Oncolytic viral therapy as promising immunotherapy against glioma
Abstract
Glioma is a common primary central nervous system malignant tumor in clinical, traditional methods such as surgery and chemoradiotherapy are not effective in treatment. Therefore, more effective treatments need to be found. Oncolytic viruses (OVs) are a new type of immunotherapy that selectively infects and kills tumor cells instead of normal cells. OVs can mediate antitumor immune responses through a variety of mechanisms, and have the ability to activate antitumor immune responses, transform the tumor microenvironment from “cold” to “hot,” and enhance the efficacy of immune checkpoint inhibitors. Recently, a large number of preclinical and clinical studies have shown that OVs show great prospects in the treatment of gliomas. In this review, we summarize the current status of glioma therapies with a focus on OVs. First, this article introduces the current status of treatment of glioma and their respective shortcomings. Then, the important progress of OVs of in clinical trials of glioma is summarized. Finally, the urgent challenges of oncolytic virus treatment for glioma are sorted out, and related solutions are proposed. This review will help to further promote the use of OVs in the treatment of glioma.
1 INTRODUCTION
Glioma were considered to originate from glial cells and neuronal cells.1 It is a common intracranial malignant tumor, accounting for 30% CNS tumors and around 80% malignant primary brain tumors.2 Histologically, gliomas are classified into four grades (I–IV) by the World Health Organization (WHO).3 In general, grades are generally associated with worse prognoses. After comprehensive treatment, patients with grade I gliomas can be potentially curable, patients' median survival with grade II ranged from 8 to 10 years, in patients with aggressive glioma (grade III) between 3 and 4 years, whereas in patients with WHO grade IV grade (such as glioblastoma) between 14.6 and 17 months.4 A new tumor classification for the CNS was released by the WHO in 2021. Through the comprehensive analysis of genetic characteristics, molecular patterns, and histopathological characteristics, the tumor classification is more specific, and the diagnosis level of brain tumors and the choice of treatment plan are improved.5 High-grade gliomas have biological characteristics such as rapid invasive growth, abnormal blood vessel proliferation, and easy destruction of brain tissue,6 which makes the treatment of malignant gliomas difficult and the prognosis is poor. Besides traditional surgery and chemoradiotherapy, immunotherapy has also been gradually applied in the treatment of glioma. Among them, oncolytic virus is the most promising therapy for glioma.
Oncolytic viruses (OVs) are natural or genetically engineered viruses that selectively infect and kill tumors without affecting normal cells. Although virotherapy for cancer started in 1902,7 it was not until the 1990s that huge progress was initiated with the development of recombinant DNA technology and viral genome engineering. Martuza8 used genetically engineered HSV without thymidine kinase (TK) to treat nude mice with U87 glioma in situ, prolonging survival. At present, four oncolytic virus products have been approved for clinical tumor treatment (Table 1),9-12 more than 300 oncolytic virus products have entered the clinical trial stage. To date, there are two dominant mechanisms of the antitumor effect of the OVs. On the one hand, oncolytic may directly lyse tumor cells. Normal host cells can recognize the virus and clear them. However, the abnormal antiviral machinery in tumor cells allow the virus to replicate massively within the cells, thereby lysing cancer cells.13, 14 Furthermore, the large amount of viruses produced by viral replication can spread to adjacent tumor tissues, leading to an enlargement of the oncolytic effect.15 On the other hand, infected tumor cells can lead to tumor regression by triggering an antitumor immune response, including innate and adaptive immunity.16 Different forms of immune-genic cell death (ICD), such as apoptosis, necrosis, and autophagy, can be triggered by Ovs.17 The release of damage-associated molecular patterns (DAMPs), tumor-associated antigens (TAAs), viral pathogen-associated molecular patterns (PAMPs) and pro-inflammatory cytokines can activate innate immunity response and kill virus-infected cells.18-20 Many studies have shown that tumor-associated antigens and neoantigens released by OVs-infected tumor cells can induce the activation of major histocompatibility class I (MHC-I) molecules and costimulatory molecules and promote the maturation of DC, reactivating and recruiting T cells (Figure 1).21-25 What's more, OVs can kill tumor cells from multiple dimensions by increasing immune cell infiltration, triggering inflammation within the TME, breaking immune tolerance and converting “cold” tumors into “heat” tumors.26-28 A highly immunosuppressive TME is a huge challenge for malignant glioma treatment. Oncolytic virotherapy can help to overcome some of this challenge, making it a promising new therapy for glioma.29
| Name | Virus type | Modification | Indications | Institution | Year |
|---|---|---|---|---|---|
| Rigvir | ECHO-7 | - | Melanoma | Latvia | 2004 |
| Oncorine | Adenovirus | E1B-55K and E3 regions deleted | Head and neck cancer | NMPA | 2005 |
| Imlygic | HSV-1 | ICP34.5 and ICP47deleted; hGM-CSF inserted | Melanoma | FDA | 2015 |
| Delytact | HSV-1 | Deletion of ICP34.5 and ICP47; inactivation of ICP6; insertion of lacZ | Malignant glioma | MHLW | 2021 |
- Abbreviations: ECHO-7, enteric cytopathic human orphan virus type 7; FDA, the Food and Drug Administration; hGM-CSF, human granulocyte-macrophage colony stimulating factor; HSV-1, herpes simplex virus type 1; MHLW, the Japanese Ministry of Health, Labour and Welfare; NMPA, the China National Medical Product Administration.
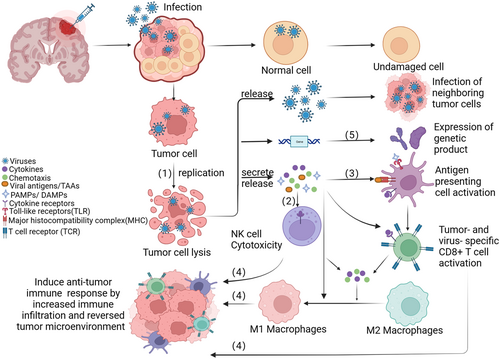
Here, we discuss current glioma treatments, the application of oncolytic virus in glioma including OVs of different vectors and genetic modifications, recent progress in clinical, challenges and solutions, and future prospects. Among them, the application of different OVs in the treatment of glioma is the part we focus on. The aim of this review was to give an outline of the latest advancements in oncolytic virus research in glioma. We hope that researchers can draw experience and ideas from this review to better solve the problems encountered by OVs in brain organic tumors at this stage.
2 CURRENT STATUS OF GLIOMA THERAPIES
Glioma is a type of tumor with a very high mortality rate, and it is urgent to find a suitable treatment plan. A variety of different treatment strategies are currently under investigation, and here we summarize the current status of the treatment of glioma with important advances in clinical research (Table 2).
| Treatment | Patients | Phase/Status | Results | Identifier | |
|---|---|---|---|---|---|
| Monoclonal antibodies | |||||
| Bevacizumab/irinotecan | 167 | Phase П completed | The 6-month PFS: 42.6% and 50.3%; the objective response rates: 28.2% and 37.8%; the mOS times: 9.2 months and 8.7 months | NCT00345163 | |
| Bevacizumab/etoposide | 59 | Phase П completed | The 6-month PFS:40.6% and 44.4%, the radiographic response rates: 22% and 37%; the median survivals: 63.1 and 44.4 weeks. Combined use has increased toxicity. | NCT00612430 | |
| Bevacizumab/radiotherapy | 25 | Pilot study completed | ORR:50%;6-month PFS: 65%; mOS:12.5 months;1-year survival: 54% | NCT00595322 | |
| Nimotuzumab/standard therapy | 150 | Phase Ш completed | 12-month PFS: 25.6%; 20.3% | NCT00753246 | |
| ABT-414/TMZ or Lomustine | 266 | Phase П completed | The 2-year survival:19.8%, 5.2% | NCT02343406 | |
| 125I-mAb425 | 192 | Phase П completed | The survival:15.7 months; and treatment was safe and well tolerated | NCT00589706 | |
| Immune checkpoint inhibitors | |||||
| Nivolumab/ipilimumab | 40 | Phase I completed | Three patients achieved a partial response and 8 had stable disease for ≥12 weeks | CheckMate143 | |
| Nivolumab/bevacizumab | 529 | Phase Ш active, not recruiting | The 12-month OS was 42% in both groups | NCT02017717 | |
| Chimeric antigen receptor T cell therapy | |||||
| HER2-CAR CMV-T cells | 16 | Phase Ⅰ completed | NCT01109095 | ||
| HER2-Specific CAR T cell | 41 | Phase Ⅰ recruiting | NCT03500991 | ||
| 4-1BB-modified IL-13Rα2 CAR-T cells therapy | 30 | Phase Ⅰ recruiting | NCT04661384 | ||
| 4-1BB-modified IL-13Rα2 CAR-T cells therapy | 82 | Phase Ⅰ active, not recruiting | NCT02208362 | ||
| Vaccines | |||||
| Rindopepimut/TMZ | 745 | Phase Ш completed | The mOS: 20.1 months; 20 months | NCT01480479 | |
| DCVax-L | 348 | Phase Ш Active, not recruiting | For methylated MGMT patients, mOS was 34.7 months after surgery, and 3-year survival rate is 46.4% | NCT00045968 | |
| (DC) vaccine pulsed with lysate derived from a GBM stem-like cell line | 39 | Phase Ⅰ completed | Newly diagnosed GBM: median PFS was 8.75 months, and mOS was 20.36 months. Recurrent GBM: median PFS was 3.23 months, 6-month PFS was 24%, and median survival was 11.97 months | NCT02010606 | |
| Physical therapy | |||||
| NovoTTF-100A | 236 | Phase Ш completed | Median survival was 6.6 versus 6.0 months, 6-month PFS rate was 21.4% and 15.1% | NCT00379470 | |
| NovoTTF-100A/TMZ | 695 | Phase Ш completed | In the TTFields-TMZ group, median PFS was 6.7 months and mOS was 20.9 months; in the TMZ-alone group, median PFS was 4.0 months and mOS was 16.0 months | NCT00916409 | |
| NovoTTF-200A/TMZ/Radiation | 30 | Early phase Ⅰ completed | 90% patients had progression, with a median PFS of 9.3 months. The 1-year PFS was 23% and the mOS was 15.8 months | NCT03477110 | |
| NovoTTF-100A/TMZ/Radiation | 950 | Phase Ш recruiting | NCT04471844 | ||
| NovoTTF-100A/bevacizumab | 3 | Phase П terminated | This study stopped accrual early | NCT02743078 | |
| NovoTTF-100A/marizomib | 66 | Phase Ⅰ completed | NCT02903069 | ||
| NovoTTF200A/nivolumab/ipilimumab | 5 | Phase П terminated | The PFS was 62.5 days | NCT03430791 | |
- Note: Clinical data source: ClinicalTrials.gov.
