Multifunctional nanoparticle for cancer therapy
Abstract
Cancer is a complex disease associated with a combination of abnormal physiological process and exhibiting dysfunctions in multiple systems. To provide effective treatment and diagnosis for cancer, current treatment strategies simultaneously focus on various tumor targets. Based on the rapid development of nanotechnology, nanocarriers have been shown to exhibit excellent potential for cancer therapy. Compared with nanoparticles with single functions, multifunctional nanoparticles are believed to be more aggressive and potent in the context of tumor targeting. However, the development of multifunctional nanoparticles is not simply an upgraded version of the original function, but involves a sophisticated system with a proper backbone, optimized modification sites, simple preparation method, and efficient function integration. Despite this, many well-designed multifunctional nanoparticles with promising therapeutic potential have emerged recently. Here, to give a detailed understanding and analyzation of the currently developed multifunctional nanoparticles, their platform structures with organic or inorganic backbones were systemically generalized. We emphasized on the functionalization and modification strategies, which provide additional functions to the nanoparticle. We also discussed the application combination strategies that were involved in the development of nanoformulations with functional crosstalk. This review thus provides an overview of the construction strategies and application advances of multifunctional nanoparticles.
1 INTRODUCTION
Cancer is a complicated disease involving a combination of abnormal physiological processes affecting multiple systems, including DNA repair, apoptosis, and immune function.1-3 Supported by abnormal signal transduction pathways, tumor tissues simultaneously manage multiple biological activities, including proliferation, metastasis, and immune escape, rendering cancer one of the most challenging diseases to treat. Current cancer treatment strategies focus on different tumor targets, including proliferation, metastasis, and immune suppression.4, 5 Meanwhile, new technologies are also emerging to facilitate the transportation and function of therapeutic agents.
In this context, nanoparticles provide several advantages for cancer treatment and have proven efficacy in solving the delivery issue of hydrophobic agents, protecting biosensitive cargos, and promoting controlled drug-release.6-8 Nanoparticles are also reliable platforms for tumor monitoring and microenvironment-responsible technologies.9, 10 Besides, the nanoparticle carrier itself is also capable of stimulating an immune response, further showing therapeutic potential.11 However, cancer is complicated in terms of its mechanisms of development, which involves genes, various cells, and physiological processes. Cancer development encompasses several processes simultaneously, including proliferation, metastasis, and immune escape. In this regard, nanoparticles with single functions are not sufficient in all tumors. Therefore, there is a high demand to develop multifunctional nanoparticles.
A multifunctional nanoparticle is not simply an upgraded version of the original function. Instead, multifunctional nanoparticles integrate different functions to further expand the carrier's application, thus achieving two or more capacities.12, 13 For cancer therapy, this design can achieve tumor suppression, tracking, and microenvironment modulation, ultimately providing a more comprehensive monitoring and controlling strategy for tumor management. However, in addition to these advantages, multifunctional nanoparticles also have higher construction requirements. Except for their basic drug delivery ability, multifunctional nanoparticles must be capable of loading extra functions. The backbones of multifunctional nanoparticles are categorized as organic (polymeric, liposomes, and protein) or inorganic backbone (metal and nonmetallic), as well as biomimetic backbone (cell membranes and exosomes). Their backbones are responsible for joint external function groups or motifs with various chemical structures. Therefore, the backbones of these nanocarriers are expected to be more flexible in both the inner and surface layers, making them more amenable to covalent or noncovalent reactions. As multifunctional nanoparticles are designed to improve clinical treatment, their formulation and preparation procedures should be relatively simple. It is also challenging to have the two or more involved functions cooperate smoothly and even synergistically on one nanoplatform. With the realization of these conditions, multifunctional nanoparticles are expected to represent an efficient form of cancer therapy. Despite the above technical difficulties, many newly developed multifunctional nanoparticles have emerged in recent research. Indeed, these well-constructed multifunctional nanocarriers have demonstrated therapeutic potential in cancer treatment with varied function design.14-16
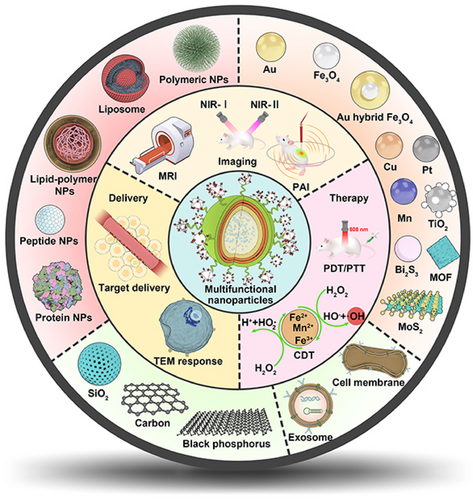
In this review, we provide a comprehensive understanding and detailed analyzation of the currently developed multifunctional nanoparticles; their platform structures were summarized in the aspect of organic, inorganic, and biomimetic-derived backbones. We especially emphasized on the functionalization and modification strategies that provide additional functions to the nanoparticle including drug delivery, in vivo imaging, and advanced therapy. We also discussed the application combination strategies that were involved in the development of discussed nanoformulations. The mechanisms that facilitate the functional crosstalk were also investigated, and their advanced applications in cancer diagnosis and treatment were highlighted (Figure 1).
