Causality between COVID-19 and female reproductive function: A Mendelian randomization study
Bowen Zhang, Jixue Xu, and Junzhi Liang contributed equally to this study.
Abstract
Coronavirus disease 2019 (COVID-19) has experienced a global pandemic, and currently, the emergence of its variants has posed challenges in terms of prevention and treatment. Nonetheless, the effect of COVID-19 infection on female reproductive function is unclear. This study aimed to systematically evaluate for the first time the causal effect of COVID-19 on female reproductive function. Genetic correlations were assessed using linkage disequilibrium score regression. Mendelian randomization (MR) analysis was performed using summary statistics of two variables, including COVID-19 severity and eight female reproductive traits. The three degrees of severity had genetically significant associations with sex hormone-binding globulin (SHBG) concentrations (rg = –0.153, p = 0.004; rg = –0.187, p < 0.001; rg = –0.180, p = 0.003). Additionally, MR showed that SHBG (β = –0.020, p = 0.040) and total testosterone levels (β = –0.061, p = 0.009) followed a decreasing trend, as the COVID-19 infection higher. No significant genetic association was found between COVID-19 infection and total estradiol concentrations, menstruation, and female infertility. Simultaneously, MR found no causal relationships between COVID-19 infection and total estradiol concentrations, menstruation, and female infertility (all p > 0.05). In conclusion, COVID-19 was causally associated with lower SHBG and total testosterone concentrations, offering invaluable insights that will help guide clinical decision-making.
1 INTRODUCTION
Coronavirus disease 2019 (COVID-19), a severe pandemic caused by severe acute respiratory syndrome coronavirus 2, has become a threat to global health, with over 767 million diagnosed cases reported as of July 12, 2023, according to the World Health Organization (https://covid19.who.int/). Recent studies have indicated that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron sublineages, such as XBB.1, exhibit enhanced antibody evasion, presenting a significant challenge in the prevention and treatment of COVID-19.1 Furthermore, it is essential to understand the effect of COVID-19 on the reproductive system because it affects fertility policies in multiple regions.2
Multiple studies have suggested that COVID-19 might influence female reproductive characteristics, including the menstrual cycle, ovarian reserve, and sex hormone levels.3, 4 Two observational studies reported that 28% and 16% of female patients with COVID-19 experienced irregular menstruation respectively, which was associated with disease severity.4, 5 Two cases of premature ovarian failure secondary to COVID-19 infection have been reported.6, 7 In addition, an observational study also found that females after COVID-19 infection had lower serum anti-Müllerian hormone levels and higher levels of prolactin and testosterone.8
In contrast, some studies have reported that COVID-19 does not affect women's reproductive function. The findings from two observational studies failed to substantiate the influence of mild SARS-CoV-2 infection on female fertility and ovarian reserve.9, 10 Furthermore, one additional study has provided evidence that SARS-CoV-2 infection does not disrupt ovarian follicular function.11 This inconsistency may be attributed to biases, such as a small sample size, reverse causality, and confounding effects of lifestyle and environmental factors. Currently, the causal relationships between COVID-19 and female reproductive function are still unclear and require further investigation.
Mendelian randomization (MR) is widely used to assess the causal relationships between exposures and outcomes using single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs).12 This approach can substitute randomized controlled trials effectively and minimize the interference of confounders and reverse causation.12
In the present study, a two-sample MR analysis was employed for the first time to comprehensively investigate the causal relationships between three distinct severity levels of COVID-19 infection (including severely ill COVID-19, hospitalized COVID-19, and SARS-CoV-2 infection) and eight female reproductive functions, respectively. The findings of the present study unveiled a causal association between COVID-19 and lower concentrations of sex hormone-binding globulin (SHBG) and total testosterone, offering invaluable insights that will help guide clinical decision-making.
