Novel nanomaterials as photo-activated cancer diagnostics and therapy
Abstract
The word “theranostics” describes an emerging trend in medicine in which the distinction between diagnosis and therapy blurs. Light or photo is used in theranostics to obtain high precision and personalised treatment. As only malignant tissues need to be spared, photo-triggered theranostics provide highly selective targeting using real-time imaging. Using nanotechnology to organise a dual-modality approach is an efficient way to circumvent pharmacokinetic limitations. Photodynamic therapy has been used successfully in the clinic for a while now, and this has paved the path for photo-triggered theranostics to be developed. The use of light-activated theranostic nanoforms has progressed from preclinical studies in animals and labs to clinical trials in humans. As both nanomaterials and their methods of manufacture advance, the theranostic approach becomes more nuanced and may be used in a wider range of real-time imaging and therapy modalities. The depth of anatomical access is also expanding because of developments in light delivery technologies. Combined, these innovations will hasten early cancer diagnosis and make tailored treatment more feasible. A non-invasive assessment approach also increases patient compliance and reduces risk. Researchers constantly make strides in their effort to create more versatile photo-sensitive nanoparticles. With any luck, photo-triggered theranostics may significantly reduce toxicity. In order to provide a better and safer clinical outcome in cancer therapy, this review aims to highlight the latest and greatest innovation research in the domain of nanotheranostics and its photo-triggering, and to sketch the possibilities for further progression and integration of nanoconstructs and photo-delivery, and trying to target approach in photo-triggered theranostics.
Abbreviations
-
- BBB
-
- Blood-brain barrier
-
- CTAB
-
- Cetyltrimethylammonium bromide
-
- DDS
-
- Dose-dense-schedule
-
- DOX
-
- Doxorubicin
-
- ECM
-
- Extracellular matrix
-
- EGFR
-
- Epidermal growth factor receptor
-
- GNRs
-
- Gold nanoparticle
-
- HAase
-
- Hyaluronidase
-
- ICG
-
- Indocyanine green
-
- MMPs
-
- Matrix metalloproteinases
-
- MPS
-
- Monolayer phospholipid system
-
- MRI
-
- Magnetic resonance imaging
-
- MWCNTs
-
- Multi-walled carbon nanotubes
-
- NIR
-
- Near-infrared fluorescence
-
- NS
-
- Nanostructures
-
- PDT
-
- Photodynamic therapy
-
- PTCAs
-
- Photo thermal conversion agents
-
- PTT
-
- Photo-triggered theranostics
-
- QDs
-
- Quantum dots
-
- SPR
-
- Surface plasmon resonance
-
- SWNT
-
- Single-walled nanotube
-
- TME
-
- Tumour microenvironment
-
- UCNPs
-
- Upconversion nanoparticles
-
- UV
-
- Ultraviolet
1 INTRODUCTION
Due to its minimally invasive nature, rapid recovery time, and low risk of side effects, phototherapy has become more popular in recent years [1-3]. The “father of contemporary phototherapy” Niels Ryberg Finsen won the Nobel Prize in 1903 for his work in using sunlight and ultraviolet (UV) radiation to treat a skin disease (lupus vulgaris) [4]. Since then, biomedical engineers have made significant advancements in the use of phototherapy for the treatment of a wide variety of medical and mental health ailments, including but not limited to eczema, psoriasis, and itchy skin. Circadian rhythm disturbances include SAD [5, 6], non-seasonal depression [7], and insomnia [8]. Phototherapy may be an even more successful strategy for the ablation of tumours when used in conjunction with phototherapeutic drugs such photosensitizers and photo thermal conversion agents (PTCAs) [9].
Cancer phototherapy makes use of ultraviolet (UV) light (100–400 nm), visible (400–760 nm), and near-infrared (760-1350 nm) light. Surface malignancies respond well to conventional phototherapy; nevertheless, both ultraviolet (UV) and visible (visible) light have modest penetration into tissues due to absorption by biological tissues [10]. The likelihood of a recurrence or spread of cancer is raised as a result [11]. As poor targeting results from nonspecific dispersion of phototherapeutic medicines, this is another key issue that must be addressed. Treatment procedures that include the inefficient or untargeted administration of phototherapeutic substances might have negative consequences beyond reduced bioavailability and efficacy.
The aforementioned challenges to cancer phototherapy have been overcome with the help of a variety of phototherapeutic nanoagents based on flexible nanomaterials [12-14]. This is because the EPR effect causes nanodrugs in a certain size range (20–200 nm) to accumulate in tumour tissue [15-17]. Hence, nanomaterials have been suggested as carriers for photosensitizers, PTCAs, medications, and genes for targeted treatment and combination therapy in tumour tissue [18-20]. As a result, nanomaterials that are sensitive to NIR light have been developed as a means of overcoming the issue of limited depth of penetration that has historically plagued phototherapy. As an example, PTCAs have been used in NIR-triggered photothermal therapy because of their capacity to directly absorb NIR light and generate heat (PTT). For deeper penetration, researchers have turned to a transducer called upconversion nanoparticles (UCNPs) [21, 22]. To emit narrowband UV or visible light, UCNPs take in longer-wavelength NIR light. Hence, despite phototherapy's drawbacks, light-responsive nanomaterials provide promise for its future.
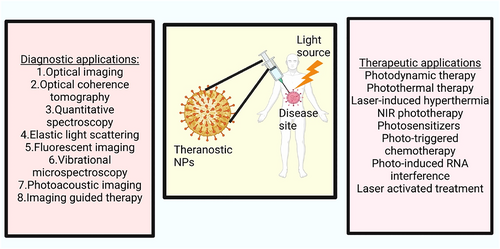
This article provides a review of the most recent research on the use of light-responsive nanoparticles in cancer therapy. There are a wide variety of therapies that might be improved by using these nanomaterials (Figure 1) [23-28]. The author also discusses the present state of phototherapy and its potential future development [29-37]. The purpose of this work is to draw attention to the value of light-responsive nanomaterials in cancer phototherapy and to suggest avenues for further investigation that might expand their use.