2 BACKBONES AND STRUCTURES OF MULTIFUNCTIONAL NANOPARTICLES
According to the composition of nanoparticles, the backbones of multifunctional nanoparticles are categorized as organic (polymeric, liposomes, lipid–polymer hybrid, protein, and peptide) or inorganic backbone (Fe3O4, gold [Au], mesoporous silica, and graphene), as well as biomimetic backbone (cell membranes and exosome). Due to the plasticity and modification, these backbones are mainly prepared by covalent or noncovalent modification to obtain multifunctional nanoparticles for cancer therapy. Here, we highlight the modification strategies of organic, inorganic, and biomimetic backbones and the summary of multifunctional nanoparticle construction strategies is in Table 1.
| Category | Backbone | Multifunction construction strategies | Functions | References |
|---|---|---|---|---|
| Polymeric nanoparticles | Poly(lactic-co-glycolic) acid (PLGA) | Physical coating |
Drug delivery/H2O2 catalytic/ Immune response/photoacoustic imaging/ Ultrasound imaging/photosensitive/ Thermal-sensitive |
14, 16, 23-25 |
| Encapsulation | ||||
| Crosslinking with carboxyl groups | ||||
| Polyethylene glycol (PEG) | Crosslinking with hydroxyl groups |
Drug delivery/hypoxia-sensitive/ Subsequent nuclear targeting |
33-35 | |
| Polydopamine (PDA) | Crosslinking with amine groups |
Drug delivery/prolong blood circulation/ pH-sensitive/photothermal-sensitive/ Tumor targeting/MRI |
46-48 | |
| Crosslinking with catechol groups | ||||
| Crosslinking with the carbon-carbon double bonds | ||||
| Mesoporous polydopamine | Physical absorption |
Drug delivery/photothermal-sensitive/ GSH-sensitive/tumor targeting |
50, 51 | |
| Crosslinking with amine groups | ||||
| Crosslinking with catechol groups | ||||
| Crosslinking with the carbon-carbon double bonds | ||||
| Dendrimer | Crosslinking with terminal amine groups | Drug delivery/PET-CT Imaging/photosensitive/ GSH-Sensitive/H2O2-sensitive | 62-65 | |
| The inner core substitution | ||||
| Repeating units controlling | ||||
| Polyethyleneimine (PEI) | Crosslinking with amine groups |
Drug delivery/pH-sensitive/ ATP-sensitive/tumor targeting/ SPECT imaging/photosensitive |
71-73 | |
| Cyclodextrin (CDs) | Crosslinking with hydroxyl groups |
Drug delivery/redox-sensitive/ Tumor targeting/pH-sensitive/immune response |
15, 80, 81, 84 | |
| Polymeric nanoparticles | Chitosan | Crosslinking with amino groups |
Drug delivery/tumor targeting/pH-sensitive/ Photosensitive/ultrasound-sensitive/ Ultrasound imaging/ROS-sensitive/ Fluorescence imaging/photothermal-sensitive |
90, 91, 93 |
| Crosslinking with hydroxyl groups | ||||
| Dextran | Crosslinking with hydroxyl groups |
Drug delivery/pH-sensitive/ Redox-sensitive/tumor targeting/ Immune response/photothermal-sensitive |
101, 102 | |
| Hyaluronic acid (HA) | Crosslinking with carboxyl groups |
Drug delivery/tumor targeting/ pH-sensitive/GSH-sensitive/ Photothermal-sensitive |
109, 110 | |
| Crosslinking with hydroxyl groups | ||||
| Liposomes | Liposomes | Encapsulation |
Drug delivery/photosensitive/ Immune response/pH-sensitive/ Tumor targeting |
123-127 |
| Polymerization with polymers | ||||
| The head phospholipid substitution | ||||
| Lipid–polymer hybrid nanoparticles | Lipid–polymer hybrid | Encapsulation |
Drug delivery/tumor targeting/ Photosensitive/photoacoustic imaging/ US imaging/ROS-sensitive/ Ultrasound-sensitive |
133, 134 |
| Physical coating | ||||
| Protein and peptide nanoparticles | Protein | Physical adsorption |
Drug delivery/fluorescence imaging/ Photothermal-sensitive/pH-sensitive/MRI |
148, 149 |
| Crosslinking with carboxyl group | ||||
| Peptide | Crosslinking with amine groups |
Drug delivery/fluorescence imaging/ Tumor targeting |
142, 143 | |
| Crosslinking with carboxyl group | ||||
| Metal nanoparticles | Fe3O4 | Physical adsorption |
Drug delivery/magneto-thermal sensitive/ Photothermal-sensitive/magnetic targeting/ Fluorescence imaging/MRI/ Fenton-like reaction |
158, 159, 161, 162 |
| Physical coating | ||||
| Crosslinking with carboxyl group | ||||
| Mesoporous Fe3O4 | Physical adsorption |
Drug delivery/magneto-thermal sensitive/ Photoacoustic imaging/ultrasound imaging/ MRI |