- Abbreviations: DCVax-L, an autologous tumor lysate-pulsed dendritic cell vaccine; mOS, median overall survival; ORR, overall response rate; PFS, progression-free survival.
2.1 Standard therapy: Surgery, chemotherapy, and radiotherapy
The standard treatment of glioma is dominated by surgical abscission, supplemented by radiotherapy and chemotherapy therapy.30 Surgery is the most important step and the focus of it is to maximize the removal of the tumor while protecting normal brain tissue around the tumor. But limitations of functional areas of the brain and indistinguishable tumor boundaries are major factors affecting the extent of glioma resection, which need advanced technology to assist surgical treatment, such as intraoperative magnetic resonance imaging (iMRI)31 diffusion tensor imaging (DTI)32 and fluorescence guidance resection (FGR).33 The aggressive nature of gliomas makes it impossible to completely cure them with surgical treatment alone.34 To delay tumor recurrence, radiotherapy and chemotherapy are used as adjuvant therapies.35 Radiation therapy is an important supplement to glioma treatment, with conventional radiation doses of 50–60 Gy.36-38 It may be possible to conduct radiotherapy using an external source, an internal source, or radioactive monoclonal antibodies.39 Temozolomide (TMZ) is a first-line agent for GBM therapy,40 but some patients with TMZ-resistance have less than ideal therapeutic effect.41
However, even with surgical resection and chemoradiotherapy, the prognosis for gliomas is not ideal, with only a slight improvement in overall survival (OS). Therefore, more novel therapies need to be actively explored.
2.2 Monoclonal antibodies
Monoclonal antibodies include bevacizumab, cetuximab, nimotuzumab, and MAB-425. Bevacizumab can inhibit vascular endothelial growth factor (VEGF) to prevent tumor angiogenesis,42 which has been approved for GBM treatment by FDA since 2009.43 In addition to bevacizumab (NCT00345163), it has also been used in combination with irinotecan (NCT00345163),44 etoposide (NCT00612430),45 or with concurrent radiotherapy (NCT00595322).46 The evidence suggests that bevacizumab can prolong the progression-free survival (PFS) of patients with glioma within 18 months but can't prolong the OS.47 Cetuximab and nimotuzumab target epidermal growth factor receptor (EGFR), which can block its ligand activity, induce apoptosis, antiangiogenesis, and thus achieve the effect of inhibiting GBM cells.48 In a phase II clinical study of cetuximab treated in patients with relapsed GBM, cetuximab did not benefit patients from PFS and OS, regardless of whether accompanied by EGFR amplification.49 The addition of nimotuzumab to standard therapy was not found to improve OS in a phase III open-label trial of newly diagnosed gliomas (NCT00753246).50 In addition to several monoclonal antibody drugs described above, there are also monoclonal antibodies binding isotopes or toxins in the clinic, such as ABT-414 (NCT02343406),51, 52 and 125I-mAb425 (NCT00589706).53 But monoclonal antibody therapy has limited survival benefit and is highly susceptible to drug resistance.
2.3 Immune checkpoint inhibitors (ICIs)
An immune checkpoint plays a crucial role in the development of immune tolerance in tumors.54 An ICI works by regulating immune cell activity through pathways such as co-inhibition or costimulation to kill tumor cells.55 It has been shown in previous studies that combining anti-PD-1 (nivolumab) with anti-CTLA-4 blocking Abs (ipilimumab) does not improve the OS during the exploratory phase I (CheckMate 143).56 Nivolumab and bevacizumab did not meet the primary endpoint of a Phase III trial of patients with recurrent GBM, because patients treated with nivolumab did not survive longer than patients with bevacizumab (NCT02017717).57 The emergence of ICIs has brought new way to the treatment of GBM, but its current research is still not optimistic. At present, the main difficulty lies in its “cold tumor” characteristics, which have blood–brain barrier (BBB)58 and strong heterogeneity,6 low tumor mutation burden,59 and immunosuppressive microenvironment.30, 60, 61 In summary, ICIs in the treatment of GBM have multiple challenges and potential, requiring more in-depth research and clinical trials.
2.4 Chimeric antigen receptor (CAR) T cell therapy
In refractory hematological cancers, CAR T cell therapies have produced sustained therapeutic effects, but in solid tumors, they have not been as successful.62, 63 Three antigens are currently being targeted with CAR T cells for the treatment of glioblastoma: epidermal growth factor receptor variant III (EGFRvIII), human epidermal growth factor receptor 2 (HER2), and interleukin-13 receptor alpha 2 (IL-13Rα2).64 The EGFRvIII is an active mutant of EGFR and is present in about 30% GBM patients.65 EGFRvIII CAR-T cells showed excellent tumor growth inhibition in immunodeficient mouse models.66 However, there are no meaningful effects in clinical for EGFRvIII-CAR T cells in GBM patients.67 The HER2 is a GBM-associated antigen that expressed in 80% GBM patients.68 HER2-specific CAR-T cells have shown potent antitumor activity in multiple tumor models.69 Several clinical trials have recently reported that HER2-specific CARs have no serious systemic toxicity In GBM (NCT01109095, NCT03500991).70, 71 Interleukin-13 (IL-13) can modulate immune response and inflammation by binding to the IL-13 receptor α1 (IL-13Rα1) and IL-13Rα2.72 IL-13Rα2, expressed in over 75% of GBMs, is related to what tumor aggressiveness and poor prognosis.73 And IL-13Rα2 was the first GBM target for CAR T cell therapy in 2004, which showed effective tumor lysis in human xenografts in vitro and in vivo studies.74 Two-thirds of treated patients had excellent tolerance of CAR T cells and the great antitumor response in clinical trial.75 Further improvements, IL-13Rα2 CAR-T cells are modified with 4-1BB or IL-15,76, 77 and 4-1BB-modified IL-13Rα2 CAR-T cell therapy is currently tested to treat medulloblastoma, ependymoma, and GBM (NCT04661384) in clinical, while intratumoral delivery is being assessed in refractory or recurrent malignant glioma (NCT02208362).
In addition to the cell targets above, there are some new targets such as GD2, chlorotoxin, EphA2, P32, CD133, B7-H3, and CD147.78 The above studies have evaluated that the use of CAR T cells for brain tumors is safe and efficacious. CAR-T cell therapy is moderately effective, however, due to heterogeneous antigen expression and limited brain T cell function.79 New strategies have been created to modify CAR-T cell function64 such as development CAR T cells targeting multiple Tas,80 transgenic expression of cytokines and chemokine receptors77, 81 and combination with radiotherapy, chemotherapy,82 and immune checkpoints.83
2.5 Vaccines
Tumor vaccine is another kind of immunotherapy with clinical transformation prospects, based on tumor-specific antigen targets, which can instigate an immune response that is both tumor-specific and patient-specific.84 Peptide vaccines, dendritic-cell-based (DC) vaccines, and mRNA vaccines are under investigation for glioma therapy. Peptide vaccines can induce antitumor immune responses by using small tumor-specific antigen sequences. Some peptide vaccines have achieved promising results in the study of malignant glioma, such as rindopepimut (EGFRvIII), R132H (IDH1), and Wilms tumor 1 (WT1) peptide vaccine.85-88
Conceptually, DC-based vaccines require the patient's own DC cells, loaded with tumor lysates or peptides, and then reinfused into the patient.84 Tumor-derived mRNA, viral antigens, and cancer stem cells also can be loaded into DCs.89 DC vaccines are typically loaded with glioma-associated antigens (GAAs) or GAA-derived peptides, such as WT1, survivin, and IL-13Rα2.90 More than 6 years of survival were achieved by five patients with GBM treated with a combination of chemotherapy and five synthetic peptide-pulsed DC vaccines.91 Glioma lysate-pulsed DC vaccines have shown promising results in early clinical trials. A phase III glioma clinical trial reported OS of 23.1 months in postoperative patients treated with tumor-lysate pulsed DC vaccine (NCT00045968).92 DC vaccines pulsed with glioma stem cells (GSCs) are able to induce interferon (IFN)-γ production to initiate T cell-mediated antitumor immunity.93 Autologous DC vaccine pulsed with GBM stem-like cell lysates were used in 11 newly diagnosed patients with GBM and 25 recurrent patients, results demonstrated improved clinical benefits in OS (NCT02010606).94
While clinical studies have demonstrated that peptide vaccines and DC vaccines can exert certain antitumor effects, the heterogeneity of gliomas is a challenge to overcome. To overcome these limitations, it may be important to combine the vaccine with surgical treatment, chemoradiotherapy, and ICIs on the basis of optimizing the DC vaccine.95
2.6 Physical therapy
The tumor treating field (TTFieds) can inhibit tumor cell division and proliferation by interfering with the normal process of mitosis and inhibiting the normal septin localization.96 TTFieds has been approved by the FDA for the diagnosis and recurrent GBM due to multiple great clinical trial results. A pilot clinical trial was initiated after TTFields were found to inhibit cancer growth in multiple animal tumor models. The median time to disease progression and OS are more than double historical control patients.97 The clinical trial results for NovoTTF-100A demonstrated no significant improvement in the median OS and PFS but the less toxic and favored quality of life than chemotherapy (NCT00379470).98 Another multicenter phase III clinical trial proved that the addition of TTFields to maintenance temozolomide chemotherapy can significantly improve the survival rate of patients with glioblastoma (NCT00916409).99 Additionally, the synergy of the triple combination of RT, TTFields, and TMZ was explored in the clinical phase I trial(NCT03477110). Treatment with TTFields combined with scalp-sparing chemoradiation is feasible, well-tolerated, and relatively safe.100 A phase III clinical trial (NCT04471844) is currently recruiting to investigate the clinical benefit of TTFields with chemoradiation treatment. Other clinical studies also found that the combination of TTFields therapy and drug therapy can improve PFS and OS in GBM, such as Bevacizumab (NCT02743078), marizomib (NCT02903069), nivolumab, and ipilimumab (NCT03430791). Although TTFields therapy is a helpful adjuvant therapy to chemotherapy in patients with GBM, its extensive application is limited by the expensive treatment cost and inconvenient equipment.