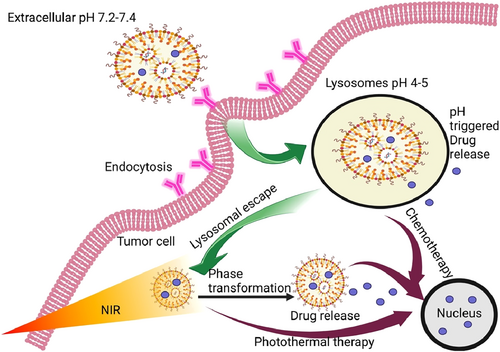
Schematic representation of the destruction of cancer cells through lysosomes with the help of phototherapy.
2 COMMON PHOTO-TRIGGERED MEDICAL NANOPARTICLES
Biomaterial and technological advancements in the past decades have opened up promising new avenues for the diagnosis and treatment of a broad variety of diseases. Biomedical scientists are still very interested in “smart” materials that can be altered by their surroundings, such as pH, temperature, or light [38-43]. The creation of novel polymers or therapeutics systems that take the advantage of light-based stimuli has increased in recent years. There are several benefits to using light-responsive materials, such as the ability to use a wide range of materials, excellent spatial or focus control, minimally invasive procedures, and so on since light-based diagnosis and diagnostics do not require physical contact, NIR light is better suited for biomedical activities, and the oral bioavailability of entrapped agents can be precisely controlled by an external light source, and they are preferred over other available sensory perception modalities [44-47]. The extraordinary absorptive qualities of the materials may allow them to react to changes in illumination. Plasmonic metal complexes are now the most popular kind of light-responsive materials because of their superior optical features in the near-infrared range, where tissue infiltration is feasible [48]. Various applications of nanoparticles in healthcare is shown are Figure 2 [48].
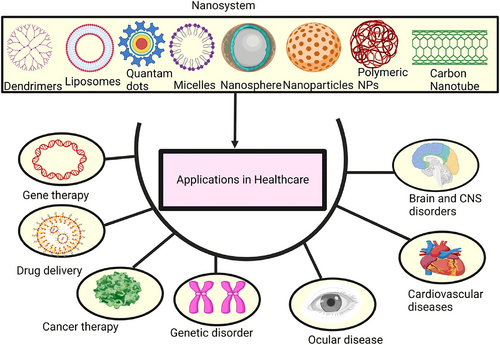
Pictorial representation of nanoparticles in the application of healthcare [48].
2.1 Gold-based NPs
Gold and other alloys with a high gold content are often used for photothermal treatment (PTT). Because of their high ratio of surface area to volume, noble metal NPs are effective detectors of surface plasmon resonance (SPR) [49], the phenomenon responsible for gold's distinctive optical and physical features. Gold also has the capacity to transform NIR light into heat, is malleable in surface and structure, and is simple to synthesise. In contrast to quantum dots (QDs) and other fluorophores, gold nanocarriers are robust and will not fade or flicker underneath the light, making them ideal for use in biological applications. A variety of nanoparticles with promising features, including gold nanoparticle (GNRs), nanotubes (NCs), nanostructures (NS), hollow gold nanoshells (HGNS), and gold-sulfide NPs [50-53], have been produced and investigated for application in photo-triggered therapies.
As rods, GNRs have the unusual property of absorbing SPR resonance frequencies in opposite directions along the rod, resulting in two absorption bands. As a result of their greater absorption and scattering efficiency, GNRs are better contrast agents [54]. Fine-tuning the structural organisation of gold nano-systems may have beneficial optical effects. Although GNRs have many potential applications in medicine, one major downside is that a surfactant employed in their manufacture, cetyltrimethylammonium bromide (CTAB), has been associated with cell cytotoxicity [55]. To enhance photodynamic treatment and hyperthermia, Kuo et al. [56] created biodegradable gold-poly (styrene-alt-maleic acid) green indocyanine (gold-(PSMA)-ICG) nanorods.
Gold nanocages, like GNRs, offer a number of appealing properties that make them well-suited to the application of photo-based imaging and treatments [57]. Bio-inert, permeable, and hollow gold nanocages boost optical imaging's temporal and spatial accuracy and free it from the limitations of ultrasonic imaging. Nanocages of the smart polymer poly-N-isopropyl acrylamide were used by Yavuz et al. [58] to enclose pharmaceuticals and control their release dependent on environmental temperature (PNIPAAm). To release the encapsulated doxorubicin (DOX), the nanocages absorbed the NIR light, which was subsequently transformed into heat through the photothermal effect. More duration spent under NIR irradiation was associated with a greater DOX release and a higher proportion of cancer cell death in in vitro tests. A recent study by Chen et al [59]. Indicates that gold nanocages accumulate in tumours and cause persistent damage to tumour cells, suggesting that they may be useful light sensors for cancer therapy. To create thiolated PEG nanocarriers, gold and Raman reporter molecules are encapsulated in the lipid bilayer. This paves the way for tumour localization and Raman scattering enhancement at the surface. These particles were coupled with a single-chain variable fragment (ScFv) antibody unique to epidermal growth factor channels upregulated on tumour cells, allowing them to light brighter than QDs when exposed to an NIR laser and to concentrate preferentially inside tumours in vivo [55]. Researchers Lapotko et al. [60] conducted the first in vitro investigations using gold nanoparticles for photothermal theranostics, effectively nano-thermalizing leukaemia cells by encasing them in microbubbles and irradiating them with a laser. The same team has also shown success in treating cancer using single-cell theranostics in which a gold nanoparticle is paired with anti-epidermal growth factor receptor (epidermal growth factor receptor) antibody. Once within the cell, gold nanoparticles may be used as diagnostic probes or even cause cell death through laser-induced membrane disruption in the form of transient plasmonic nanobubbles (PNB) [61]. In addition, the efficacy of these gold nanoparticles was validated in vivo using human prostate cancer cell-carrying zebrafish embryos. Formerly, C225-conjugated gold nanoparticles were needed to label these cells. It was shown that targeted cellular ablation is possible using PNBs generated by metal nanoparticles when exposed to laser pulses. By generating a temporary PNB around the gold nanoparticles with a specific instance laser pulse, the same group has recently employed this approach for both localised delivery of pharmacological payload and mechanical death of cells. When used to “inject” a molecular payload into cells, tiny PNBs may briefly breach the cell membrane. On the contrary, large PNBs may directly trigger mechanical cell death in their target. Several gold nanocarriers have been found to be more effective for various in vitro diagnostic and therapeutic uses. Larger surfaces are preferred for imaging applications owing to their high dispersion efficiency, in contradiction to the photothermal effect, in which the majority of light is transformed locally to heat to kill diseased tissues [62].