163, 164 | |
| Superparamagnetic iron oxide (SPIONs) | Drug delivery | MRI/activate ferroptosis | 167 | |
| Gold (Au) | Crosslinking with Au-S bond |
Drug delivery/fluorescence imaging/ Redox-sensitive/photothermal-sensitive/ Electrical conductivity |
169, 176-179 | |
| Crosslinking with electrostatic interaction | ||||
| Fe3O4/Au hybrid | Crosslinking with Au─S bonds |
Drug delivery/photothermal-sensitive/MRI/ CT imaging/pH-sensitive/magnet-sensitive |
184 | |
| Copper (Cu) | Crosslinking with coordination bond | Drug delivery/GSH-sensitive | 190 | |
| CuS | Crosslinking with Cu─SH bond | Drug delivery/photothermal-sensitive | 193 | |
| Hollow porous CuS | Physical absorption |
Drug delivery/Tumor targeting/ Immune response |
194 | |
| Metal nanoparticles | Titanium (TiO2) | Crosslinking with ionic bond |
Drug delivery/immune response/ Photosensitive/Fenton-like reaction |
204-206 |
| Crosslinking with hydrogen groups | ||||
| UiO-66 | Physical absorption |
Drug delivery/pH-sensitive/ Immunosensor/photosensitive |
216, 217 | |
| Crosslinking with carboxyl groups | ||||
| MOF-5 | Substituting with other metal icons | Drug delivery/immune response | 218 | |
| MOF-74 | Crosslinking with metal-sulfur bond | Drug delivery/conductivity/redox-sensitive | 219 | |
| Platinum (Pt) | Crosslinking with coordination bonds |
Drug delivery/photosensitive/ pH-sensitive/immune response/ Tumor targeting |
233, 234 | |
| Physical absorption | ||||
| Mesoporous Pt | Physical absorption |
Drug delivery/photothermal sensitive/ CT imaging |
235 | |
| Molybdenum disulfide (MoS2) | Crosslinking with S─S bond |
GSH-sensitive/photothermal sensitive/ Tumor targeting/pH-sensitive/PET imaging |
242, 246, 247 | |
| Crosslinking with C─S bond | ||||
| Manganese atom | Encapsulation |
Drug delivery/MRI/fluorescence imaging/ Fenton-like reaction/immune response |
253 | |
| MnCO3 | Physical absorption |
Drug delivery/pH-sensitive/MRI/ Ultrasound imaging |
254 | |
| MnO2 | Crosslinking with polymers |
Drug delivery/redox-sensitive/ Photothermal sensitive |
255 | |
| Metallic nanoparticles | Bismuth sulfide (Bi2S3) | Physical adsorption |
Drug delivery/radiosensitive/ Tumor targeting/photoacoustic imaging/ CT imaging/photothermal-sensitive |
269, 270 |
| Encapsulation | ||||
| Nonmetallic nanoparticles | Mesoporous silica (MSNs) | Physical absorption |
Drug delivery/tumor targeting/ Photothermal sensitive/pH-sensitive/ Immune response |
|
| Crosslinking with silanol groups | ||||
| Physical absorption | ||||
| Hollow mesoporous silica (HMSNs) | Encapsulation |
Drug delivery/photothermal sensitive/ Fluorescence imaging/ PET imaging/ Chemiluminescence imaging |
288 | |
| Crosslinking with silanol groups | ||||
| Graphene | Intercalating with other metals |
Drug delivery/photosensitive/ Fluorescence imaging/MRI |
299 | |
| Physical absorption | ||||
| Graphene oxide | Crosslinking with hydroxyl groups |
Drug delivery/fluorescence imaging/ Photothermal-sensitive/tumor targeting |
302, 303 | |
| Crosslinking with epoxy groups | ||||
| Crosslinking with carboxyl groups | ||||
| Crosslinking with carbonyl groups | ||||
|
Carbon quantum dots |
Physical adsorption | Drug delivery/fluorescence imaging/MRI | 310, 311 | |
| Crosslinking with amine groups | ||||
| Crosslinking with hydroxyl groups | ||||
| Nonmetallic nanoparticles | Black phosphorus nanosheets | Physical coating |
Drug delivery/tumor targeting/ Photothermal-sensitive/pH-sensitive/ Immune response |
316-319 |
| Physical adsorption | ||||
| Charge coupling | ||||
| Biomimetic nanoparticles | Cell membranes | Encapsulation |
Drug delivery/photoacoustic imaging/ Photothermal imaging/ Fluorescence imaging/pH-sensitive/ Fenton-like reaction/ Immune response/tumor targeting |
330-332, 336, 337 |
| Crosslinking with amine groups | ||||
| Crosslinking with thiol groups | ||||
| Crosslinking with carboxyl groups | ||||
| Fusing with other cell membranes | ||||
| Exosome | Encapsulation |
Drug delivery/tumor targeting/ Fenton-like reaction/immune response/ Photosensitive |
342, 343 | |
| Crosslinking with amine groups | ||||
| Crosslinking with thiol groups | ||||
| Crosslinking with carboxyl groups | ||||