2 RESULTS
2.1 Genetic correlations between COVID-19 and female reproductive function
We estimated the genetic correlations between COVID-19 and female reproductive function. Since linkage disequilibrium score (LDSC) regression cannot avoid interference from confounding factors, we used genetic correlations that remained significant after multiple testing corrections as supplements to MR. The LDSC analysis revealed a significant and negative association between COVID-19 and SHBG concentrations (severely ill COVID-19: rg = –0.156, p = 0.006; hospitalized COVID-19: rg = –0.197, p < 0.001; SARS-CoV-2 infection: rg = –0.188, p = 0.002). These findings were consistent when using standard data (severely ill COVID-19: rg = –0.153, p = 0.004; hospitalized COVID-19: rg = –0.187, p < 0.001; SARS-CoV-2 infection: rg = –0.180, P = 0.003). In addition, the genetic association between COVID-19 and age at menopause was revealed as well. We only found suggestive causal correlations between age at menopause and three different levels of COVID-19 infection in the ReproGen Consortium (Reprogen) data (severely ill COVID-19: rg = 0.120, p = 0.029; hospitalized COVID-19: rg = 0.172, p = 0.010; SARS-CoV-2 infection: rg = 0.156, p = 0.011) (Figure 1 and Supporting Information: Tables 1–3).
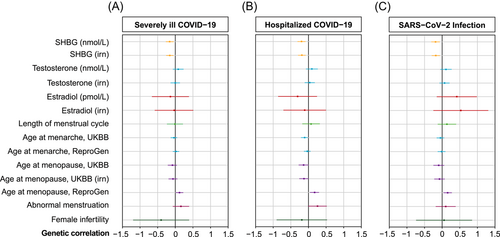
2.2 IVs were strictly screened and shown to possess significant statistical power
The number of patients with severely ill, hospitalized, and infected was 378,521, 876,382, and 1,253,716, respectively (Supporting Information: Tables 4–6). Eight, seven, and four SNPs were selected as IVs from genome-wide association study (GWAS) for these groups, respectively (Table 1). These independent SNPs reached genome-wide significance (p < 5 × 10−8, r2 = 0.001, clumping window size = 10 MB), and minor allele frequency (MAF) > 1% indicated the absence of rare SNPs. During MR analysis, rs2237698 was unavailable in UK Biobank (UKBB) and thus was excluded when analyzing this data set. In addition, two data sets from the Reprogen database—“age at menarche” and “age at menopause”—were included to increase the reliability of the results. However, rs35081325 was ambiguous as a palindromic SNP and was removed from the second data set. All SNPs had an F-statistic greater than 10 in the range of 31–239.
| Exposure | SNP | Effect allele | Other allele | β | SE | p Value | MAF | F-statistic |
|---|---|---|---|---|---|---|---|---|
| Severely ill COVID-19 | rs35081325 | T | A | 0.625 | 0.046 | 1.13E−41 | 0.08 | 183 |
| rs111837807 | C | T | 0.318 | 0.044 | 7.31E−13 | 0.13 | 51 | |
| rs622568 | C | A | 0.255 | 0.039 | 6.86E−11 | 0.17 | 43 | |
| rs2237698 | T | C | 0.239 | 0.041 | 6.59E−09 | 0.10 | 34 | |
| rs10735079 | A | G | 0.211 | 0.029 | 7.91E−13 | 0.30 | 51 | |
| rs77534576 | T | C | 0.467 | 0.079 | 2.93E−09 | 0.04 | 35 | |
| rs2109069 | A | G | 0.276 | 0.030 | 1.63E−20 | 0.33 | 86 | |
| rs2834163 | A | G | −0.189 | 0.030 | 4.16E−10 | 0.36 | 39 | |
| Hospitalized COVID-19 | rs41264915 | G | A | −0.205 | 0.036 | 1.02E−08 | 0.08 | 33 |
| rs35081325 | T | A | 0.546 | 0.035 | 7.93E−54 | 0.09 | 239 | |
| rs111837807 | C | T | 0.216 | 0.034 | 3.22E−10 | 0.10 | 40 | |
| rs622568 | C | A | 0.178 | 0.030 | 4.36E−09 | 0.18 | 34 | |
| rs1859330 | A | G | 0.156 | 0.023 | 4.91E−12 | 0.30 | 48 | |
| rs2109069 | A | G | 0.187 | 0.024 | 1.96E−15 | 0.32 | 63 | |
| rs13050728 | C | T | −0.186 | 0.024 | 6.72E−15 | 0.36 | 61 | |
| SARS-CoV-2 infection | rs35508621 | C | T | 0.191 | 0.020 | 1.92E−22 | 0.09 | 95 |
| rs579459 | T | C | −0.088 | 0.013 | 4.51E−11 | 0.21 | 43 | |
| rs1859330 | A | G | 0.072 | 0.012 | 1.26E−09 | 0.32 | 37 | |
| rs2109069 | A | G | 0.070 | 0.013 | 3.01E−08 | 0.32 | 31 |
- Note: The β coefficient represents the change in exposure associated with the effect allele compared to the other allele. All the instrumental variables of COVID-19 included exhibit strong correlations with exposure (p < 5 × 10−8), and the MAF exceeds 0.01, thereby ruling out rare SNPs. The F-statistic of all instrumental variables is greater than 10, mitigating the presence of weak instruments.