Despite extensive research, treatments that truly benefit patients have not yet been developed. Traditional therapy is the most common, but surgical methods are extremely complex, elaborate and expensive, and chemoradiotherapy has serious side effects. For high-grade gliomas, even after standard treatment with surgery and chemoradiotherapy, the median OS is between 15 and 23 months and 5-year survival rate is low.101 Monoclonal antibodies are guidelines recommended for GBM targeting with high targeting specificity. However, due to their large molecular size, they may not easily pass through the BBB. Multiple monoclonal antibodies have shown no advantage in prolonging survival.102 ICIs have shown promising results in preclinical experiments, but clinical trial results have not been satisfactory. Clinical trials evaluating ipilumamab, pembrolizumab, and nivolumab did not meet the primary endpoint.103 No significant clinical benefit has been observed in clinical trials of CAR-T cell drug candidates for glioblastoma. The Immunotherapies for glioblastoma described above are still limited and need to be further optimized. The TTFieds is a new and safe method, however, the device is expensive and requires prolonged wear, which can affect the quality of life of patients. Multiple clinical trials have demonstrated no OS improvement with NovoTTF alone, but combining it with other treatment strategies can improve the treatment effect.104 Oncolytic virus therapy is a promising cancer treatment with positive results from many clinical trials. Delytact is the first oncolytic virus approved for the treatment of glioma. Clinical results have shown a significant improvement in survival and safety compared to standard therapies. The 1-year survival rate of the oncolytic virus treatment group was 92.3% compared with the 15% 1-year survival of standard treatment.105 Therefore, oncolytic viral therapy is likely to be a cost-effective modality to treat gliomas.
3 ONCOLYTIC VIROTHERAPY FOR BRAIN GLIOMA
Heretofore, the oncolytic virus therapy is considered a promising strategy for treating cancer by selectively replicating within cancer cells and killing them.106 Some encouraging data were obtained in many preclinical trials and clinical trials. Delytact for glioma approved in Japan has greatly promoted the application of oncolytic virus in glioma. In this section, we describe the most common OVs and their genetic modifications, then provide an overview of OVs associated with gliomas that have entered clinical trials, and finally list novel OVs for the treatment of gliomas.
3.1 Modification of OVs
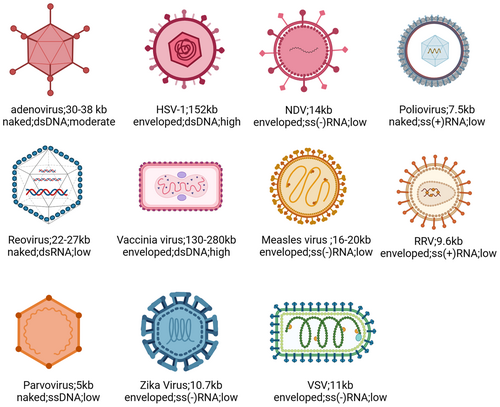
In the study of glioma, many viruses have been proposed including Adenovirus, Herpes simplex virus (HSV), poliovirus, vaccinia virus (VV), measles virus (MV), retroviruses, Newcastle disease virus (NDV), reovirus, parvovirus, and so on (Figure 2). Although early OVs have a degree of oncolytic activity, their specificity is not very high. Therefore, scientists have begun to improve tumor-specific selection and safety of OVs using various methods, such as deleting virulence genes, loading tumor-specific promoters, and carrying tumor suppressor gene miRNAs.107 HSV-1 is often deleted by the ICP34.5108 and ICP47 genes109 to attenuate the pathogenicity of the virus; oncolytic adenoviruses often delete E1B-55K or E1AΔ24 to ensure that the virus selectively replicate in tumor cells110, 111; oncolytic poxviruses increase its selectivity to tumor cells by deleting the TK gene.112 Expression of oncolytic virus genes initiated by tumor-specific promoters, including human telomerase reverse transcriptase promoter (hTERT), E2F-1 and glial fibrillary acidic protein (GFAP) promoter can significantly improve oncolytic virus' anti-tumor specificity.113, 114 With the continuous development of viral biology, tumor immunology, and molecular genetics, the modification of OVs is also constantly advancing. There are currently five strategies for arming OVs as follows115: (1) encoding T cell co-stimulating molecules to improve APC function116-118; (2) Arming OVs with chemokines to recruit tumor lymphocytes in TME119-121; (3) Arming OVs with cytokines to improve the function of antitumor lymphocytes in TME122-124; (4) Arming with antigens with OVs, which can be used as a tumor vaccine125, 126; (5) Arming OVs with ICIs to relieve immunosuppression of TME.127-129 We have compiled the main modifications of these OVs (Table 3).
| Virus Family | Name | Modification |
|---|---|---|
| Adenovirus | ONYX-015 | E1B-55K (−) |
| Delta-24 | E1A∆24 | |
| DNX-2401 | E1A∆24; RGD-4C (+) | |
| DNX-2440 | E1A∆24; RGD-4C (+); OX40L (+) | |
| CRAd-S-pk7 | Survivin promoter (+); poly-lysine (pk7) (+) | |
| ICOVIR-5 | E2Fp (+); E1A∆24; RGD-4C (+) | |
| ICOVIR-17 | E2Fp (+); E1A∆24; RGD-4C (+); PH20 (+) | |
| VCN-01 | E2Fp (+); E1A∆24; RGDK (+); PH20 (+) | |
| Herpesvirus | Dlsptk | Tk (−) |
| HSV-1716 | 759 bp deletion in each copy of the ICP34.5 gene | |
| G207 | γ34.5 (−); LacZ gene (+); inactivation of ICP6 | |
| G47Δ | γ34.5 (−); LacZ gene (+); inactivation of ICP6; ∆ICP47 (−) | |
| rQNestin34.5 | Nestin-1 promoter for expression of ICP34.5 | |
| KNE | EGFR-retargeted | |
| FGE-4: T124 | KEN; with miR-124 recognition site | |
| R-613 | EGFRvIII-retargeted | |
| R-LM113 | Nectin 1/HVEM-detargeted, hHER2-retargeted | |
| R-LM249 | Nectin 1/HVEM-detargeted, hHER2-retargeted | |
| R-115 | Nectin 1/HVEM detargeted, erbB2-retargeted; mIL-12 (+) | |
| G47Δ-mIL-12 | G47Δ; mIL-12 (+) | |
| M002 | γ34.5 (−); mIL-12 (+) | |
| oHSV-Flt3-L | γ34.5 (−); LacZ gene (+); inactivation of ICP6; Flt3-L (+) | |
| oHSV-TRAIL | γ34.5 (−); LacZ gene (+); inactivation of ICP6; TRAIL (+) | |
| Poliovirus | PVSRIPO | Internal IRES is replaced by HRV2 |
| Vaccinia virus | TG6002 | J2R gene (−); I4L gene (−); FCU1 gene (+) |
| Measles virus | MV-Edm | Attenuated Edmonston strain |
| MV-CEA | Carcinoembryonic antigen gene (+) | |
| Retroviruses | Toca511 | FCU1gene (+) |
| Newcastle disease virus | MTH-68/H | Moderately pathogenic (mesogenic) strain |
| NDV-HUJ | Avirulent (lentogenic) strain | |
| Reovirus | Reolysin | None |
| Parvovirus | ParvOryx | None |
| Zika virus | ZIKV | None |
| Vesicular stomatitis virus | VSV Δ51 | A deletion of amino acid at the 51st position of its M protein |
| RVSV (GP) | Envelope GP is replaced by LCMV-GP | |
| VSV-EBOV | EBOV (GP) gene (+) | |
| GLESS-FAST-VSV | G protein less-fusion-associated small transmembrane-VSV | |
| Myxoma virus | vMyx-M011L-KO | M011L (−) |
| vMyx-IL15Rα-tdTr | IL15Rα-IL15 gene (+); tdTomato Red gene (+) | |
| Seneca valley virus | SVV-001 | None |
- Abbreviations: −, deletion; +, insertion. E1AΔ24, a deletion of 24 base pairs within the E1A region; E2Fp, E2F-responsive promoter; Flt3L, FMS-like tyrosine kinase 3 ligand; HRV2, human rhinovirus type 2; IRES, internal ribosome entry site; LCMV-GP, glycoprotein of lymphocytic choriomeningitis virus; M011L, an antiapoptotic Bcl-2 homolog; OX40L, the immune costimulator OX40 ligand; PH20, PH20: human sperm PH20 hyaluronidase; RGD-4C, arginyl-glycil-aspartic acid motif in the HI loop of the fiber; RGDK, RGDK in the putative heparin sulfate-glycosaminoglycans binding domain KKTK in the fiber shaft; TK, thymidine kinase gene; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Oncolytic adenovirus is a commonly used oncolytic viral vector, and its most important modifications are the deletion of viral genes and the insertion of RGD, tumor-specific promoters, and functional proteins gene.130 ONYX-015 is an oncolytic adenovirus with a deletion of the E1B-55K gene.131 The E1B-55kD protein can bind to p53 to inhibit p53-mediated apoptosis.132 The lack of E1B-55K enables the virus to replicate in p53-deleted tumor cells. ONYX-015 demonstrated significant antitumour activity in xenograft models of glioblastoma.133 E1A-delta24 (Delta-24), a tumor-selective adenovirus, had a 24-bp deletion in the Rb-binding region of the E1A