| Crosslinking with amine groups |
2.1 Organic backbone
2.1.1 Polymeric nanoparticles
PLGA: Poly(lactic-co-glycolic) acid (PLGA) is a copolymer of polylactic acid and polyglycolic acid.17 Due to its biodegradability and biocompatibility, several drugs based on PLGA have been approved by the United States Food and Drug Administration (US FDA) for clinical cancer application, such as Lupron Depot®, Sandostatin Lar®, and Trelstar®.18, 19 As PLGA nanoparticles possess both hydrophilicity and hydrophobicity, they have the potential to form a water/oil/water (W/O/W) core–shell structure by the compatibility principle.20, 21 Additionally, PLGA nanoparticles possess free carboxyl end-groups and can be modified as multifunctional nanoparticles, by connecting other bioactivation agents through covalent bonds.22 Based on the delivered ability of PLGA, there are three main modification strategies for cancer therapy, namely encapsulation, coating, and covalent modification. Initially, these water-soluble agents can be encapsulated in the PLGA core, while the hydrophobic agents are loaded in the PLGA shell, forming a core–shell structure. Chen et al.14 used water-soluble catalase (Cat) and hydrophobic imiquimod (R837) to prepare multifunctional nanoparticles based on PLGA (PLGA–R837@Cat). The Cat was encapsulated in the PLGA core and R837 was loaded in the PLGA shell. Under X-ray radiation, these nanoparticles can decompose H2O2 to generate O2 to relieve tumor hypoxia and activate the immune response. In this work, they used the classic shell–core structure of PLGA, the Cat and drugs were coencapsulated and formed multifunctional PLGA nanoparticles to realize the immunotherapy of cancer. Similarly, Li et al.23 reported that PLGA nanoparticles encapsulated with sinoporphyrin sodium and perfluoropentane (PFP) in the core, and IR780 loaded in the shell form multifunctional nanoparticles (DIPP-nanoparticles) for achieving cancer treatment with dual-mode imaging. Additionally, these bioactivation agents can be coated on the surface of PLGA nanoparticles to provide the function of coating substances, such as polydopamine (PDA). Indeed, Peng et al.24 prepared a multifunctional near-infrared responsive material based on PLGA nanoparticles (docetaxel [DTX]–PLGA nanoparticles@PDA-TPGS), in which PLGA was loaded with DTX and coated with PDA by an oxidative polymerization reaction. After intravenous treatment with these nanoparticles, the tumor was suppressed under chemo-photothermal therapy (PTT). Furthermore, multifunctional PLGA nanoparticles can also be prepared by direct covalent, forming of a block copolymer, such as PLGA–PEG. Yu et al.25 reported that PEG and peptide functional-modified PLGA nanoparticles could be used to deliver IR780, forming aNP@IR780. PLGA–PEG–maleimide was loaded with IR780 and then conjugated with PD-L1 binding peptide; as a result, aNP@IR780 showed a strong CT26 antitumor effect by activating immune cells following near-infrared irradiation (NIR). Additionally, Zhou et al.16 reported that using PLGA–PEG–poly(N-isopropylacrylamide) and PEG–PLGA–biotin to deliver photochemical agent (ATT-2), forming PATA nanoparticles, facilitated effective tumor targeting and the property of phase transition temperature. Moreover, PLGA nanoparticles can be also modified by target peptide to enhance cancer therapy or by codelivery with magnetic nanoparticles for increasing T2 contrast magnetic resonance imaging (MRI).26
PEG: Polyethylene glycol (PEG), also known as polyethylene oxide and polyoxyethylene, has a linear polymer structure.27, 28 PEG nanoparticles can prolong the systemic circulation time of the drug in the body and reduce immunogenicity, resulting in their wide use in cancer therapy.29 Due to their nonimmunogenic and biodegradable properties, PEG nanoparticles are regarded as a “stealth” polymer and several, including DOXil®, Asclera®, and Movantik®, are approved by the US FDA.30-32 Multifunctional modification strategies of PEG nanoparticles are mainly conducted to activate the two terminal hydroxyl groups and change the structure to introduce functional groups. To avoid the formation of self-crosslinking complexes, PEG