- Abbreviations: COVID-19, coronavirus disease 2019; MAF, minor allele frequency; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SE, standard error; SNP, single-nucleotide polymorphism.
2.3 Causal association between COVID-19, and SHBG and total testosterone concentrations
In this investigation, the LDSC analysis encompassed the complete GWAS data, while the MR analysis focused on genome-wide significant SNP (Figure 2).
We used raw and standard data to improve the reliability of the results. Three causal relationships were identified. In particular, MR suggested that severely ill COVID-19 was causally linked with SHBG levels (β = –0.605, SE = 0.273, p = 0.027); similar results were obtained using standard data: β = –0.020, SE = 0.010, p = 0.040. The estimates of MR Egger (raw data: β = –1.326, SE = 0.706, p = 0.119; standard data: β = –0.040, SE = 0.026, p = 0.183), and weighted median (raw data: β = –0.490, SE = 0.280, p = 0.080; standard data: β = –0.013, SE = 0.008, p = 0.137) were not significant but were in the same direction. In addition, no causal relationship was found between hospitalized COVID-19 and SHBG (raw data: β = –0.423, SE = 0.315, p = 0.180; standard data: β = –0.013, SE = 0.010, p = 0.211) or total testosterone (raw data: β = 0.004, SE = 0.010, p = 0.660; standard data: β = 0.009, SE = 0.021, p = 0.677).
Furthermore, SARS-CoV-2 infection might casually affect the concentrations of SHBG (raw data: β = –2.000, SE = 0.981, p = 0.041) and testosterone (standard data: β = –0.061, SE = 0.023, p = 0.009) (Table 2). However, the results were inconsistent between the raw and standard data (standard data of SHBG: β = –0.060, SE = 0.035, p = 0.084; raw data of testosterone: β = –0.024, SE = 0.015, p = 0.095). For sensitivity analyses, the direction of β values obtained by weighted median and MR Egger was the same as that obtained using inverse-variance weighted (IVW). Moreover, the causal effects were similar between the weighted median (raw data of SHBG: β = –2.127, SE = 0.923, p = 0.021; standard data of SHBG: β = –0.062, SE = 0.029, p = 0.031; standard data of testosterone: β = –0.067, SE = 0.028, p = 0.016) and IVW but different from those obtained with MR Egger (p > 0.05 of all). After multiple testing corrections, all causal effects of COVID-19 on SHBG and total testosterone concentrations were null (p < 0.00625) and were regarded as potentially significant.