gene, which can render it cancer-specific. Delta-24 inhibited tumor growth in nude mice with D-54 MG by 66.3% with a single dose, and by 83.8% with multiple injections.134 Delta-24-RGD (DNX-2401) has an RGD-4C peptide motif of the fiber knob compared to Delta-24, which allows the adenovirus to enter cells by binding directly to integrins. To enhance the virus-mediated immune response, Delta-24-RGDOX (DNX-2440) was constructed to express the immune co-stimulator OX40 ligand (OX40L). Compared with its predecessor (DNX-2401), Glioma mouse models treated with DNX2440 exhibit increased T-cell responses and a prolonged survival rate.135 This virus is currently being tested in a phase I clinical trial in patients with recurrent GBM by stereotactical injection (NCT03714334). CRAd-S-pk7 was created by inserting survivin promoter (S) and poly-lysine (pk7) inserting fiber region to target glioma.136 CRAd-S-pk7 can inhibit xenograft tumor growth and 67% of treated mice were long-term survivors.136 ONYX-015, DNX-2401, CRAd-S-pk7 are currently being explored in clinical trials. ICOVR-5, ICOVIR17, and VCN-01 had shown good anti-glioma activity in preclinical experiments. ICOVIR-5 encompassed E1A partial deletion in Rb protein–binding region, RGD-4C insertion and E2F1-responsive promoter to improve the selectivity and effectiveness of viral replication.137 ICOVIR-5 significantly prolonged the median survival and 37% without relapse in xenografted mice.138 ICOVIR-17 is a modified version of ICOVIR15 expressed human hyaluronidase PH20.139 By enhancing the degradation of HA and viral spread, ICOVIR-17 showed significant antitumor effects and prolonged survival in mice.140 Compared with ICOVIR-17, VCN-01, improved the infectivity, because its RGD was inserted in the fiber shaft instead of the fiber knob. VCN-01 displayed a pronounced cytotoxic effect on glioma cells and increased OS significantly in orthotopic glioma models in vivo.141 There have been no clinical trials of these viruses for gliomas.
HSV is a common candidate viral vector for OV due to its safety, large gene capacity, and ability to infect a variety of cells.142 Oncolytic herpes simplex viruses (oHSVs) are usually designed by deleting one or more virulence genes to improve their safety. Dlsptk, the HSV-1 mutant with the deletion of the TK gene, was the first genetically engineered HSV to be used in the treatment of glioblastoma.8 HSV-1716, G207, G47Δ, and rQnestin34.5 are members of oHSVs in which γ34.5 has been deleted or modified.143 In addition to the loss virulence genes, they might be further modified to express different immunomodulatory or anticancer transgenes in pursuit of better treatment. A mIL-12 expressing OHSV, M002, have demonstrated the antitumor efficacy in intracranial syngeneic neuroblastoma murine model and human glioma xenograft tumor models.144, 145 A clinical trial is underway to determine whether IL-12-encoding virus (M032) can be effective in treating glioblastoma (NCT02062827). HSVs can also be engineered to improve infection effects based on cancer cells' specific receptor expression. The EGFR, EGFRvIII, HER2, which are highly expressed on the surface of glioblastomas, have received significant attention. EGFR-retargeted HSV (KEN, FGE-4), EGFRvIII-retargeted HSV (R-613, KMMP9), and HER2-retargeted oncolytic HSV (R-LM249, R-115) not only conferred tumor specificity but also showed promising anti-GBM efficacy.146
Poliovirus is an envelope-free single-stranded RNA virus, a natural neuro-pathogen with inherent neuro-aggression.147 Poliovirus invades cells via the receptor CD155 on the cell surface, which has strong antitumor potential due to its wide expression in solid tumors such as gliomas.148 Since the internal ribosome entry site (IRSE) is a key neuro-causative factor for the poliovirus, it needs to be modified to reduce neurovirulence. PVSRIPO, which is the IRES is replaced by human rhinovirus type 2 (HRV2).149 Preclinical experiments have proved that PVSRIPO can infect and lyse glioma cells and can effectively inhibit tumor growth and improve survival rate in animal models of glioma.150, 151
VV is a double-stranded DNA virus that replicates in the cytoplasm of host cells, which has the advantages of wide range of infection, strong tumor targeting, and large gene capacity.152 Although unmodified VV has oncolytic effects, it has serious side effects. TK-knockout VV has high tumor targeting and safety due to the deletion of the TK gene. To further improve the oncolytic effect of VV, double-, triple-, and quadruple-deletion mutant VVs have been generated, such as TG6002, GL-ONC, and T601.153 TG6002 is an attenuated VV with targeted deletions of the J2R gene and the I4L gene engineered to express the yeast FCU1 gene, which encodes the ribonucleotide reductase.154 TG6002 is the only modified VV for glioma clinical trials. The combination of TG6002 and 5-fluorocytosine (5-FC) has shown significant antitumor effects in a variety of human xenograft tumor models.154 The safety and efficacy of TG6002/5-FC for local chemotherapy in patients with recurrent glioblastoma are being tested in phase I/II clinical trial (NCT03294486).
MV is an enveloped virus with unsegmented negative-stranded RNA, belonging to the paramyxoviridae family.155 MV relies on CD150, CD46 and nectin-4 cell membrane receptors to enter tumor cells.156 The high expression of CD46 receptor on the surface of malignant glioma cells makes MV a good oncolytic viral vector for the treatment of glioma. Engineered oncolytic MVs derived from attenuated strains, are engineered to express either human sodium–iodine transporter (NIS) or human carcinoembryonic antigen (CEA).157 Compared with MV-CEA, MV-INS has more significant antitumor activity in vitro and in vivo. More importantly, MV-INS can be used in combination with local radiotherapy to treat gliomas.158 MV-INS only conducted clinical studies in children and young adults with recurrent medulloblastoma or recurrent ATRT (NCT02962167), but MV-CEA is currently in clinical testing for gliomas.
In addition to the above genetically modified OVs, there are also many viruses with natural oncolytic activity. NDV is an enveloped, single-stranded RNA virus, which can cause local infections in humans with mild symptoms.159 Various animal models and human clinical tests have confirmed that NDV is nonpathogenic, relatively safe, and has no side effects in humans. Thus, attenuated strains of NDV are currently used in the study of OVs.160 At present, other NDV-based strategies for the treatment of malignant glioma are being explored, such as autologous tumor vaccine modified with nonlytic NDV (ATV-NDV).161 Reovirus are nonenveloped virus with 9–12 linear double-stranded RNA that occurs naturally in the mammalian respiratory and intestinal systems.162 Reovirus has a targeted lytic effect on cells activated by the RAS pathway, such as malignant gliomas cells and breast cancer cells.163 Clinical trials and preclinical studies of Reovirus have shown great promise.164 H-1 parvovirus (H-1PV) is a self-propagating virus with oncolytic and tumor-suppressing activity and is nonpathogenic in humans.165 H-1PV interacts with galactactagglutinin-1 to enter cancer cells, and the LGALS1 gene (encoding galectin-1) is overexpressed in glioblastoma cells.166 In immunocompetent rat models of rat (RG-2 cell-derived) and immunodeficiencies rat models of human (U87 cell-derived) glioma, a single stereotactic intratumor-injected H-1PV and multiple systemic (iv) application of H-1PV significantly improved survival in rat and human glioma models.167
3.2 Oncolytic virus in clinical trials
The progress of OVs in preclinical research of glioma experiments has further promoted the translation into clinical trials. Among them, DNX-2401 and G207 play an important role in the research of glioma treatment. Next, we focus on OVs that have entered clinical trials. Table 4 provides a summary of clinical trials for OVs for gliomas.