with only one terminal hydroxyl group is synthesized, and another terminal hydroxyl group is modified by other functional groups, including the methoxy (MeO) group and amine groups. For example, Liu et al.33 prepared tumor-targeted IDM nanomedicine based on a PEG block copolymer. To this end, they used MeO–PEG–NH2 conjugated with β-benzyl-L-aspartate N-carboxy-anhydride form PEG-b-PBLA and modified it with 6-(2-nitroimidazole) hexylamine to codeliver with doxorubicin (DOX) and indocyanine green (ICG) to efficiently suppress 4T1 cancer by inducing an immunogenic response and PDT/PTT. Multifunctional modification of hydroxyl groups at the both ends of PEG realized tumor immunotherapy and photodynamic therapy (PDT). The terminal hydroxyl group of MeO–PEG (mPEG) is considered to be active at the COOH for covalent binding to hydroxy-containing drugs, forming a pH-sensitive ester bond. Jing et al.34 prepared multifunctional nanoparticles (PECL/DA-Tat-M) based on the PEG backbone. The amine groups on NH2–mPEG–PCL were conjugated with the carboxyl terminal groups on Tat peptide, and modified by 2,3-dimethylmaleic anhydride (DA) for targeting 4T1 breast tumors with a pH response. Additionally, the structure of PEG can be changed to form PEG derivatives, such as 8-arm PEGs. Indeed, Zhang et al. prepared a new PEG–Ce6–Fe2+-gossypol metal-phenolic networks (PFGs) based on the PEG backbone.35 To this end, they used 8-arm PEG–succinimidy-(N-methyl-polyethylene glycol (NHS groups) to conjugate with Ce6, Fe2+, and gossypol, forming PFGs to efficiently inhibit tumor growth by combining with chemo-photodynamic therapy (PDT). In other studies, PEG was modified by bifunctional groups for covalent binding to other bioactive agents to enhance target cancer therapy, such as NHS–PEG–mal, NH2–PEG–mal, and mal–PEG–COOH.36-38
PDA: PDA nanoparticles are prepared by the oxidative polymerization of dopamine hydrochloride.39 Given the abundance of catechol, quinine, and amine groups in the structure, PDA nanoparticles exhibit hydrophilic properties and are easy to modify.40, 41 Due to the advantages of excellent biocompatibility and photothermal conversion ability, PDA nanoparticles are regarded as a near-infrared absorbing materials and are widely applied in cancer therapy.42-44 Based on the dopamine hydrochloride structure of PDA, the modification design strategies of PDA involve active catechol groups, amine groups, and carbon–carbon double bonds by covalent modification.45 Specifically, the carbon–carbon double bonds in PDA nanoparticles have the potential to be covalently modified by reactants containing amine groups by Schiff base reaction, such as PEG–NH2. Indeed, Zhu et al.46 developed a multifunctional nanoplatform based on PDA nanoparticles (Fe(III)PP@SAS nanoparticles), in which the PDA nanoparticles were covalently modified by mPEG–NH2 by a Schiff base reaction to load with FeCl3·6H2O and sulfasalazine. These nanoparticles can trigger the cancer cell ferroptosis, with pH-sensitive and near-infrared light irradiation. Fe(III)PP@SAS nanoparticles utilized the carbon–carbon double bonds in PDA with covalent modification and realized multifunctional PDA nanoparticles treated cancer with photo-ferrotherapy. Moreover, the high number of double bonds in PDA can be covalently modified by reactants containing thiol groups (SH) using the Michael addition reaction for further modification, such as mPEG–SH. Zhou et al.47 reported a dual peptide (RGD and beclin 1) and mPEG–SH functional modified by PDA to obtain PPBR nanoparticles for targeting tumor cells by recognition of αvβ3 receptors and causing cell autophagy by beclin 1 peptide. Additionally, due to the catechol structure, PDA can directly conjugate with metal or nonmetal ions by coordinate covalent bonds, such as Fe. Wang et al.48 prepared multifunctional PDA nanoparticles doped with ferric ion and conjugated with alendronate (ALN) to form PDA/Fe–ALN nanoparticles. The catechol groups on PDA are centered on ferric ions by coordinate covalent bonds and are conjugated with the amino groups on ALN. The doping with Fe to generate PDA/Fe–ALN nanoparticles achieved successful MRI for bone tumors.
