowledgement
This work was supported by a grant from Japan Society for the Promotion of Science (JSPS) KAKENHI (No. 16H05527 and 25463011).
Disclosure
None declared.