| Exposure | Outcome | nSNP | β | 95% CI | p Value |
|---|---|---|---|---|---|
| Severely ill COVID-19 | |||||
| SHBG (nmol/L) | 7 | −0.605 | −1.140, −0.070 | 0.027 | |
| SHBG (irn) | 7 | −0.020 | −0.038, −0.001 | 0.040 | |
| Testosterone (nmol/L) | 7 | 0.002 | −0.012, 0.017 | 0.753 | |
| Testosterone (irn) | 7 | 0.004 | −0.025, 0.033 | 0.799 | |
| Hospitalized COVID-19 | |||||
| SHBG (nmol/L) | 7 | −0.423 | −1.040, 0.195 | 0.180 | |
| SHBG (irn) | 7 | −0.013 | −0.033, 0.007 | 0.211 | |
| Testosterone (nmol/L) | 7 | 0.004 | −0.015, 0.024 | 0.660 | |
| Testosterone (irn) | 7 | 0.009 | −0.032, 0.049 | 0.677 | |
| SARS-CoV-2 infection | |||||
| SHBG (nmol/L) | 4 | −2.000 | −3.923, −0.078 | 0.041 | |
| SHBG (irn) | 4 | −0.060 | −0.129, 0.008 | 0.084 | |
| Testosterone (nmol/L) | 4 | −0.024 | −0.053, 0.004 | 0.095 | |
| Testosterone (irn) | 4 | −0.061 | −0.107, −0.015 | 0.009 | |
- Note: The β coefficient represents the amount of change in the dependent variable when the independent variable increases or decreases by one standard deviation. Suggestive causal relationships between the exposure and the outcome are indicated by bold p values. Mendelian randomization was performed using the inverse-variance weighted method.
- Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; irn, rank-based inverse normal transformation; nSNP, number of single-nucleotide polymorphism; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SHBG, sex hormone-binding globulin.
Heterogeneity was corrected after removing outliers, except for the association between severely ill COVID-19 and SHBG levels in the standard data (p = 0.025). No directional pleiotropy was detected using MR-Egger intercept test (p > 0.05). For these potential causal effects, leave-one-out sensitivity analysis showed that no single SNP significantly contributed to the relationship between COVID-19 and the concentrations of SHBG and total testosterone (Figure 3). MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) analysis showed that no outliers in IVs existed in MR analysis of COVID-19 on SHBG and total testosterone (Supporting Information: Tables 7 and 8).
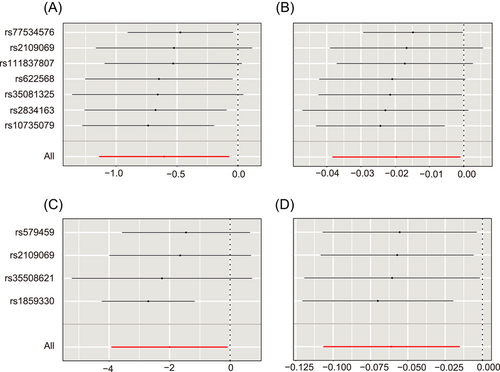
In summary, these results indicated that severely ill COVID-19 had a potential causal relationship with lower concentrations of SHBG and that SARS-CoV-2 infection had a potential causal relationship with lower concentrations of SHBG and total testosterone.
2.4 COVID-19 was not associated with total estradiol levels, menstruation, and female infertility
Regardless of disease severity, MR analysis found no causal relationships between COVID-19 and total estradiol concentrations (β = 2.699; β = 6.081; β = 15.132), menstruation (length of menstrual cycle [β = –0.008; β = –0.007; β = –0.085], age at menarche [UKBB: β = 0.0002; β = 0.001; β = 0.032; Reprogen: β = –0.019, β = –0.016, β = 0.002], age at menopause [UKBB: β = 0.080; β = 0.102; β = 0.232; Reprogen: β = 0.003, β = 0.018, β = –0.013], excessive, frequent, and irregular menstruation [odds ratio, OR = 1.000; OR = 1.001; OR = 1.003]), and female infertility (OR = 1.000; OR = 1.000; OR = 1.001) (all p > 0.05) (Table 3). We used data from the UKBB and Reprogen databases for two variables—age at menarche and age at menopause—to increase the reliability of the findings. Standard data were also provided when accessible. There was high consistency between outcome data.