Mesoporous PDA is a mesoporous structure that was first introduced based on PDA nanoparticles. Compared with PDA nanoparticles, mesoporous PDA can load multiple drugs into the porous structure. Mesoporous PDA has significantly improved drug loading capacity and high photothermal conversion efficiency, which provides a new idea for developing multifunctional PDA nanoparticles.49 Based on the mesoporous structure of PDA, the multifunctional design strategies of mesoporous PDA mainly include codelivery of multiple drugs and covalent modifications with catechol, amine groups, and carbon–carbon double bonds. Typically, mesoporous PDA can codeliver two or more drugs by absorption in a porous channel modified by SH groups. Hu et al.50 reported that using folic acid (FA) PEG thiol (FA–PEG–SH) modified on the surface of mesoporous PDA (MPPD), and codelivered with perfluorooctane and IR820 in the porous channels could enhance the cellular uptake with GSH response. Similarly, Dai et al.51 reported that mesoporous PDA could also be modified by AS1411 aptamer and deliver DTX for targeting prostate cancer cells with chemo-PTT. Furthermore, other substances can be used to coat the surface of PDA based on its strong adhesion property; this would serve to improve cancer therapy with PDT, fluorescence optical imaging, and MRI guidance, such as cationic coating layer, covalent organic frameworks, and metal ions.44, 52, 53
Dendrimers: Dendrimers are nanosized polymeric nanoparticles composed of an inner core, repeating units, and terminal functional groups, forming three-dimensional (3D) spherical nanoparticles with a regular dendritic structure.54-56 Dendrimers can load various hydrophobic drugs through electrostatic interactions and hydrophilic drugs with covalent bonds or chelation, which can enhance the solubility of drugs and is widely used in cancer therapy.57, 58 Based on the delivery capability of the dendrimer, multifunctional dendrimer nanoplatforms can be designed by modifying terminal functional groups, changing the inner core, and altering the controllability of the number of repeating units.59, 60 Poly(amidoamine) (PAMAM) is a commonly used dendrimer, and the terminal amine groups of PAMAM can be conjugated with NHS groups and carboxyl.61 Indeed, Konopka et al.62 prepared a multimodal tracer (64Cu–Rho–G4–CML) based on the PAMAM G4 dendrimer. The amine groups of the PAMAM G4 dendrimer modified by NOTA chelator, amine-reactive tetramethyl rhodamine, and Nε-(carboxymethyl) lysine via NHS groups. The prepared 64Cu–Rho–G4–CML nanotracer could target the RAGE ligand and rapidly clear in blood for optical and positron emission tomography (PET) imaging. They prepared multifunctional dendrimers by covalently modifying the amine group at the end of PAMAM to realize PET imaging-guided tumor therapy. Similarly, Nabi et al.63 reported the preparation of 67Ga-DTPAPAM–PEG/GEF@MUC-1 to achieve imaging guiding of breast cancer. In this case, the amine groups of PAMAM G2 were conjugated with the carboxyl groups in diethylenetriaminepentaacetic acid through a condensation reaction, before being modified by PEG2000-Br to load gefitinib (GEF) and chelate with 67Ga to selectively kill cancer cells with a combination of chemotherapy to provide an imaging guide for breast cancer. Additionally, multifunctional dendrimers can be synthesized with a peptide core, such as glutamic acid and lysine. Indeed, Zhou et al.64 synthesized multifunctional dendrimer nanoparticles ((PBA4–E2E)2–PpIX–LA2) based on a peptide. This study synthesized a G2 glutamic acid dendrimer and reacted it with protoporphyrin IX to form the (OtBu4–E2E)2–PpIX compound, which was modified by primary amine-lipoic acid and conjugated with pinacol boronic ester to load paclitaxel (PTX). These dendrimers could control the drug release by GSH and H2O2 in response to cancer therapy. Additionally, Zheng et al.65 designed a PEGylated dendritic peptide conjugate (PDPP) based on glutamic acid and lysine to kill cancer cells under PDT. However, compared with multifunctional nanoparticles of PLGA and PEG, dendrimers are poor drug loading, have cellular toxicity, and high cost in covalently modification synthesis. Therefore, dendrimers should overcome the above disadvantages and construct multifunctional modification dendrimers nanoparticles with biocompatibility, safety, and easy synthesis properties for cancer therapy.