The optical signals generated by gold-based nanocarriers are, however, far weaker than those released by QDs and other fluorophores. As a solution, NIR-absorbing gold nanocarriers might be utilised to increase image contrast by soaking up radiation at a longer wavelength that is longer than what is used by tissue chromatophores [63].
2.2 Carbon nanotubes
Many newly produced carbon-based nanotubes that combine inorganic elements have shown theranostic promise, increasing the variety of materials available for photo-based therapy and diagnostics. The most well-known member of this family is the single-walled nanotube (SWNT), which exhibits exceptional NIR absorption. These carbon nanostructures show great potential for theranostic applications because they can be tailored for selective uptake by cells. Another recent research has demonstrated that SWNTs may be oxygen-doped covalently to improve their absorption of the NIR emission wavelength, resulting in sharper pictures [64]. In comparison to single-walled carbon nanotubes (SWNTs), multi-walled carbon nanotubes (MWCNTs) and metal-filled MWCNTs exhibit improved physical, chemical, and optical properties [65]. Fisher et al. [66] highlight the benefits of multi-walled carbon nanotubes for their prospective application in cancer therapy. Cancer cells were shown to have a lower chance of survival when exposed to multi-walled carbon nanotubes, and tumours produced less heat shock protein as a result. These findings provide more evidence that multi-walled carbon nanotubes may be useful as a safe method for administering drugs. Whilst these results are encouraging, further study is required to ensure that the carbon nanomaterial is biocompatible. Single-walled carbon nanotubes are one example of a new kind of carbon-based nanostructures that are hybrids (SWNTs). Carbon nanotubes are ideal moulds for connecting metals due to their specific chemical and physical properties. When used as dark field photon scattering agents in imaging, those metal-filled carbon nanotubes display intriguing optical features [67].
2.3 Inorganic photosensitizers
Titanium oxide nanotubes have been extensively researched due to their biocompatibility and their potential in medication delivery applications [68]. Titanium oxide nanotubes, as shown recently by Hong et al. [69], offer great promise in photothermal therapy (PTT) for the treatment of various cancers. In order to ensure the specific irreversible destruction of tumour cells without hurting healthy cells, researchers employed TiO2 nanotubes as a thermal coupling agent. TiO2 nanotubes have a substantial photothermal effect. Our results showed a strong correlation between TiO2 nanotube concentration and in vitro cell mortality. Laser power, laser duration, and the total quantity of TiO2 nanotubes in suspension may all be utilised to fine-tune the interaction between NIR light and the nanotubes as shown by in vivo animal research.
2.4 Capturing photosensitizers and dyes inside of polymeric nanocarriers
Many different types of polymers and biocomplexes are being studied for their potential to enclose light-responsive molecules to be utilized for photo-triggered therapy and imaging. The low water solubility, low toxicity, and fast clearance from the body of these materials make up for the shortcomings of currently available photosensitizers, making them more attractive for use. For good measure, these substances provide accurate dosing of capsuled medicines or detecting dyes [70]. To counteract the cellular toxicity [71] and lack of specificity of metal-based NPs like QDs and gold NPs, tailored and controlled delivery using biodegradable surface treatments and targeting ligands may be an effective solution. Methods, such as liposomes, nanowires, microcapsules, conductive polymers, polymeric NPs, and capsules, are discussed among others. Ultra-deformable liposomes containing zinc porphyrin as a photocatalyst were produced by Montanari et al. [72] to treat early stage Plasmodium braziliensis infections. According to in vitro research, immunological responses, not the selective effects of photodynamic treatment, are responsible for the leishmanicidal action. These data highlight the need for further research to establish the therapeutic benefits of ultra-deformable liposomes photosensitizing and non-photodynamic properties. In addition, polysomes with intrinsic fluorescence characteristics for photothermal and fluorescence imaging may be made using porphyrin-lipid conjugates. After 2 days of intravenous administration, these porphyries aggregated at the tumour site, producing bright fluorescence, in mice bearing KB cancer xenografts. Lymphatic imaging was also possible following intradermal injection of the polysomes due to the strength of their photoacoustic emissions.
Because of their many useful properties, including biocompatibility, small size, simplicity of manufacture, and simple surface functionalization, aptamers are another kind of elastomeric carriers now in use. Recent work by Shen et al. [73] demonstrates that nano-silica frameworks patched with 4, 4′-Azobenzene dibenzoyl chloride (ADC) and B3-type monomer triethylamine (TEA) may be utilised to create highly photosensitive degradable polymeric nanocapsules. Dendrimers may be effective in photodynamic treatment if they are coated with photosensitizers. The toxicity problems prevent their widespread use as drug delivery agents. There is evidence that dendrimers with positive electrode surface groups are hazardous to cells and this toxicity increases with time.
Aziropolymers based on amphiphilic polysiloxane or poly(chloromethyl) styrene were linked to micelles by Moleavin et al. [69], allowing the construction of amphiphilic macromolecular micelles. The amphiphilic character of these micelles considerably reduces their adverse consequences. Very little effort is needed for drug testing, and the inside patient structures necessary for precise dosing and sustained release of therapy are very stable over extended periods of time. Micellar nanocarriers containing pH-sensitive tetramethyl rhodamine dye were developed by Zhou et al. [74], which when activated at a certain pH release more light 5 min after the first activation. One intriguing possibility is that in hypoxic circumstances, PEG-based electron-rich micelles containing the photosensitizer tetrakis(meso-hydroxyphenyl)porphyrin (mTHPP) might produce increasing quantities of O2 through the energy transfer mechanism, thereby competing with 1O2 synthesis. It enhances photoactivation and, by extension, phototoxicity when given to cancer cells in a hypoxic environment.
Polymer materials are being studied for their potential use in the creation of nanocarrier housing compounds that respond to extrinsic light stimulation [75, 76]. Poly (lactic-co-glycolic acid), poly (ethene glycol), dextran, and poly(azobenzene) are only a few examples of these polymers (PLGA). Among the polymer materials that might be used as an optically triggered switch for intravenous drug administration is polyazobenzene, which has remarkable physical and chemical light-induced interconversion characteristics. Poly azobenzene polymers are effective dispersants for single-walled carbon nanotubes as discovered by Umeyama et al. [77]. Because of their great degree of tunability through intermolecular interactions within the polymer, polymers reinforced with carbon nanotubes have been shown to be a promising foundation for medicinal delivery systems.