| Virus | Combination | Delivery | Condition | Phase/status | Enrolled patients | Results | Reference |
|---|---|---|---|---|---|---|---|
| Adenovirus | |||||||
| ONYX-015 | i.t | Recurrent MG | Phase I completed | 24 | No serious toxicity with 1010 pfu of virus; Median survival: GBM patients: 4.9 months; AA and AO patients:11.3 months | [168] | |
| DNX-2401 | i.t. | Recurrent MG | Phase I completed | 37 | No dose-limiting toxicities; A decrease in tumors was observed in 18 of 25 patients, with a mOS of 9.5 months regardless of dose | NCT00805376 | |
| i.t./CED | Recurrent GBM | Phase I/II completed | 20 | NA | NCT01582516 | ||
| TMZ | i.t. | Recurrent GBM | Phase I completed | 31 | NA | NCT01956734 | |
| IFN-γ | i.t. | Recurrent GBM or GSM | Phase Ib completed | 37 | NA | NCT02197169 | |
| pembrolizumab | i.t. | recurrent GBM or GSM | Phase II completed | 49 | NA | NCT02798406 | |
| i.t./CED | Recurrent GBM | Phase I completed | 20 | CED technique of Delta24-RGD is safe; median PFS: 82 days; mOS: 129 days; 6 of 19 patients had an OS of more than 6 months, of which 2 achieved long-term survival of 7.5 and 2.5 years | [169] | ||
| i.t. | Naive DIPG | Phase I active, not recruiting | 12 | NA | NCT03178032 | ||
| MSC-DNX-2401 | Surgery | i.a. | Recurrent HGG | Phase I recruiting | 36 (estimated) | NA | NCT03896568 |
| DNX-2440 | i.t. | Recurrent Glioblastoma | Phase I recruiting | 16 | NA | NCT03714334 | |
| NSC-CRAd-S-pk7 | i.t. | Newly Diagnosed MG | Phase I completed | 13 | No formal dose-limiting toxicity and no treatment-related deaths; the median PFS was 9.1 months and mOS was 18.4 months | NCT03072134 | |
| Herpesvirus | |||||||
| HSV-1716 | Surgery | i.t. | HGG | Phase I completed | 12 | No associated toxicity; 3 of 12 patients surviving more than a year | [170] |
| Dexamethasone Surgery | i.t | Refractory or Recurrent HGG | Phase I terminated | 2 | NA | NCT02031965 | |
| G207 | i.t. | Malignant glioma | Phase I completed | 21 | No associated toxicity or serious adverse events | [171] | |
| i.t. | Recurrent GBM | Phase Ib completed | 6 | doses of 1 × 109 pfu can be without the development of significant AEs or toxicity | [172] | ||
| i.t. | Recurrent GBM | Phase Ib completed | 6 | Safety of multiple injections | [173] | ||
| Tumor resection cavity | Recurrent BC | Phase Ib/II completed | 65 | NA | NCT00028158 | ||
| 5-Gy radiation | i.t. | Recurrent MG | Phase 1 completed | 9 | Six of the nine patients had stable disease or PR for a least one time point | NCT00157703 | |
| 5-Gy radiation | i.t. | Children With Progressive or Recurrent Supratentorial BT | Phase I active, not recruiting | 13 | Twenty grade 1 adverse events were possibly related to G207; the mOS was 12.2 months; 4 of 11 patients were still alive 18 months after G207 treatment | NCT02457845 | |
| 5-Gy radiation | i.t. | Children With Recurrent HGG | Phase II not yet recruiting | 40 (estimated) | NA | NCT04482933 | |
| 5-Gy radiation | i.t. | Recurrent or Refractory Cerebellar BT | Phase I recruiting | 15 (estimated) | NA | NCT03911388 | |
| G47Δ | i.t. | Recurrent GBM | Phase I/IIa completed | 21 | No toxicity or serious adverse events | UMIN000002661 | |
| i.t. | Recurrent GBM | Phase II completed | 19 | The 1-year survival rate of 84.2% and the median OS and PFS of 20.2 months and 4.7 months | UMIN000015995 | ||
| rQNestin34.5 | Cyclophosphamide | i.t. | Recurrent MG | Phase I recruiting | 62 (estimated) | NA | NCT03152318 |
| M032 | i.t. | Recurrent MG | Phase I active, not recruiting | 24 | NA | NCT02062827 | |
| C134 | i.t. | Recurrent GBM | Phase I active, not recruiting | 24 (estimated) | NA | NCT03657576 | |
| Poliovirus | |||||||
| PVSRIPO | i.t./CED | Recurrent GBM | Phase I completed | 61 | 21% of patients achieved 24 months of overall survival | NCT01491893 | |
| i.t./CED | Recurrent MG in Children | Phase 1b active, not recruiting | 12 (estimated) | NA | NCT03043391 | ||
| i.t./CED | Recurrent MG | Phase II active, not recruiting | 122 (estimated) | NA | NCT02986178 | ||
| Pembrolizumab | i.t. | Recurrent GBM | Phase II active, not recruiting | 30 | NA | NCT04479241 | |
| Vaccinia virus | |||||||
| TG6002 | 5-FC prodrug | i.v. | Recurrent GBM | Phase I/II recruiting | 78 (estimated) | NA | NCT03294486 |
| Measles virus | |||||||
| MV-CEA | Surgery | i.t. | Recurrent GBM | Phase I completed | 23 | No dose-limiting toxicity to date | NCT00390299 |
| Retroviruses | |||||||
| Toca511 | 5-FC | Tumor resection cavity | Recurrent MG | Phase I completed | 58 | Durable complete responses in some recurrent HGG patients | NCT01470794 |
| 5-FC | i.t./i.v. | Recurrent MG | Phase I completed | 54 | NA | NCT01156584 | |
| Lomustine | Tumor resection cavity | Recurrent HGG | Phase II/III terminated | 403 | Did not improve OS or other efficacy end points | NCT02414165 | |
| Temozolomide | |||||||
| Bevacizumab | |||||||
| Newcastle disease virus | |||||||
| MTH-68/H | i.v. | HGG | Phase I completed | 4 | The survival rates of 5–9 years | [174] | |
| NDV-HJU | i.v. | Recurrent GBM | Phase I/II completed | 11 | No major side effects and the complete tumor response in one patient | [175] | |
| Reovirus | |||||||
| REOLYSIN | i.t. | Recurrent MG | Phase I completed | 18 | NA | NCT00528684 | |
| i.t. | Recurrent MG | Phase I completed | 18 | No severe adverse events;10 patients had stable disease; one had a partial response, and four had progressive disease. Median survival was 140 days | [176] | ||
| i.v. | BT | Phase Ib completed | 9 | Intravenous infusion leads to infection of brain tumors, infiltration by cytotoxic T cells, and upregulation of PD-L1 | 2011-005635-10 (EudraCT)177 | ||
| Sargramostim | i.v. | High-grade relapsed or refractory BT | Phase I active, not recruiting | 6 | NA | NCT02444546 | |
| Parvovirus | |||||||
| ParvOryx | i.v./i.t. + tumor resection cavity | Progressive primary or recurrent GBM | Phase I/II completed | 18 | No dose-dependent side effects or dose-limiting toxicity; 12 patients showed progressive disease or died | NCT01301430 | |
| i.t./i.v. | Recurrent GBM | Phase I/IIa completed | NA | Enhanced immune response and extend median survival | [178] | ||
- Note: Clinical data source: ClinicalTrials.gov, UMIN Clinical Trials Registry (UMIN-CTR) and European Union Drug Regulating Authorities Clinical Trials (EudraCT).
- Abbreviations: 5-FC, 5-fluorocytosine; AT/RT, atypical teratoid rhabdoid tumor; BC, brain cancer; BT, brain tumors; CED, convection enhanced delivery; DIPG, diffuse intrinsic pontine gliomas; GBM, glioblastoma; GSM, gliosarcoma; HGG, high-grade glioma; i.a., intraarterial; i.t., intratumoral; i.v., intravenous; MB, medulloblastoma; MG, malignant gliomas; mOS, median overall survival; NBM, neuroblastoma; PFS, progression-free survival.
ONYX-015 is one of the first OVs for cancer therapy. Upon testing ONYX-015 on 24 patients with recurrent gliomas, good safety, and immune cell infiltration were observed, but no antitumor effects were observed.168 However, encouraging effects were seen in head and neck cancer patients by the combination of ONYX-15 and chemotherapy.179 In 2005, H101 (Oncorine), similar to ONYX-015, was approved for head and neck cancer in China.180
DNX-2401 is a recombinant oncolytic adenovirus that carries two genetic modifications. One is the absence of 24 bp in E1A gene. The second is the insertion of the RGD-4C motif in the HI ring of the fiber. Adenoviruses enter cells by binding to Coxsackievirus and adenovirus receptor (CAR) on the cell surface.181 However, glioma cells express lower levels of CAR than normal cells.182 Inserting the sequence encoding the RGD peptide into the HI loop of the fiber knob can improve the efficiency of oncolytic adenovirus glioma cells.183 In U87MG xenografted mice, DNX-2401 resulted in longer survival than Delta-24.184 Naiara et al. evaluated the anti-glioma effect of DNX-2401 in pediatric high-grade glioma (pHGG) and diffuse intrinsic pontine glioma (DIPG) models. Preclinical results shown that DNX-2401 has a significant antitumor effect in pHGG and DIPG orthotopic immunosuppressed and immunocompetent models. DNX-2401 administration triggers an immune response against tumors in addition to its oncolytic effects.185 A clinical trial first assessed the safety and maximum tolerated dose (MTD) injected directly around the glioma tumors (NCT00805376). DNX-2401 improved long-term survival in recurrent HGG, which may be due to the direct oncolytic effect and the anti-glioma immune response triggered by increasing cytotoxic T-cell infiltration.186 Preclinical trials have shown synergistic effects between DNX-2401 and TMZ.187 The tolerance of DNX-2401 along with TMZ was tested in malignant glioma patients (NCT01956734). DNX-2401 and temozolomide was well tolerated and showed therapeutic activity. Interestingly, fibroblast growth factor 2 (FGF2) can be a prognostic biomarker of DNX-2401 treatment, increased FGF2 expression was correlated with OS lengthening.188 DNX-2401 was well tolerated as monotherapy while the addition of IFN-γ did not provide additional benefits or improve survival rates (NCT02197169).189 DNX-2401 combined with pembrolizumab has been evaluated in a phase II trial (NCT02798406), but no results have been released. A phase I clinical trial tested the safety of convection-enhanced delivery (CED) of DNX-2401 in patients with recurrent GBM. CED of DNX-2401 was proved to be safe and feasible in the tumor and in surrounding brain with a local inflammatory reaction.169 A phase I clinical trial of allogeneic bone-marrow-derived human mesenchymal stem cells (BM-hMSCs) loaded with the DNX-2401 via intra-arterial injection in patients with recurrent high-grade glioma tested the ability of BM-hMSCs to deliver DNX-2401 (NCT03896568). The new technique of perfusion guided-ESIA injections (PG-ESIA) enhances the ability of BM-hMSCs-DNX-2401 to perform targeted delivery to brain tumors.190
CRAd-S-pk7 is a new oncolytic virus that is being studied in clinical trials. A phase I clinical trial will determine the safety and MTD of neural stem cells loaded with CRAd-S-pk7 (NSC-CRAd-S-pk7) in newly diagnosed malignant glioma patients combined with standard chemoradiotherapy (NCT03072134). The median PFS was 9.1 months and median OS was 18.4 months. The NSC-CRAd-S-pk7 trial proved feasible and safe, and 1.50 × 108 NSCs loading 1.875 × 1011 viral particles were recommended for further trial.191 Jennifer et al. proposed a regimen by in-brain administration of multiple doses, which achieved good therapeutic results in IND studies (IND 19532).192
HSV-1716 has a 759 bp deletion in each copy of ICP34.5 gene.193 ICP34.5 is a neurovirulent protein of HSV, the ICP34.5 gene mutation may be useful for glioblastoma selectivity.194 Phase I clinical trial have demonstrated the safety of HSV1716 when injected into the brain tumor resection cavities after surgical resection in HGG.170 However, another phase I study for refractory or recurrent HGG in younger patients has been terminated without reason (NCT02031965).