| Exposure | Outcome | nSNP | Estimatea | 95% CI | p Value |
|---|---|---|---|---|---|
| Severely ill COVID-19 | |||||
| Estradiol (pmol/L) | 7 | 2.669 | −8.782, 14.120 | 0.648 | |
| Estradiol (irn) | 7 | −0.004 | −0.029, 0.022 | 0.782 | |
| Length of menstrual cycle | 7 | −0.008 | −0.032, 0.016 | 0.510 | |
| Age at menarche, UKBB | 7 | <0.001 | −0.018, 0.019 | 0.982 | |
| Age at menarche, Reprogen | 7 | −0.019 | −0.05, 0.011 | 0.215 | |
| Age at menopause, UKBB | 7 | 0.080 | −0.058, 0.217 | 0.256 | |
| Age at menopause, UKBB (irn) | 7 | 0.013 | −0.014, 0.039 | 0.354 | |
| Age at menopause, Reprogen | 8 | 0.003 | −0.065, 0.07 | 0.937 | |
| Excessive, frequent, and irregular menstruationb | 7 | 1.000 | 0.997, 1.003 | 0.983 | |
| Female infertilityb | 7 | 1.000 | 0.999, 1.001 | 0.737 | |
| Hospitalized COVID-19 | |||||
| Estradiol (pmol/L) | 7 | 6.081 | −8.259, 20.422 | 0.406 | |
| Estradiol (irn) | 7 | −0.002 | −0.031, 0.028 | 0.910 | |
| Length of menstrual cycle | 7 | −0.007 | −0.038, 0.023 | 0.638 | |
| Age at menarche, UKBB | 7 | 0.001 | −0.022, 0.024 | 0.936 | |
| Age at menarche, Reprogen | 6 | −0.016 | −0.062, 0.029 | 0.481 | |
| Age at menopause, UKBB | 7 | 0.102 | −0.071, 0.274 | 0.247 | |
| Age at menopause, UKBB (irn) | 7 | 0.017 | −0.016, 0.05 | 0.320 | |
| Age at menopause, Reprogen | 7 | 0.018 | −0.052, 0.087 | 0.615 | |
| Excessive, frequent, and irregular menstruationb | 7 | 1.001 | 0.998, 1.005 | 0.501 | |
| Female infertilityb | 7 | 1.000 | 0.999, 1.001 | 0.750 | |
| SARS-CoV-2 infection | |||||
| Estradiol (pmol/L) | 4 | 15.132 | −29.129, 59.392 | 0.503 | |
| Estradiol (irn) | 4 | 0.041 | −0.046, 0.129 | 0.352 | |
| Length of menstrual cycle | 4 | −0.085 | −0.215, 0.046 | 0.204 | |
| Age at menarche, UKBB | 4 | 0.032 | −0.02, 0.084 | 0.223 | |
| Age at menarche, Reprogen | 4 | 0.002 | −0.053, 0.057 | 0.945 | |
| Age at menopause, UKBB | 4 | 0.232 | −0.061, 0.526 | 0.121 | |
| Age at menopause, UKBB (irn) | 4 | 0.046 | −0.011, 0.103 | 0.111 | |
| Age at menopause, Reprogen | 4 | −0.013 | −0.175, 0.148 | 0.870 | |
| Excessive, frequent, and irregular menstruationb | 4 | 1.003 | 0.995, 1.012 | 0.421 | |
| Female infertilityb | 4 | 1.001 | 0.998, 1.004 | 0.572 | |
- Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; estradiol, total estradiol; irn, rank-based inverse normal transformation; nSNP, number of single-nucleotide polymorphism; Reprogen, the ReproGen Consortium; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UKBB, UK Biobank.
- a Odds ratio for binary variables and the β coefficient for all other outcomes. The β coefficient represents the amount of change in the dependent variable when the independent variable increases or decreases by one standard deviation.
- b Binary variables. MR was performed using the inverse-variance weighted method.
The directional pleiotropy of IVs for MR analysis was not supported by the MR-Egger intercept test (p > 0.05). There was no significant change in the observed correlation; furthermore, the results of Cochran's IVW Q test showed no significant heterogeneity (p > 0.05) after the removal of outliers detected by MR-PRESSO (Supporting Information: Tables 8 and 9); this indicated the robustness of the findings. The leave-one-out analysis showed no single large effect dominating the analysis and identified the stability of SNPs included in each MR analysis.