PEG has been used in a number of applications, such as surface modification and the creation of complex nanocarrier complexes for light-activated drug delivery. PEG has been used to precisely modify the surface of gold nanoparticles (NPs) [78] to improve their stability, water solubility, and cell targeting. PEGylation of QDs has been shown to allow for greater biodistribution after injection, inhibiting the quick removal of these nanoparticles from the system [79]. In 1991, Umeda et al. [80] developed a photothermal system using PEG. By attaching a PEG to PAMAM dendrimers and then coating them on gold NPs [81], the photothermal characteristics of the gold NPs were significantly improved. Furthermore, the findings suggest that such structures may function as effective tools for selective cell targeting while retaining the advantages of PTT. N-(2-hydroxypropyl)meth acrylamide (HPMA) is another chemical with potential use in imaging and medication delivery. Packaging and coupling of medicinal and/or diagnostic probes are both possible using HPMA either on its own or as a copolymer [82]. Using the application of HPMA, Ren et al. [83] synthesised copolymer conjugates labelled with scanning reagents, such as IR dye 800CW, with the goal of preventing and treating particle-induced inflammation, which may be a sign of aseptic implant loosening. Ultra-high molecular weight polyethene particles were implanted into male Swiss Webster mouse models, leading to inflammation in vivo (UHMWPE). NIR optical imaging demonstrated that cells at the site of inflammation had taken up HPMA copolymers following administration.
Polymeric materials have several potential applications beyond imaging and cell targeting. Most recently, Bogart et al. [84] employed dextran polymer-coated iron oxide NPs for photothermal microscopy. Improved imaging sensitivity and resolution are good news for imaging live samples as shown by their results. PLGA polymer has been widely implemented across several Nano carrier systems. In a recent study, Cheng et al. [85] synthesized poly (lactic-co-glycolic acid) (PLEA) NPs loaded with Foo and QI-conjugated Taxol, which were subsequently coated with poly (styrene sulfonate). These NPs have the potential to be used in photothermal ablation of cancer cells utilizing near-infrared light in addition to chemotherapy. There in vitro and in vivo studies show that using these NPs to deliver both chemotherapy and photothermal therapies is more successful against cancer than each treatment method used alone [86-89]. Our findings highlight the potential for future combinational therapy techniques to use a wide range of photo-triggered materials in the treatment of cancer [90-94]. The present research is centered on the synthesis of multi-functional NPs that might be used for concurrent imaging and therapy. Many imaging approaches, drug release strategies, and photothermal treatments may benefit from the newly synthesized polymers and systems.
Several photosensitizers and their applications are tabulated in Table 1 [95]. Macrophages are a double-edged sword in the tumour microenvironment. As a prominent component of tumour stromal cells, macrophages can gather around blood vessels, induce angiogenesis and promote tumour invasion. On the other hand, they could also phagocytose cancer cells and remodel the tumour microenvironment. Fortunately, the polarization of macrophages can be repolarized. The transformation from M2- to M1-phenotype macrophages is sufficient to have a tumor-suppressive effect. Of note, the polarization of macrophages is independent of T cells, while M1 macrophages are able to induce Th1 immune responses, and M2 macrophages can trigger Th2 immune responses. This provides an opportunity to target macrophages in cancer immunotherapy. More importantly, the direction of macrophages to T or B cells does not rely on the existence of tumor-specific antigens. While IFN-γ from M1 macrophages is an incentive for Th1 responses, TGF-β and IL-10-derived M2 macrophages cause the generation of Treg cells [96].
| No. | Photosensitizer | Application | Stage completed |
|---|---|---|---|
| 1 | Photofrin | PDT of various carcinomas | FDA-approved |
| 2 | Levulan | PDT for actinic keratosis treatment | FDA-approved |
| 3 | Metvix | PDT for actinic keratosis treatment | FDA-approved |
| 4 | Visonac (methyl aminolevulinate) | PDT of moderate acne by killing bacteria and action on sebaceous gland | Phase IIb |
| 5 | Verteporfin | PDT of neovascular age-related macular degeneration | Phase IIIb |
| 6 | δ-aminolaevulinic acid | PDT for basal cell carcinoma | Phase III |
| 7 | Zinc phthalocyanine tetrasulfonate | PDT for naturally occurring tumours in dogs | Phase I |
| 8 | Radachlorin | PDT of skin cancer | Phase II |
| 9 | HPPH (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a) | PDT of obstructive oesophageal tumours | Phase I/II |
| 10 | Silicon phthalocyanine | PDT of cutaneous neoplasms | Phase I |
| 11 | Hexaminolevulinate (Hexvix) | PDT of intermediate or high-risk urothelial cell | Phase I |
| 12 | BF-200 5- aminolaevulinic acid | PDT of actinic keratosis | Phase III |
| 13 | Hemoporfin | PDT for port-wine stain | Phase IIa |
3 STRATEGIES TARGETING TME
Tumour microenvironment (TME)-targeted strategies offer a further avenue to pursue. The rapid growth of new blood vessels, known as angiogenesis, is a hallmark of every malignancy [97]. Positive results from research on this character flaw were found. Sengupta developed an NP system where combretastatin and doxorubicin (DOX) are co-encapsulated into the PLEA core to prevent tumour angiogenesis [98]. Schematic diagram of immunotherapy through nanomaterial, tumour microenvironment and destruction of cancer cells is shown in Figure 3. Due to combretastatin's fast blocking of malignant arteries, DOX was quickly taken up by the tumour, resulting in a higher therapeutic index and lower toxicity [99]. The extracellular matrix (ECM) has been studied for its potential role in cancer therapy alongside aberrant vasculature. The extracellular matrix (ECM) serves as a scaffold that promotes cancer cell growth, motility, invasion, and angiogenesis [100]. Major contributors to these carcinogenic properties include collagen, HA, and a wide range of enzymes. Cancer cells are able to navigate through the ECM with the help of collagen, the principal structural protein, and HA increases IFP, which impedes medication diffusion and penetration [101]. The TME may be regulated by matrix metalloproteinases (MMPs) and other enzymes by affecting the activity of molecules other than the extracellular matrix (ECM), such as growth factors, receptors, and cytokines [102]. Nanocarrier development must take ECM into account. Patients with metastatic pancreatic cancer, especially those with high hyaluronidase expression, benefited from the addition of PEGylated recombinant human hyaluronidase (PEGPH20) that targets ECM hyaluronic acid [103]. Hyaluronidase (HAase) carrier coating has been found to increase the ability of nanocarriers loaded with chemical therapies to enter solid tumours. The efficiency of antitumor treatments is enhanced by using this simple technique.