G207 was derived from HSV-1 by deleting ICP34.5 gene and inserting LacZ gene into ICP6 gene, which can increase glioblastoma selectivity.195 In some preclinical trials, the antitumor activity and safety of G207 were proved in different tumor models, especially glioblastoma.196-198 Phase I/II and phase Ib studies (NCT00028158) also demonstrated the safety and effectiveness of G207 in recurrent malignant gliomas, even within a high dose of 3 × 109 plaque-forming units (pfu).171-173 Another phase I study of G207 alone or with radiation in recurrent GBM shows safety and radiographic responses (NCT00157703).199 The combination of G207 and radiotherapy in patients with recurrent or progressive pHGG prolonged the median OS(NCT02457845).200, 201 Additionally, G207 alone or combined with a single low dose of radiation in recurrent or progressive pHGG was tested to assess the efficacy and tolerability of G207 and to survey for virologic shedding following G207 (NCT04482933). Phase I trial of G207 of refractory or recurrent cerebellar brain tumors is currently recruiting (NCT03911388).
G47Δ, originated from G207 by a lack of the nonessential α47 gene, showed a greater replication capability and a higher antitumor efficacy than G207.202 G47Δ exhibited antitumor efficacy in several tumor types, including malignant glioma.203-205 It was designated as Sakigake breakthrough therapy by the MHLW in February 2016, which allowed its expedited approval.206 G47∆ showed no toxicity or serious adverse events in progressive GBM patients (UMIN000002661).207 A phase II study tested the efficacy of G47∆ in 19 patients with residual or recurrent, supratentorial glioblastoma after radiation therapy and TMZ (UMIN000015995). This study showed that the 1-year survival rate of 84.2% and the median OS and PFS of 20.2 months and 4.7 months, which compared favorably with other treatments.208 The good survival benefit and safety profile led to the approval of G47∆ as the first oncolytic virus product for malignant glioma by MHLW on 11 June 2021 in Japan.
rQNestin34.5 can control the expression of ICP34.5 via the nestin-1 promoter, allowing it to replicate selectively in glioma cells.209 rQNestin34.5 showed anticancer efficacy and safety in mouse GBM.210 This virus is now assessing safety in malignant glioma patients in clinical trial (NCT03152318).
The C134 virus is a replication-competent HSV that express the human cytomegalovirus (HCMV) IRS1 genes. IRS1 protein can allow viruses to evade PKR-mediated protein shutoff and maintain late viral protein synthesis, resulting in improving virus replication/production and killing ability.211 Compared with γ34.5-deleted HSV, C134 reduced tumor volumes and improved survival in two murine brain tumor models.212 C134 directly administered in the CNS demonstrated safety in HSV-susceptible CBA/J mouse and nonhuman primates.213 The safety of C134 is testing in recurrent GBM administered intratumorally (NCT03657576).
Many clinical trials of PVSRIPO for gliomas have been carried out. Sixty-one patients with recurrent GBM treated with PVSRIPO in a phase I clinical trial. Results showed that PVSRIPO had no neurotoxicity, and the survival rate of 24 and 36 months among patients was extended (NCT01491893).214 PVSRIPO was tested to determine the efficacy and potential toxicity in two ongoing clinical trials (NCT03043391, NCT02986178), where PVSRIPO will be delivered to the tumor by CED. A phase II open-label, single-arm study of PVSRIPO and pembrolizumab for recurrent glioblastoma is currently ongoing (NCT04479241).
Since MV-CEA showed potent antitumor activity and was shown to have synergistic effects with radiotherapy in vitro malignant glioma models.215, 216 MV-CEA was studied in treating glioblastoma multiforme patients (NCT00390299) to determine the side effects and dosage. The order of surgery and oncolytic virus injection was different between the two groups. The first group of patients undergo tumor resection on Day 1, followed by MV-CEA administered. In the second group, patients receive MV-CEA inside the tumor on Day 1, and the tumor mass is removed on Day 5 and then administered MV-CEA into the resection cavity. There was no DLT after intracranial MV-CEA doses of up to 107 TCID50 were used, but the final experimental results were not published.217
Vocimagene amiretrorepvec (Toca511) is derived from a gamma retroviral replicating vector that encodes cytosine deaminase, which converts the prodrug 5-FC into the potent anticancer drug 5-fluorouracil (5-FU) in infected tumors.218 Animal tests have shown that Toca511 replicated stably in glioblastoma xenograft models and after 5-FC administration resulted in complete regression of tumor and prolonged survival.219 A study of recurrent HGG treated with Toca511 and 5-FC (NCT01470794) has shown durable complete responses. The median duration for responders is 35.7+ months.220 The study of Toca511/5-FC in patients with recurrent malignant glioma has ended, but the results have not yet been published (NCT01156584). A phase II/III study (NCT02414165) of comparing Toca511/5-FC with standard therapy for recurrent HGG has been terminated since neither improved OS nor other efficacy endpoints compared to the control group.221
MTH-68/H and NDV-HUJ are attenuated strains of NDV currently used to treat gliomas. Four patients with high-grade glioblastoma multiforme were treated with MTH-68/H after the failure of traditional antitumor therapy, and the quality of life was improved, and the survival time was extended to 5–9 years.174 A Phase I/II trial was conducted to determine the safety and tumor response of repeated intravenous administration of NDV-HUJ. Five of the 11 participants developed Grade I/II constitutional fever and one had complete remission.175 In general, NDV treatment of malignant glioma has been preliminarily confirmed to be safe and reliable, and has certain efficacy, but there are few relevant clinical studies and no major breakthroughs have been made.
Reolysin is a wild-type reovirus serotype-3 strain.222 A Phase I clinical trial of intra-tumoral Reolysin infusion for treating recurrent malignant gliomas in adults has been completed (NCT00528684). Although dose-limiting toxicity was not identified and MTD was not reached, anti-glioma activity was shown.176 Samson et al. achieved breakthrough success in the treatment of malignant glioma through intravenous injection of Reolysin, which proved for the first time that reovirus can pass through the BBB, replicate and kill tumor cells, and stimulate T cell activation to exert antitumor immunity.177 Reolysin in combination with sargramostim in treating younger patients with high-grade relapsed or refractory brain tumors is currently being evaluated in phase I clinical trial (NCT02444546).
ParvOryx is a H-1 parvovirus (H-1PV). Due to good preclinical experimental data showing that H-1PV has strong cytotoxicity and tumor suppressor effects in glioma cells, it led to the phase I/IIa trial of ParvOryx01 in patients with recurrent GBM to tested the safety, tolerance, and efficacy (NCT01301430). It was found that ParvOryx can pass through the BBB and induce the establishment of immunogenic tumor microenvironment (TME).223 Another phase I/IIa study also demonstrated the ability of parvovirus to switch the immunosuppressed TME toward immunogenicity, even when administered systemically.178
3.3 Novel OVs
A number of other novel oncolytic viral vectors have also been investigated for gliomas, but no clinical trials have been conducted yet. The safety and efficacy of different OV have been validated in preclinical trials, and these favorable results will provide valuable data for clinical translation.
The Zika virus (ZIKV) is a flavivirus of the Flaviviridae family. The virus tends to infect neural progenitor cells, which can cause severe neuropathy.224 Multiple studies have confirmed that ZIKV preferentially targeted GSCs, exerted oncolytic effects, and prolonged the survival time of tumor-bearing mice in a dose-dependent manner.225, 226 Kaid et al. also analyzed the safety and antitumoral effect of Zika virus intrathecal injections in three dogs bearing spontaneous CNS tumors. The results showed that the tumor regressed without adverse reactions.227 A recent study indicated that ZIKV induced a strong pro-inflammatory response and increased CD4 and CD8 T cell intra-tumoral infiltration and activation in GBM mouse models, which can improve immunotherapy efficacy.228 Taken together, Zika virus has shown good therapeutic prospects in brain tumors.