In conclusion, there was no causal association between COVID-19 and total estradiol levels, menstruation, and female infertility.
3 DISCUSSION
To our knowledge, this is the first study using MR analysis to comprehensively investigate the causality between COVID-19 and female reproductive function. In this study, genetic evidence supported that COVID-19 was causally associated with lower SHBG and total testosterone concentrations but had no causal relationship with total estradiol concentrations, menstruation, and female infertility.
Studies have investigated the mechanisms underlying the effect of COVID-19 on female reproduction, which can be quite complicated. One statement is that SARS-CoV-2 may damage ovarian function by impairing angiogenesis and follicular development in the follicular microenvironment.13 This virus may infect ovary cells expressing the angiotensin-converting enzyme 2 receptor.14 Follicular fluids past COVID-19 decreased the expression of steroid production parameters and vascular endothelial growth factor in granulosa cells and damaged the migration of vascular endothelial cells.13 Another statement is that SARS-CoV-2 infection may indirectly affect gonadal function by causing inflammation15 which could further decrease sex hormone levels.16 This may partly explain the causal effect of COVID-19 infection on the levels of total testosterone and SHBG. Additionally, the release of inflammatory factors may also lead to an increase in testosterone clearance, and the substantial releases of proinflammatory cytokines caused the upregulation of aromatase enzyme production, which accelerated the conversion of testosterone into estradiol,17 which might also result in reduction of testosterone and SHBG after COVID-19 infection. However, these changes in hormone levels may only be present in recent exposures, and soon disappear in the short run,2 and the underlying mechanisms still require to be further studied.
We found no causal relationship between COVID-19 and total estradiol concentrations, menstruation, and female infertility, consistent with several previous studies.11, 18, 19 Nonetheless, the results on the relationship between COVID-19 and female reproduction are inconsistent,3-5, 11, 18-21 which may be due to several factors. First, psychological stress and anxiety caused by COVID-19 may lead to temporary changes in female reproductive function. Consistent with this hypothesis, Li et al.5 observed that menstruation returned to normal in 99% of patients with COVID-19 within 2 months after infection. Second, there may be a reverse causal relationship between COVID-19 and sex hormones. For instance, patients with COVID-19 are generally older.19 Moreover, estradiol has an anti-inflammatory effect on SARS-CoV-2; thus, lower estradiol levels in postmenopausal women may increase the susceptibility to COVID-19.22-24 Third, observational results may be affected by confounding factors, such as differences between populations and research methods, small sample size, or the high incidence of COVID-19 in certain populations.
This study has several strengths. First, we used MR and LDSC simultaneously. While MR uses strongly linked and independent SNPs,25 LDSC employs summary statistics of GWAS,26 thus preventing the loss of crucial information caused by linkage disequilibrium pruning in MR. Furthermore, MR, but not LDSC, can reduce the interference of confounding factors on the results by searching the Phenoscanner database, removing pleiotropic genetic variants, and rejecting reverse causation because genetic variation begins at birth. Consequently, our findings are based on MR analysis augmented by LDSC results. Second, several sensitivity and heterogeneity analyses were conducted to evaluate the effectiveness of IVs, and the results were highly consistent, further increasing the reliability of MR results. Third, patients with COVID-19 included in this study were refined to severely ill, hospitalized, and infected in the context of illness severity, and eight indicators of female reproductive function were analyzed in these three groups, improving the reliability of the results. However, this study also has limitations that may affect the interpretation of our results. The genetic data were obtained from European cohorts, potentially leading to bias due to population stratification, limiting the applicability of the results to other populations.
4 CONCLUSION
In summary, MR analysis demonstrated that COVID-19 was causally associated with lower SHBG and total testosterone concentrations but not with total estradiol concentrations, menstruation, and female infertility. Additionally, the impact of COVID-19 on female reproductive function was largely independent of severity. COVID-19 infection does not significantly affect female reproduction. Future studies should assess the effects of COVID-19 on pregnant women, embryos, and fetuses and the long-term effects of COVID-19 on female reproductive function.