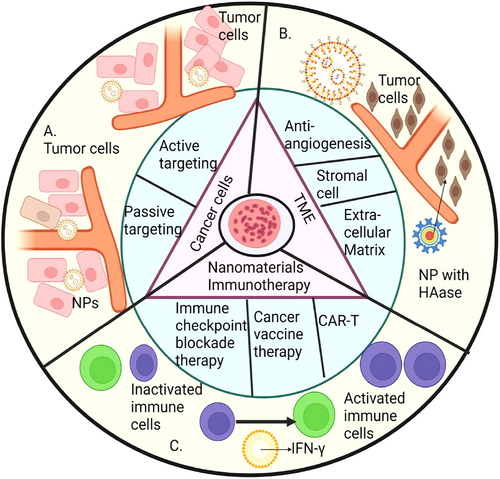
Schematic diagram of immunotherapy through nanomaterials, tumour microenvironment and destruction of cancer cells.
4 BLACK PHOSPHOROUS NANOSHEETS FOR CANCER THERAPY
Black phosphorous nanosheets (BP NSs), a kind of attractive two-dimensional materials, have been widely used in optoelectronics, transistors and photocatalysis. Recently, growing interest has been attracted to explore the application of BP NSs for cancer therapy in view of their unique properties. BP NSs have a high surface area and negative charge, which can load drugs, targeting molecules, photosensitizers, and magnetic nanoparticles. They are also potential candidates for cancer phototherapy, including photothermal therapy (PTT) and photodynamic therapy (PDT), by virtue of their extensive near-infrared absorbance. Furthermore, BP NSs exhibit a biodegradable and compatible nature to avoid toxicity in vivo [104].
5 NANOMATERIALS AND CANCER IMMUNOTHERAPY
Cancer's origins and early stages may be traced back to malfunctions in the immune system. Immunotherapy has a wide range of applications, some of which include cancer vaccine treatment, immune response modulator therapy, or immunological checkpoint blockade therapy [105]. These cancer immunotherapies employ either naturally occurring or synthetic chemicals to enhance or restore the immune system activity and have the anti-tumor effect. Research into loading PD-1 and PD-L1 from the immune checkpoint pathway onto nanocarriers for the treatment of cancer is ongoing. Although traditional immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 showed inconsistent outcomes, Bu et al. [106] hypothesised that overexpression of PD-1 allowed cancer cells to avoid antitumor immunity. Bonding between PD-L1 and ICIs was ensured by the author's use of multivalent poly (amidoamine) dendrimers, which increased drug accumulation at tumour locations and enhanced PD-L1 blocking action [107]. One of the functions of CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) is to suppress immune responses [108]. Examples of such chemicals include antibodies, small molecule inhibitors, and proteins. To get these moles to their destinations, drug delivery vehicles like nanoparticles are important. These techniques might be utilised to develop novel nanoplatforms that, with any hope, will outperform existing therapies in terms of efficacy and bioavailability.
5.1 Advantages and challenges of nanomaterial applications in cancer therapy
Nanomaterials as an alternative to conventional chemical drugs for the treatment of cancer: pros and cons. Persistent regenerative activation, evasion of growth suppression, resistance to cell death, homologous recombination longevity, induced vasculature, activating invasion and metastasis, irritation, genomic instability, and mutation are all hallmarks of tumourigenesis and tumour formation [109, 110]. A schematic representation of cancer immunotherapy through the activation of nanoparticles is shown in Figure 4. Conventional chemotherapy and radiation are inefficient and dangerous because of their non-targeted dispersion and cytotoxicity to healthy cells as well as malignant ones. Finding the right dose-dense-schedule (DDS) balance in cancer treatment is thus essential [111]. Chemical treatments, whether taken orally or intravenously, must circumvent several "fortifications," such as those posed by the tumour environment and vasculature, the monolayer phospholipid system (MPS), the blood-brain barrier (BBB), and renal filtration. Barriers such as the microenvironment, blood flow, the Renal Enhanced Selectivity for Proteins (RES), the BBB, and healthy kidney function all contribute to the body's natural resistance to infections. With cancer treatment, however, these defences may reduce the efficacy of chemical therapies that target cancer. Different from normal cells, cancer cells multiply at a much faster pace. Cancer tissues have a dense extracellular matrix and exhibit overactive angiogenesis due to an overabundance of angiogenic factors and excessive interstitial fluid.
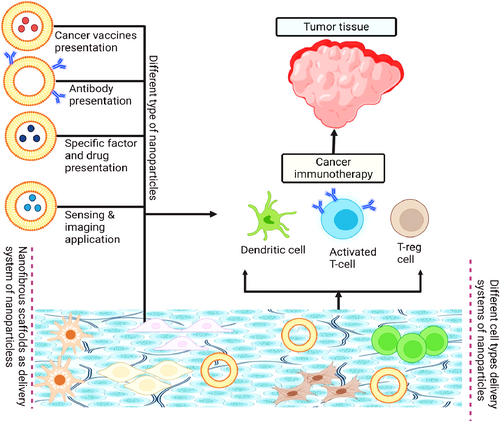
Schematic representation of cancer immunotherapy through activation of nanoparticles.
5.2 Nanomaterial and drug metabolism
Drug metabolism is very complex, and its difficulties cannot be overstated. Macrophages in the tissues, granulocytes in the blood, and other immune cells make up what is called the monocyte-polymorphonuclear-lymphocyte (MPS) system [112] Immune cells in the liver, spleen, and lungs of an organism may respond to external compounds like chemical medicines, activating macrophages or leucocytes, which then quickly remove the medications from the body, resulting in a short drug half-life [24]. There is a decrease in MPS clearance and an increase in the half-life of the medications carried by nanocarriers with surface modifications, such as PEG or a specific peptide [113]. Among the kidneys' many crucial functions is filtration. A greater clearance rate in the kidneys is associated with a number of characteristics, including particle size, shape, and surface charge. It was crucial to take into account renal clearance while giving out older chemical drugs [114]. The toxicity of nanocarriers is mitigated by adequate renal clearance. These issues plague many conventional drug delivery strategies, decreasing treatment efficacy at oncological sites and unintentionally increasing dosage and toxicity for normal tissue [115-118].