PEI: Polyethyleneimine (PEI) is a cationic polymeric that consists of repeating ethylamine units with linear and branched types.66, 67 Due to the strong positive charge on the surface of PEI, it is possible to condense nucleic acid by electrostatic interactions, facilitating high drug delivery efficiency for cancer therapy.68, 69 The multifunctional construction methods based on PEI are mainly mediated by covalent coupling amine groups.70 For example, the amine groups of PEI can be covalently coupled with carboxyl, such as 4-(bromomethyl) phenylboronic acid (PBA). Ma et al.71 reported that using PBA, Cetuximab (C225), and DA multifunctionally modified PEI, forming PEI–DMA–C225. The amine groups in PEI were modified by PBA via a substitution reaction to form PEI–PBA. After being conjugated with C225, these nanoparticles exhibited both a pH response and ATP response. After treatment with miR146a by tail vein injection, these nanoparticles could target androgen-independent prostate cancer and inhibit the growth of tumor cells. PEI–DMA–C225 nanoparticles utilized the amine groups of PEI to multifunctional modified with other bioactive groups, which added the capability of tumor environment response in PEI-based nanoparticles. Additionally, the amine groups of PEI could be covalently coupled with NHS groups, such as mPEG–NHS. Indeed, Zhu et al.72 prepared PEI with alkoxyphenyl acylsulfonamide (APAS) and 131I to form multifunctional nanoparticles (APAS–131I-PNPs/DOX). The amine groups in PEI were conjugated with mPEG–NHS and APAS to improve the cancer cell uptake. By loading DOX and labeling 131I, the prepared nanoparticles inhibited cancer cells in combination with chemotherapy and radiotherapy. Moreover, PEI can be self-assembled with other polymeric nanoparticles to form block polymers, such as PEI–PCL. Wang et al.73 prepared MPPD@IR825 nanoparticles based on poly (ethylene imine)-poly(ε-caprolactone) block polymers (PEI–PCL). The dimethyl maleic anhydride-modified PEGylated shell could increase the drug release of DOX and IR825; these nanoparticles exhibited efficient breast tumor ablation with chemo-PTT.
CDs: Cyclodextrins (CDs) are degradation products of amylase and consist of glucopyranose forming cyclic oligosaccharides.74 CDs are widely applied as delivery vectors for cancer therapy because of their hydrophilic properties and ability to form host-guest structures with drugs.75-77 Due to the multiple glucopyranoside units in the structure of CDs, the multifunctional design strategies based on CDs mainly modify the free hydroxyl groups by covalent coupling.78 β-Cyclodextrin (β-CD) is a commonly used CD with seven glucopyranose units, while the free hydroxyl groups can be activated in carboxyl groups such as N, N′-carbonyl diimidazole (CDI).79 Mousazadeh et al.80 prepared novel redox-sensitive folate-appended-polyethylenimine-β-CD host-guest supramolecular nanoparticles (HGSNP) for pH-dependent sustained intracellular drug release and simultaneous efficient gene transfection. The hydroxyl groups in β-CD were activated by CDI, forming carboxyl groups and conjugated with the amine groups in PEI to modify FA and load with DOX, forming HGSNP. The prepared HGSNP nanoparticles used the free hydroxyl groups in β-CD to multifunctional modified with FA, which realized the tumor targeting and pH response for cancer therapy. Additionally, the hydroxyl groups of β-CD could be covalently coupled with carbonyl groups through nucleophilic substitution. Similarly, Zhang et al.15 also used CDI to active the hydroxyl groups in β-CD to conjugate to the amino groups of PBA pinacol ester and self-assembled with DSPE–PEG to obtain OCD NP to regulate the proinflammatory microenvironment and target colon cancer. Moreover, the hydroxyl groups of β-CD could be activated by anhydride in order to introduce carboxyl groups, such as succinic anhydride. Rodell et al.81 used succinic anhydride to activate β-CD in carboxyl groups to conjugate with l-lysine for delivery of the immune agonist R848 to achieve cancer immunotherapy. However, carboxymethyl–β-CD (CM–β-CD) is a CD derivative formed by connecting the carboxymethyl group to the CD cavity through an ether bond.82 The carboxymethyl group is linked to the edge of the CD cavity through the ether bond, which can be dissociated to carboxyl groups for covalent coupling, such as to amine groups in cell-penetrating peptide.83 Wei et al.84 reported using a pH-responsive cell-penetrating peptide (R6H4)-modified CM–β-CD obtained by a condensation reaction and loaded curcumin (CUR) to form multifunctional RCC nanoparticles. These nanoparticles could target delivery and enhance the CUR anticancer effects safely and with no toxicity.