5 MATERIALS AND METHODS
5.1 Study design
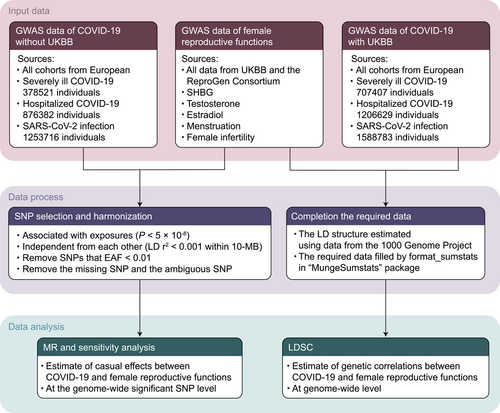
Figure 2 provides a concise overview of our study design. To explore the association between COVID-19 and female reproductive function, we employed two distinct methods: LDSC and MR. LDSC was utilized to investigate the genetic correlation using full GWAS data, while MR allowed us to examine the association at the genome-wide significant SNP level. These complementary approaches enabled a comprehensive exploration of the relationship between COVID-19 and female reproductive function, shedding light on potential genetic factors underlying this association.
5.2 Exposure data from GWAS
Exposure data were obtained from the COVID-19 Host Genetics Initiative GWAS.27 Data were obtained only from the European cohort, which was included to reduce racial bias. A total of 13, 20, and 34 cohorts were included for severely ill COVID-19, hospitalized COVID-19, and SARS-CoV-2 infection, respectively, encompassing case counts of 4297, 7703, and 22,581. The total numbers of individuals across the cohorts were 378,521, 876,382, and 1,253,716, respectively. The UKBB cohort was excluded from the analysis to avoid sample overlap. In COVID-19 cases, patients who required respiratory support due to SARS-CoV-2 infectious symptoms or whose primary cause of death was COVID-19 were defined as severely ill COVID-19; the hospitalized COVID-19 cases were those who were hospitalized with SARS-CoV-2 infectious symptoms; all the control groups were selected as genetically ancestry-matched samples.
5.3 Outcome data from GWAS
The outcomes consisted of eight indicators from UKBB28 and the ReproGen Consortium,25, 29 including SHBG (n = 166,235), total testosterone (n = 154,364), total estradiol (n = 40,997), length of menstrual cycle (n = 32,847), age at menarche (UKBB: n = 188,644; Reprogen: n = 252,514), age at menopause (UKBB: n = 111,593; Reprogen: n = 200,309), excessive, frequent, and irregular menstruation (n = 194,174), and female infertility (n = 194,174). SHBG, total testosterone, and total estradiol were individually measured using a Beckman Coulter Unicel Dxl 800 through a two-step sandwich immunoassay analysis, a one-step competitive analysis, and a two-step competitive analysis, respectively. Information regarding the length of the menstrual cycle, age at menarche, and age at menopause was obtained in UKBB through the administration of touchscreen questions to participants. The phenotypes of excessive, frequent, and irregular menstruation, as well as female infertility, were defined based on the International Classification of Diseases codes. Additionally, we obtained standardized (rank-based inverse normal transformed) data of continuous outcomes, when available.
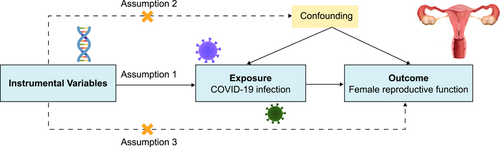
5.4 MR assumption and IV selection
MR was based on three assumptions: (1) IVs were significantly correlated with the exposure variable (COVID-19 infection); (2) IVs were not correlated with potentially confounding factors for female reproductive function; and (3) IVs were only associated with outcomes resulting from the exposure variable (Figure 4).25
Beneficial SNPs were selected as follows:
Inclusion criteria: (1) as IVs, SNPs had significant genome-wide associations with the exposure variable (p < 5 × 10−8) and were further filtered using a clumping r2 cutoff of 0.001 on a 10-Mb window; (2) all SNPs had a minor allele frequency of greater than 1%; (3) no association between SNPs and the confounding factors for outcomes was found in the PhenoScanner database (p < 5 × 10−8); (4) there was no strong correlation between the SNPs and the outcomes (p < 5 × 10−8); and (5) SNPs had an F-statistic value of greater than 10.