6 TARGETING STRATEGIES OF NANOMATERIALS APPLIED TO CANCER THERAPY
Targeting cancer cells directly is an integrative method for treating this illness. To aid in the delivery of chemical treatments or biomaterials to cancer cells, individualised nanocarriers may use EPR and active targeting. Overexpressed antigens on the surfaces of cancer cells are often targeted with antibodies. Tumour cells endocytose the encapsulated payload, which may then trigger cytotoxicity (as in the case of chemical therapies) or cell death (in the case of nucleic acid materials). Exosomes, lipid nanoparticles (NPs), and dendrimers are all being explored as potential vehicles for nucleic acid delivery in cancer therapy.
To combat tumour development, doctors use a technique called "targeted treatment" in which they zero in on the specific genes or proteins responsible for the disease. Targeted therapy often focuses on molecules involved in apoptosis and angiogenesis. Two common types of tools used in targeted therapy are small molecule antagonists and monoclonal antibodies. If an antibody is conjugated with its antigen, its specificity may increase. The only cells targeted by targeted therapy are the cancer cells, as opposed to the rapidly multiplying normal cells that are the focus of free chemical medicines and other non-targeted therapies [119]. However, NPs loaded with therapeutic medications or modified with specially targeted mAbs in the interface gain better efficacy and decreased toxicity than nanocarriers supplied with anti-tumor chemical treatments.
As an essential part in directing nanocarriers to their destinations, the EPR effect is becoming more accessible. The nanoplatform must interact with TME, MPS, and barriers in the human body for EPR-based passive targeting to be possible. MPS, the immunological response, and other nanoemulsion interactions all have an effect on active targeting, which uses conjugated verbs with antibodies, peptides, aptamers, and molecules. DDSs are built with both passive and active targeting in mind. Existing targeted therapies may benefit from the created nanoplatform by loading the Nano carrier with therapeutic chemicals or changing the surface.
7 PHOTO-TRIGGERED THERAPY USING NANOCARRIERS
Nanomaterials have been designed for a wide range of light-based therapeutic applications, not only imaging and diagnostics [120, 121]. Light is used to activate drugs in photodynamic treatment (PDT), which is then used to treat cancer and other disorders. Light, photosensitizers, and oxygen all play critical roles in the therapy. Images of a photosensitizer, a chemical substance that converts light into a type II chemical reaction, are shown. Porphyrins, chlorophylls, and dyes are the three primary chemical categories of photosensitizers utilised in medicine. One kind of photosensitizers, metallico-phthalocyanine, has received a lot of interest due to the versatility of its photodynamic (PD) features, which may be altered by changing the metal ion utilised. Strong combined photodynamic (PD) and photothermal (PT) effects were seen when zinc-phthalocyanine nanowires made by Moon et al. [122] were subjected to a near-infrared (NIR) laser. Because of their biocompatibility and capacity to target tumours, human serum albumin nanoparticles (NPs) that have been linked with photosensitizers have been found to be excellent photodynamic drug delivery alternatives.
The noninvasive cancer treatment known as photothermal therapy (PTT) has lately gained a lot of attention. By transforming electromagnetic energy (through NIR) into heat, the photoabsorbers cause harm to the tumour. Selecting a wavelength at which the unhealthy tissue absorbs lighter than the sick tissue is essential. Scientists have created a wide variety of photocatalytic agents, and also as carbon nanotubes (CNTs), gold nanorods (GNRs), nanoshells, and nanocages, for PTT (CNTs). The representation of cancer treatment from PDT is shown in Figure 5 [123]. Recently, QDs have gained popularity due to their superior fluorescence compared to typical dyes, size-dependent fluorescence, narrow emission spectra, and resistance to photobleaching. Once implanted in a melanoma tumour of a rat, the CdTe and CdSe QDs developed by Chu et al. [124] quickly turned light energy into heat. The findings suggested that the tumour shrank significantly. It has been shown that combining QDs with light-absorbing dyes may significantly boost the thermal damage in the targeted tumours. PTT has access to nanostructures of ICG and phospholipid-polyethylene glycol (ICG-PL-PEG) [125]. The results showed that the ICG-PL-PEG solution had a stronger NIR temperature-dependent increase than pure ICG dyes. They were much more successful in staying inside the tumour when combined with an anti-integrin (v) monoclonal antibody (3). Researchers have loaded PLEA nanoparticles (NPs) with targeting ligands such that ICG may be used for prostate cancer-specific targeting, optical imaging, and PTT LIN. Our NPs' efficiency is shown by their biocompatibility and high uptake by PC3 prostate cancer cells. Their contrast was apparent to a depth of 3 cm below in the laboratory tissue phantom, demonstrating their potential for deep tissue imaging and malignant hyperthermia, and they displayed considerable temperature rises in vitro and in phantoms, confirming their promise for both applications. Using GNRs with greater absorbance than chromophores and NIR dyes, Lin et al. [126] also successfully improved PTT in genetically engineered mice. A GNR's absorption cross section is at least five orders of magnitude bigger than that of a conventional dye. These nanorods scatter light with a great efficiency, even more so than highly fluorescent dyes [127].
Representation of cancer treatment from PDT [123].
Comparable to PDT and PTT, NIR offers a wide variety of applications in the area of applied biology. Due to its longer wavelength, blue light is more able to penetrate deep tissues than red light. It also inhibits most auto luminescence produced by neighbouring tissue and has low levels of dispersion and energy absorption [128]. Small-diameter GNRs, gold-nanocages, and gold-nanoshells have recently attracted a lot of NIR attention due to their stability or bioactivity. Using near infrared photoimmunotherapy (PIT) and a novel monoclonal antibody (mAb) [129], researchers have been able to track the rate of acute necrosis of cells in cancer patients in real time. Even before morphological changes are visible in the targeted tumour, FLT imaging may be able to monitor the deadly consequences of NIR-mediated mAb-induced PIT [130]. Lim et al. [131] utilised Pluronic NPs and phthalocyanine to develop a new NIR absorber for PTT. NIR fluorescence images of tumor-bearing animals given FPc NPs revealed significant visual contrast that was localised to the tumour. Meanwhile, the signal from the tumour grew, while the signal from the rest of the body shrank with time.