Vesicular stomatitis virus (VSV), a negative-sense single-stranded RNA virus, is extremely sensitive to the inhibitory action of interferons (IFNs).229 VSV Δ51 is a VSV mutant with a deletion of amino acid at the 51st position of its M protein.230 It could kill glioma cells efficiently and could infect and destroy the TMZ-resistant brain tumor stem cells (BTSCs) in combination with vvDD in vitro.231 RVSV (GP), without natural neurotoxicity but with potent oncolytic activity, in which envelope glycoprotein (GP) is replaced by a mutant glycoprotein of lymphocytic choriomeningitis virus (LCMV-GP), can effectively eliminate brain cancer in multiple preclinical tumor models. Remarkably, rVSV(GP) escapes humoral immunity, thus, allowing repeated virus administrations.232 VSV-EBOV virus, a chimeric VSV expressing the EBOV GP selectively targeted brain tumors and improved survival in tumor-bearing mice.233 Furthermore, a novel recombinant VSV: G protein less (GLESS)-fusion-associated small transmembrane (FAST)-VSV (GLESS-FAST-VSV) significantly prolonged the survival of normal-immunity animals harboring brain tumors through multiple intra-tumoral injections.234 As these promising experimental results, we can anticipate the clinical application of such viruses in the near future.
Myxoma virus (MXVY) belongs to the Poxviridae family and is a prototype for the genus Leporipoxvirus.235 MXVY showed efficacy in murine xenograft model of human glioma and models of medulloblastoma, and rapamycin can further enhance the oncolytic potential of MXVY.236, 237 M011L-deficient oncolytic MXVY (vMyx-M011L-KO) induced apoptosis in brain tumor-initiating cells and enhanced survival in an immunocompetent glioblastoma mouse, suggesting that it would be an ideal candidate to translate vMyx-M011L-KO to the clinic.238 More recently, IL15Rα-IL15-armed oncolytic MXVY provided potent antitumor effects, eliminating gliomas in 83% of treated mice.239
Another virus worth mentioning is the Seneca Valley virus-001 (SVV-001). Studies confirmed that SVV-001 could efficiently pass through BBB and eliminate medulloblastoma and pediatric glioma with intravenous injection.240, 241 Despite these promising results, the effect of SVV-001 in immunocompetent models was not well defined.
4 CHALLENGES IN ONCOLYTIC VIRUS TREATING FOR GLIOMA
In the past 20 years, the research on the use of OVs for gliomas has achieved encouraging results, and the application of a series of genetic engineering technologies has made the treatment of OVs have higher specificity, efficacy and safety, and more and more OVs research has entered phase I, II, and even phase III clinical trials. However, there are still many challenges in the treatment of tumors with OVs (Figure 3).
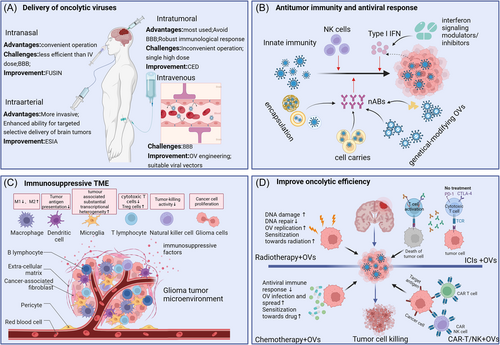
4.1 Delivery of OVs
In current experimental studies, the common route delivery of OVs is intra-tumoral injection. The intra-tumoral injection has been applied in most glioma trials, because it can overcome BBB penetration, maximize OVs concentration at the site of cancer to exert antitumor effects, protect the integrity of the OV from the immune system, and lead to robust immunological response.242 However, intra-tumoral administration often requires neurosurgery, and the complexity of surgery and uncertainty about tumor location make it difficult to multiple administrations. A single high-grade dose is often associated with risks such as bleeding, infection, and tissue damage. Therefore, the safety and convenience of intra-tumoral administration need to be further optimized. CED is a micro-invasive technique that uses a catheter to establish a pressure gradient to locally deliver therapeutics into brain.243 CED also can significantly limit systemic toxicity by directly delivering drugs to the site of cancer. Multiple experiments have proven that CED can produce effective delivery of OVs to brain tumors.
Theoretically, the systemic administration is a desirable delivery method because it is easy to operate and can be administered repeatedly, and the virus can be widely distributed to treat primary and metastatic tumors. However, the BBB is the biggest obstacle to the systematic administration of OVs for glioma. BBB plays an important role in controlling the microenvironment of the CNS to allow for proper neuronal function.244 At the same time, the BBB is a restrictive vascular barrier that limits the effectiveness of drugs delivered to brain tumors, including OVs. Even so, there are several OVs that can cross BBB to reach brain tumors, such as VV, H-1PV, SVV-001, SFV,245 and M1 virus.246 Experiments have shown that the system-injected oncolytic reovirus can be carried by immune cells to cross the BBB.177 This provides new ideas for OVs to cross the blood-brain by being loaded on brain-targeted cells, such as neural and mesenchymal stem cells.247 Currently, BM-hMSCs-DNX-2401 (NCT03896568) and NSCs-CRAd-S-pk7 (NCT03072134) are validating the feasibility of this strategy. Endovascular selective intra-arterial (ESIA) infusion can deliver drugs into glioma tumors when injected systemically, enhancing drug delivery and targeting tumor vasculature selectively.248 ESIA infusion of mesenchymal stem cells loaded with delta-24 in a canine model showed feasibility and safety and supported the clinical trial of ESIA infusion of NSCs-CRAd-S-pk7.249 Furthermore, focused ultrasound-mediated intranasal delivery (FUSIN) can circumvent the BBB and enhance the transport of intranasally administered drugs at the FUS-targeted brain location.250 Experiments have shown that FUSIN can safely and effectively deliver AAV to spatial target brain locations, showing the potential to deliver OVs to glioma tumors.251
4.2 Antitumor immunity and antiviral response
Besides the direct oncolysis, OVs can enhance innate or adaptive immune responses to exert antitumor. However, the immune response can also inhibit viral replication due to the antigenicity of the oncolytic virus itself, allowing the body to produce antibodies that neutralize the virus, thereby culminating in the clearance of viruses and reducing the therapeutic effect.252 Therefore, how to balance the immune response and the antiviral response to support the virus to exert its antitumor function is a major challenge.
Viral infection of peri-tumoral cells can trigger an innate antiviral inflammatory response that releases a variety of stimulating cytokines, such as IFN that can trigger an antiviral response in TME. Several studies have shown that upregulation of endogenous IFN signaling levels is detrimental to the treatment of OVs.253 Therefore, combination therapy with OV and interferon signaling modulators/inhibitors is a strategy to reduce antiviral response immune. Ruxolitinib, JAK/STAT pathway inhibitors that can modulate the type I IFN response, increases the oncolytic efficiency of MV in patient-derived GBM xenografts.254 In addition, rapamycin and cyclophosphamide (CPA), which can inhibit or delay antiviral activity in the body, have been used to improve therapeutic outcomes of oncolytic virus in malignant glioma tumor models.255
To protect OV from nAbs that are produced by self-immunogenicity, encapsulation of OVs has been extensively explored. Masking viral surface proteins with biomaterials such as polymers, nanoparticles, liposomes or copolymers enhances protection against nAbs during delivery and targets tumors to some extent. Polyethylene glycol (PEG), polysaccharides, cationic polymers, and cationic lipids are the most commonly used biomaterials.256 However, the strategies still have limitations. Chemical modification approaches are not usually heritable and viral bioactivity might be impaired by excessive shielding. Another method of shielding is the use of extracellular vesicles (EVs), which are lipid-bilayer membrane structures secreted by most cells. EVs can cross the BBB and placental barrier more efficiently, and have great potential as a carrier of natural medicine.257 Tumor cell-derived microparticles have proven to be effective vectors for oncolytic adenoviruses.258 Cell-based delivery vectors are among the most promising delivery vectors for OVs. OVs loaded into cells can circumvent the recognition of OVs by the main immune system of the host. Mesenchymal cells and NSCs have been shown to have tumor-homing properties so they can be used to deliver OVs to tumors.259, 260 In clinical trials, BM-hMSCs and NSCs are being applied to deliver oncolytic adenovirus to treat gliomas. Methods for genetically modifying oncolytic virus surface proteins have been developed to make OVs more resistant to the host's immune system. Surface antigens of OVs can be replaced by those of other viruses so that they escape from pre-existing immune responses. VSV-GP enhanced infectivity for glioma cells and demonstrated resistance to complement-mediated inactivation.261, 262 Besides, Ana Mato-Berciano et al generated an oncolytic adenovirus with the albumin-binding domain (ABD) on the hexon that also can evade neutralizing antibodies.263
4.3 Immunosuppressive TME
Malignant gliomas have a highly immunosuppressive TME that becomes resistant to immunotherapy. To improve antitumor immune responses, we need a thorough understanding of the TME of gliomas. Among the components of the TME are extracellular matrix (ECM), signaling molecules, stromal cells, and immune cells.264 The composition of the ECM and hardness of the tumor-associated ECM change in GBM patients.265 The spread of OVs is hindered. Tumor-associated myeloid cells (TAMCs) are key drivers of immunosuppression in the TME, constituting up to as much as 50% of the cellular mass in tumors.266 TAMs, one of the most numerous subtypes of TAMCs, can release immunosuppressive factors to exert immunosuppressive effects in the TME and negatively correlate with OS in malignant glioma patients.267 Myeloid-derived suppressor cells (MDSCs) are other cells with potent immunosuppressive effects in the TME. In addition to secreting immunosuppressive cytokines, MDSCs can inactivate CD8 cells and disable TCR.268 T cells play a vital role in the adaptive immune response to malignancies. However, cytotoxic T cells are downregulated in the TME of gliomas due to T cell aging, depletion, tolerance, and incompetence.35 NK cells are affected by immunosuppressive factors within TME. The unique MHC-I molecule expressed by glioma cells can also bind to receptors on the surface of NK cells, thereby inhibiting their function.269 DCs are also affected by immunosuppressive TME to induce the regulatory of phenotype. These regulatory DCs can in turn activate Tregs and downregulate CD8 T cells recruitment.270 To summarize, inhibition of immune effector cells and activation of immunosuppressive cells result in a highly immunosuppressive TME for gliomas.