Exclusion criteria: (1) SNPs demonstrating palindromic properties; and (2) outliers identified using MR-PRESSO (excluded during the sensitivity analysis).
5.5 LDSC regression
LDSC regression calculates genome-wide genetic correlations based on GWAS summary data.30 In this study, LDSC regression was performed to determine the genetic correlations between COVID-19 and female reproductive function.30, 31 The linkage disequilibrium structure for European populations was estimated using data from the 1000 Genome Project. Since sample overlap was not considered in the LDSC study, we used GWAS data on European cohorts with COVID-19, including the UKBB cohort (https://github.com/bulik/ldsc).
5.6 Statistical analysis
The primary study used IVW MR, which assumes a zero intercept and calculates causality using a random-effect model.32 Multiple complementary methods were applied to assess the sensitivity of the causative effects, including weighted median, MR-Egger regression, MR-PRESSO, and leave-one-out analyses. The weighted median is a reliable estimation method if a minimum of 50% of the weight is attributed to valid genetic variants.33 However, MR Egger offers pleiotropy robust estimates, even when the proportion of valid genetic variants is lower than 50%.34 MR-PRESSO employs various methods to address horizontal pleiotropy, correct for it, and assess the impact of outlier removal on causal estimates. The global test of MR-PRESSO is designed to detect the presence of horizontal pleiotropy, while outlier removal is used to mitigate this concern. By removing outliers, MR-PRESSO leads to narrower confidence intervals. Heterogeneity across studies was checked using Cochran's IVW Q-statistic, and horizontal pleiotropy was detected using the MR-Egger intercept test,35 which evaluates pleiotropy through the intercept term, and a zero intercept implies no horizontal pleiotropy, thus providing independence from the direct effect assumption.
The strength of the IVs was measured using the formula F = βexposure2/SEexposure2. High strength was indicated by F > 10.36, 37 The strength of a causal association with binary outcomes was calculated using odds ratios and 95% confidence intervals, while the β coefficient and standard error were calculated for all binary outcomes. The β coefficient represents the amount of change in the dependent variable when the independent variable increases or decreases by one standard deviation. p < 0.00625 (0.05 divided by the eight outcomes: SHBG, total testosterone, total estradiol, length of menstrual cycle, age at menarche, age at menopause, excessive frequent and irregular menstruation, and female infertility) indicated strong significance and 0.00625 < p < 0.05 indicated suggestive significance. All p values were two-tailed. Statistical analysis was performed using the “TwoSampleMR” and “MRPRESSO” packages in R version 4.2.1.
AUTHOR CONTRIBUTIONS
Bowen Zhang, Jixue Xu, and Junzhi Liang contributed to the writing. Bowen Zhang, Mingjun Hao, and Jingzan Wei participated in the data extraction and statistical analyses. Junzhi Liang, Jixue Xu, and Yuexin Yu contributed to drawing figures. Da Li, Zhijing Na, and Yuanyuan Fang contributed to literature search, study design, data collection, data analysis, data interpretation, and writing. All authors have contributed to the manuscript and approved the submitted version. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank all investigators and participants from the COVID-19 Host Genetics Initiative, UK Biobank, and the ReproGen Consortium. In addition, Adobe Illustrator was used to create certain elements in figures. This work was supported by the National Natural Science Foundation of China (No. 82071607); LiaoNing Revitalization Talents Program (No. XLYC1907071); Fok Ying Tung Education Foundation (No. 151039); Outstanding Scientific Fund of Shengjing Hospital (No. 202003); 345 Talent Project of Shengjing Hospital of China Medical University (No. M1344).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Ethical approval was waived because the study used publicly available summary statistics without accessing individual-level data. Ethical approval was acquired for all included GWAS studies.
Open Research
DATA AVAILABILITY STATEMENT
All data used in this study are available in the public repository.