One cutting-edge tool utilised in phototherapy for cancer treatment is the laser, which may be programed to cause hyperthermia. The technology has a major drawback since it cannot spatially discriminate between heating tumours and surrounding tissue. This issue was attempted to be remedied by Terentyuk et al. [132] who used plasmonic silica/gold nanoshells to generate tunable laser hyperthermia in tissues, hence increasing the photothermal effect on cancer cells. Laser surgery and other minimally invasive surgical technologies are gaining popularity due to their efficiency when used simultaneously. This is because of the pain relieving and restorative effects they have. One of the most innovative treatments now in use combines near-infrared (NIR) light, laser light (light emitting diode), and an optical absorber. In recent years, indocyanine green (ICG) dye has risen in favour for this combination technique as a result of its effectiveness and safety in human surgical operations. A lack of designs that can accurately target certain tissue types for heating has prevented several promising concepts from reaching clinical trials despite much research [133].
In the case of phototherapy, NPs may transport not just drugs but also genetic material. RNA interference has been widely used in the investigation of gene function (RNAi). Both photochemical internalisation and photoinduced RNAi were developed in 2005. The use of -cyclodextrin and polymers based on six methylene units led to the development of a light-activated polymer that entraps small interfering RNA (siRNA). The human S100A4 protein expressed by OHS cells was the subject of this study. The results showed that treatment had no effect on cell viability and that silencing efficacy was more than 90% relative to the untreated control. Because of the siRNA conjugation to the gold NPs, they are able to penetrate tissues more deeply at NIR wavelengths [134, 135]. Photo-stimulation of fluorescently tagged protein carriers has been presented as a novel method for the delivery of short hairpin (shRNA) by Endoh et al. [136].
8 PHOTO ACTIVATED THERANOSTIC NANOCARRIERS
Therapeutic nanomedicine uses advanced NPs to diagnose and cure illness. Treatment-diagnosis is the term. Light-based theranostic NPs that detect and treat illnesses are appealing. Multifunctional NPs with a gold-silver nanocage core, silica shell, and NIR photosensitizer Yb-2,4-dimethoxyhematoporphyrin were created by Khlebtsov et al. [137] using photodynamic therapy and plasmonic heating (Yb-HP). Compared to photodynamic therapy with Yb-HP, plasmonic PTT using gold-silver nanocages was more effective in killing HeLa cervical cancer cells in vitro. Tissue transparency analysis might benefit from Yb-IR HP light. Targeting circulating breast cancer cells in vivo, Galanzha et al. [138] employed gold layered-carbon nanotubes linked with folate and CD44-specific antibodies. For use in theranostics, Singh et al. [139] created coloured non-plasmonic silica NPs. To improve the NIR fluorescence imaging and light absorption, these particles included heptamethine cyanine dye and Si-naphthalocyanine heating dye. The PTT increased as a result. After direct tumour injection into mice, NIR laser irradiation caused high fluorescence and 95% tumour viability reduction.
Due to their multiple imaging and therapeutic modes, multifunctional nanoparticles (NPs) are becoming increasingly important in nanotechnology research. Researchers created folic acid-conjugated poly acrylic acid-coated iron oxide NPs with Taxol, 4-Dil, and 4-DiR to fight cancer. Optical and magnetic resonance imaging (MRI) cancer diagnostics and targeted cancer drug delivery may employ these NPs. Huan et al. [140] developed a dual-imaging NP formulation. Our team synthesized multi-functional folic acid-conjugated and silica-modified gold nanorods for in vivo imaging and radiation or photothermal treatment (GNR-SiO2-FA). These multifunctional nanorods preferentially bind to MGC803 gastric cancer cell folate receptors in vitro and in vivo using X-ray and CT imaging.
Theranostic NPs that combine two therapies are gaining popularity since they should provide a better therapeutic effect than each therapy alone. Wu et al. [141] constructed a silver-gold bimetallic core surrounded by a thermoresponsive PEG shell with hyaluronic acid-based targeting ligands. These nanogels are used for photothermal, chemotherapeutic, fluorescence imaging, and cancer-specific targeting. Photoluminescent emission intensity from the silver-gold core increases with temperature-dependent shell shrinkage, allowing optical temperature monitoring and cellular imaging. Hyaluronic acid chains bound to cluster determinant may target cancer cells (CD44). Due to its bimetallic core, irradiating B16F10 skin cancer cells' TMZ-loaded NPs with NIR light caused a photothermal response. When the thermosensitive shell was heated, more chemotherapeutic TMZ was released. During NIR irradiation, Lee et al. [142] created unique PEG-PLEA-gold half-shell NPs (120–125 nm) containing DOX for chemo-photothermal treatment and imaging. Nanoparticles (NPs) concentrated in tumours of shaved mice following intravenous or topical administration. NIR irradiation and chemotherapy eliminated the tumours without injuring the animals, and they did not return. This approach outperformed chemotherapy and PTT for cancer.
A radioisotope might be included in NPs for use in cancer diagnosis and therapy. Shell cross-linked (SCK) NPs conjugated with folate were developed by Rossin et al. [143] for use in cancer treatment and radiation detection. Poly(acrylic acid-b-methyl-acrylate) micelles radiolabelled with 64Cu showed stability in the tumour in vivo, raising the possibility that they may be used to detect and treat early stage, low-volume malignancies. With the intention of using radiation therapy, Yang et al. [144] developed a QI-photosensitizer (Photofrin) combination. In vitro investigations showed that the combination of QD-photofrin conjugates and radiation was more effective at killing H460 human lung cancer cells than either component alone (p 0.94). Compared to radiosensitizers, these conjugates pose no health risks. Gold nanoparticles (NPs) and QDs were discovered to be effective in radiation therapy by Hainfield et al. [145]. Radioresistant mouse head and neck squamous cell carcinoma was treated with hyperthermia using high X-ray doses of 42 Gy and 157 keV beam energy, leading to decreased tumour volume and improved survival. Cutting-edge research into the detection and treatment of a broad variety of illnesses may be made possible by theranostic photo-triggered NPs.
9 TARGETING STRATEGIES OF NANOMATERIALS APPLIED TO CANCER THERAPY
Targeted cancer therapies focus on proteins or pathways to eliminate the illness. Targeted medicines target apoptosis and angiogenesis. Targeted treatments use small molecule inhibitors or monoclonal antibodies. Antigen-antibody fusion increases antibody specificity. Targeted therapies only affect tumor-related molecular targets, unlike free chemical medicines and other non-targeted therapies that destroy fast-growing cells. NPs loaded with active targeting medicines or modified with precisely targeted mAbs in the surface are more effective and safer than nanocarriers packed with anti-tumor chemical therapies.