Oncolytic viral therapy disrupts immunosuppressive TME to some extent by causing tumor cells to lysis, releasing TAAs, and inducing innate immunity. Several OVs have been shown to induce immune cell responses, such as DNX-2401, ONYX-015, oncolytic reovirus, and oncolytic parvovirus. Engineering OVs to express immunostimulatory factors is a potential approach to achieve higher levels of pro-inflammatory factors in the TME to effectively turn tumors from “cold” to “hot.” Some recombinant OVs expressing cytokines or chemokines have shown good antitumor activity in glioma models. DNX-2440 stimulated T-cell responses in tumors and prolonged survival rate in glioma mouse models, which is currently undergoing in clinical (NCT03714334). G47Δ-mIL-12 has significantly enhanced survival of mouse in orthotopic U87 and human GSC-derived GBM xenograft models.271 M032, IL-12-encoding HSV, is currently being testing in a phase I trial in glioblastoma patients (NCT02062827). FLT3L is a vital growth factor for DCs that can induce of antitumor immune responses by increasing DC numbers, and FLT3L-expressing Ad or HSV improved survival in mouse glioma models.272, 273 JX-594, VV with expressing GM-CSF, has shown potential anti-glioma activities in both in vitro and immunocompetent rodent models.274
4.4 Improve oncolytic efficiency
Although OVs as a single drug has shown great promise for brain tumors, the therapeutic efficiency of OVs still needs to be improved. So far, many researchers have focused on the engineered oncolytic virus itself to improve its antitumor activity. In addition to the small space for modification and high technical difficulty, excessive modification of the virus skeleton will reduce the infectious capacity and oncolytic function of OVs. More and more evidence suggested that the infection and replication of OVs in tumors can activate antitumor immunity, turning “cold” tumors into “hot” tumors, thereby enhancing the effectiveness of treatment.12 Therefore, combining OVS with chemotherapy drugs or immunotherapy is an effective way to improve the efficacy of patients with brain tumors.
Chemoradiotherapy is the most traditional method for glioma, and their combination with oncolytic virus has shown certain application prospects. OVs combined with radiotherapy have the synergy. In pHGG and DIPG models, DNX-2401 in combination with radiotherapy produced a synergistic anti-glioma effect.275 Chemotherapy can improve the treatment effectiveness of oncolytic virotherapy by helping OVs to evade antiviral immune responses. Many studies have shown that combination therapy between OV and TMZ shows synergistic antitumor activity. TMZ in combination with DNX-2401 can improve CD8+ T cell infiltration and significantly improve survival in C57BL/6 mice carrying intracranial GL261 tumors.187 Good results have also been shown in 31 patients with recurrent Glioblastoma (NCT01956734). However, experiments have shown that TMZ does not improve the survival rate of G47Δ-IL12-treated tumor-in-situ mice, but reduces the therapeutic effect of G47Δ-IL12.276 Cyclophosphamide, an immunosuppressive drug, can improve the antitumor efficacy of Ad, HSV, MV, and reovirus by inducing apoptotic cell death of tumor cells and affecting the innate and adaptive immune responses.277 The prodrug 5-FC is also in combination with Toca511 and TG6002 to treat gliomas, which are under clinical evaluation.278 Overall, the combination of OVs with chemoradiotherapy is additive and synergistic. However, to reach its full potential, the optimal conditions for the use of the clinical combination should be carefully considered.
Oncolytic virotherapy is usually combined with ICIs to increase its potency. It is known that OVs can promote immune cell infiltration and increase PD-L1 expression in tumors. Positive results have been demonstrated in a number of preclinical and clinical trials. Belcaid et al demonstrated that Delta24-RGD combined with PD-1 blockade resulted in increased OS in both the GL261 and CT2A glioma model, with a low dose of the Delta24-RGD upregulated PD-1 expression on CD8 T cells and significantly altered the immune microenvironment in murine glioma.279 Furthermore, an ongoing phase II clinical trial is investigating DNX-2401 combined with pembrolizumab in GBM patients (NCT02798406). An increase in PD-1/PD-L1 axis expression in tumors is induced by reovirus, and PD-1 blockade enhanced systemic therapy in the GL261 glioma model by reovirus.177 Highly effective antitumor responses and improved long-term survival were demonstrated when α-PD1 therapy was combined with oncolytic VV expressing IL-21 treatment.280 MV in combination with anti-PD-1 antibody blockade overcome immunosuppression and enhance antitumor activity against GBM, thus improving therapeutic outcome.281 Saha et al. used a triple-combination approach in combination with anti-CTLA-4, anti-PD-1, and intra-tumoral G47Δ-mIL12 treatment due to the two independent immune inhibitory pathways. Indeed, triple combination therapy cured 77% of treated mice with 005-derived tumors.282 Therefore, the combined application of multiple immunotherapies may be the focus of future research to achieve improved antitumor effects.
Oncolytic virotherapy in combination with CAR T cell therapy is another strategy to improve the oncolytic potency. The viral infection-induced local inflammatory reaction may reverse the immunosuppressive TME and enhance more efficient homing and penetration of CAR-T cells into the tumor stroma.283 IL-7-loaded oncolytic adenovirus and B7H3-CAR-T combination prolongs survival and reduces tumor burden because of the enhanced T cell proliferation and reduced T cell apoptosis.284 Lp2-CAR-transduced T cells (Lp2-CAR-T) specifically targeted podoplanin-expressing glioma cells and were also able to kill patient-derived GSCs. Combining Lp2-CAR-T with G47Δ can further inhibit tumor growth and improve survival.285 The synergy between OVs and CAR-NK cells has also been assessed. Ma et al. generated an oHSV-1 expressing human IL15/IL15Rα sushi domain fusion protein (OV-IL15C). OV-IL15C plus EGFR-CAR NK cells synergistically suppressed tumor growth and significantly improved survival in an immunocompetent model by increasing intracranial infiltration and activation of NK and T cells and elevated persistence of CAR NK cells.286 Overall, combination therapy with oncolytic virus and CAR T cells may improve antitumor efficacy. However, no clinical trials have been carried out in patients with gliomas.
5 CONCLUSION
In this review, we focused on the application of OVs for gliomas in clinic. With good experimental results, OVs will become an emerging option for treating gliomas. However, due to the BBB, immunosuppressive TME, and polymer heterogeneity of glioma patients, and the delivery and efficacy of oncolytic virus, there are still certain shortcomings in the application of oncolytic virus in clinical glioma treatment. Therefore, the treatment strategies of oncolytic virus for glioma patient need to be continuously explored and optimized. First, the use of molecular biology engineering to modify OVs can further enhance the recognition and intracellular proliferation of tumor cells, weaken the antiviral effect, enhance the antitumor ability, and make oncolytic virus expected to become an important antitumor drug. In addition, modified OVs in combination with chemotherapy, radiotherapy, ICIs, and CAR-T cell therapy can maximize the therapeutic effect.
In addition to the treatment of glioma, oncolytic virus therapy also shows good treatment effects in various tumors. T-VEC was approved by FDA for unresectable stage III and IV melanoma in 2015.287 ONCOS-102, an oncolytic adenovirus expressing GM-CSF, is fast-track certified by the FDA for the treatment of PD-1 refractory advanced melanoma based on the good efficacy of ONCOS-102 clinical research.288 Other OVs such as ICOVIR-5, PVSRIPO, and coxsackievirus A21 are also performed in melanoma early clinical trials.289-291 Many OVs have shown efficacy and positive clinical outcomes in clinical trials in colorectal cancer, including enadenotucirev, reolysin, JX-594, and NDV.292-294 Reolysin was examined its effects in early breast cancer and metastatic triple-negative breast cancer (TNBC). AdV-tk and T-VEC are also in clinical trials in TNBC. AdV-tk is well tolerated and has excellent safety profile, 21.43% of patients showed clinical benefit and patients with clinical benefit had a durable response. The results suggest that the addition of TVEC may increase the complete response rate.295 Furthermore, preclinical studies and early clinical trials have found that oncolytic virus therapy in these malignant tumors such as pancreatic cancer, malignant pleural mesothelioma, cervical cancer, and bladder cancer shows certain therapeutic potential and good safety.296 These good treatment effects also show that oncolytic virus will become the main force of tumor treatment in the future.
AUTHOR CONTRIBUTIONS
Die Hu: Data curation (lead); formal analysis (lead); writing—original draft (lead); writing—review and editing (lead). Yaomei Tian: Data curation (supporting); formal analysis (equal); writing—original draft (lead); writing—review and editing (equal). Jie Xu: Writing—original draft (supporting); writing—review and editing (equal). Daoyuan Xie: Writing—original draft (supporting); writing—review and editing (equal). Yusi Wang: Writing—original draft (supporting); writing—review and editing (equal). Mohan Liu: Writing—original draft (supporting); writing—review and editing (equal). Yuanda Wang: Writing—original draft (supporting); writing—review and editing (equal). Li Yang: Conceptualization (lead); funding acquisition (lead); supervision (lead). All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
All images, including the graphical abstract image and the figures, were created with BioRender (https://www.biorender.com/). This study was supported by the Natural Science Foundation Project of Sichuan under grant number 2022NSFSC0848; by China Postdoctoral Science Foundation under grant number 2018M643466; by 1.3.5 project for disciplines of excellence (No. 39ZYGD18007), West China Hospital, Sichuan University.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
Open Research
DATA AVAILABILITY STATEMENT
The authors have nothing to report.