The EPR effect helps nanocarriers target a vital function. The EPR effect, which allows passive targeting, requires nanoplatform-TME, MPS, and body barrier interactions. EPR allows active targeting using antibodies, peptides, aptamers, and small compounds. MPS, the immune response, and other nanoparticle interactions limit active targeting effectiveness. DDS targets passively and actively. The nanoplatform may improve targeted therapies by loading the Nano carrier with therapeutic molecules or modifying the surface.
10 MEDICAL APPLICATIONS OF PHOTO-BASED THERANOSTIC NPS
A schematic diagram of cancer cell treatment via modification of the compound is shown in Figure 6 [146]. The increased incidence of cancer has prompted scientists to develop NPs with the ability to both treat and diagnose the disease. The potential of photo-based theranostic NPs to detect and eliminate circulating tumour cells and cure cancer at any stage has been established in a number of investigations. The new aptamer-QI-DOX conjugates created by Bagalkot et al. [147] are able to be imaged with dual fluorescence resonance energy transfer and target PSMA-overexpressing prostate cancer cells (Bi-FRET). When doxorubicin is taken up endocytically, QDs cease to emit light. With this revised formulation, cellular NPs may be detected. To detect and visualise prostate cancer using spectroscopy, Gao et al. [148] developed semiconductor QDs coated in an amphiphilic triblock polymer and linked to PSMA monoclonal antibody. Fluorescent imaging of PSMA-overexpressing C4-2 prostate cancer xenografts in nave mice revealed the accumulation of ligand-conjugated QDs. Tri-functionalized mesoporous silica NP were created by Cheng et al. [149] using cRGDyK proteins for v3 integrin-specific targeting, an NIR fluorescent intravenous contrast ATTO647N for sensing, and a palladium-porphyrin-based photocatalyst for PDT. Increased NP uptake and cytocompatibility were seen in the human glioblastoma cell line U87-MG as opposed to the adhesion receptor-deficient MCF-7 breast cancer cell line. Nanoemulsions containing Cy7 fluorescent dyes or iron oxide nanocrystals were developed for use in near-infrared fluorescence (NIR) imaging and MRI cancer therapy. The RGD protein and the hydrophobic glucocorticoid dexamethasone acetate valerate were both components of the nanoemulsions (PAV). Radiological examinations performed in living animals reveal that this mixture effectively reduces tumour size.

Schematic diagram of cancer cell treatment via modification of compound [146].
There is increasing interest in a nano-sized system for cancer diagnostics and therapy, while theranostic NPs have been designed to treat a wide range of ailments. For inflammatory atherosclerosis, McCarthy et al. [150] created a multifunctional covalently bonded dextran-coated iron oxide (CLIO) nanoparticle. For near infrared (NIR) fluorescence imaging, CLIO employs AlexaFluor 750. A photosensitizer based on chlorin might potentially use light to kill inflammatory cells in atherosclerotic plaques. These NPs were synthesized by Lim et al. [151] and were quickly consumed by RAW274. 7 macrophage cells; they may reflect near-infrared (NIR) light for scanning and poly (diethylene glycol) (PTG) translysis. Similarly, the FDA has authorised Visudyne, a light-activated liposome containing a photosensitizer (hydro-monobenzoporphyrin) and phospholipids (EPG and DMPC). Tissue bonding with laser-activated NPs facilitates wound closure and tissue regeneration because of the laser heat. Albumin was "soldered" to rabbit ex vivo aortic tissue using radiofrequency heated SPIO NPs (15 nm). In order to non-invasively bind skin lesions and explanted lens capsular tissues, microscopic gold NPs may be utilised as photothermal transducers [152].
Light-based NPs might cure bacteria. Gold nanoparticles (NPs) coupled with anti-protein antibodies killed gram-positive Staphylococcus aureus. After ingesting NPs, bacteria 103 were killed by focused laser pulses (420–520 nm) that converted to heat. Huang et al. [153] created multifunctional Foo-gold nanoeggs that absorb NIR for PTT and contain vancomycin adsorbed to treat bacterial infections. Magnets outside the test container helped bacteria agglomerate [154, 155].
11 CONCLUSION
Scientists are increasingly looking to nanoparticles as a means of constructing systems that can be supervised post-administration and used for targeted and localised pharmaceutical distribution in response to the rising demand for effective treatment modes to heal diseases with minimal patient suffering. Despite the tremendous study, there is still a long way from where these nano-systems can be deployed on individuals. Large-scale NP manufacturing is required prior to clinical use. To ensure that the nanoparticle system is safe for human ingestion, bioactivity and cell and tissue interaction must be thoroughly studied. Each NP component has to undergo in vivo sensitivity and toxicity testing. While the development of theranostic NPs for clinical trials is delayed, these NPs provide the potential to overcome the limitations of current treatment techniques and provide a safe and reliable method for diagnosing and treating diseases with a little impact on patients' quality of life. Since then, photo-based theranostic NPs have evolved as a promising system that combines elements of chemistry, biology, genomics, medical physics, and other disciplines into a comprehensive solution that can tackle many of today's human health concerns. While photo-based theranostic particles show great promise, they nonetheless face a long road to clinical use. Nanoparticles (NPs) including photosensitizers and metal-based imaging agents need optimal scanning depths achieved via the use of several imaging modalities and NP components. As a result, NPs placed in deep tissue may be seen. To identify NP signals, sensitive imaging techniques are required. If used correctly, photo-based therapies like PDT and PTT with photo-based theranostic NPs should have minimal side effects. Given the potentially lethal nature of certain photosensitive payloads, such as QDs, removing the NPs from the system prior to the breakdown of the polymeric shell is essential. Nano-carriers may enhance the detection and treatment of many illnesses thanks to their high spatial resolution and controlled drug release, and likely to accomplish for effective and target-specific delivery of the encapsulated agent.
AUTHOR CONTRIBUTIONS
Rishabha Malviya: Conceptualization (equal); Formal analysis (equal); Resources (equal); Writing – review & editing (equal). Deepika Yadav: Data curation (equal); Methodology (equal); Project administration (equal); Resources (equal); Writing – original draft (equal).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.