CS: Chitosan (CS) is a natural cationic polymer consisting of β-(1,4)-linked N-acetyl glucosamine units prepared by deacetylation of chitin in crustacean shells.85, 86 As CS nanoparticles have excellent biocompatibility and biodegradability, they can be used as a drug coating and drug delivery vector for cancer therapy.87, 88 Based on the N-acetyl glucosamine unit structure in CS, multifunctional design strategies of CS are active free hydroxyl and amino groups by conjugating with other agents.89 The active amino groups in CS can be covalently coupled with carboxyl groups through an amidation reaction. For example, Zhang et al.90 used glycol-modified CS (GCP) to conjugate with pyropheophorbide a (Ppa) and DOTA via an amidation reaction to form GCP-nanoparticles. They used radionuclide 99mTc/177Lu chelation with DOTA to realize radiotherapy. These nanoparticles could inhibit the growth of cancer cells in 4T1 tumor-bearing mice by laser irradiation. They utilized the free hydroxyl and amino groups in CS multifunctional modified with photosensitizes and radionuclide, which realized photodynamic radionuclide therapy of cancer based on CS nanoparticles. Additionally, the active amino groups in CS could be covalently coupled with NHS groups. Zhao et al.91 prepared an ultrasound-responsive multifunctional nanoparticle based on CS nanoparticles (SP94–DOX–NDs) for castration-resistant prostate cancer. With the help of a Mal–PEG2000–NHS linker, CS was conjugated with a SP94-HS peptide via a sulfhydryl-maleimide coupling reaction, forming SP94–PEG2000–CS to deliver DOX for effective anticancer treatment, with potential for real-time ultrasound imaging. Moreover, carboxymethyl CS, the carboxymethylated product of CS, adds a carboxyl group to CS, providing another modification method for multifunction, such as nucleophilic substitution.92 Shao et al.93 devised a multifunctional nanosystem (CMCh–BAPE–RGD@ICG) for targeted and image-guided PTT in gastric cancer. They used carboxymethyl CS (CMCh) to couple with 4-hydroxymethyl-pinacol phenyl borate (BAPE) to form an amide bond by bromination and nucleophilic substitution to obtain CMCh–BAPE nanoparticles for effectively inhibiting the tumor growth of gastric cancer under ICG-mediated near infrared imaging and PTT.
Dextran: Dextran is a hydrophilic natural polymer formed by the condensation of glucose.94 The polymer backbone consists of α-1,6 glycosidic bonds between glucose monomers.95 Similar to other natural polymers, with hydrophilic, biocompatible, and biodegradable characteristics, dextran can be widely used as scaffold, surface coating, and delivery vector for cancer therapy.96-99 Due to the glucose monomers in dextran, multifunctional strategies based on dextran mainly involve chemical modification of the active hydroxyl groups, which can increase the drug release by stimuli-responsive activity, combining immunotherapy and PTT for cancer.100 For example, the active hydroxyl groups in dextran can be covalently coupled with carboxyl groups. Indeed, Curcio et al. prepared nanoparticles (DFNPs) based on two new amphiphilic dextran derivatives.101 Condensation of cysteamine-modified dextran (DEX) with PEG600COOH could be conducted to obtain DEXssPEGCOOH. As DEXssPEGCOOH consists of hydrophobic chemicals with disulfide bridges, hydrazone, or imine, these nanoparticles showed stimuli-responsive activity to achieve the pH and redox-responsive release of anticancer drugs. The prepared DFNPs used active hydroxyl groups in dextran to multifunctional modified with bioactive agents by covalently coupling, which realized the targeting and stimuli-responsive activity for cancer therapy. Additionally, the active hydroxyl groups in dextran can be covalently coupled with aldehyde groups via an acetal reaction. Gao et al.102 reported the synthesis of a multifunctional vaccine (I-R-Ap-AcDEX nanoparticles) based on dextran for treating cancer with immunotherapy and PTT. The hydroxyl groups of dextran and 2-methoxypropene via an acetal reaction formed cyclic and acyclic acetals to obtain AcDEX nanoparticles, which were then conjugated with ICG, imiquimod (R837), and antigen peptide (Ap) to treat cancer.
HA: Hyaluronic acid (HA) is a natural polymer composed of d-glucuronic acid and N-acetyl-d-glucosamine.103 HA is a biodegradable hydrophilic polymer material that is used as a biological scaffold for cancer therapy.104, 105 Because of its tumor targeting, nonimmunogenicity, and biosafety properties, HA has been widely used in drug delivery systems for drug control and sustained release.106, 107 Due to the glucuronic acid and glucosamine in HA, multifunctional design strategies based on HA are chemically modified by active hydroxyl and carboxyl groups.108 For instance, the active hydroxyl and carboxyl groups can be separately conjugated with carboxyl and hydroxyl groups. Liu et al.109 synthesized multitarget and pH-sensitive nanoactiniaes to codeliver icariin (ICA) and curcumin (Cur) for breast cancer. The carboxyl groups in oligomeric HA were conjugated with the amine groups in adipic dihydrazide. The hydroxyl groups in oligomeric HA were modified by biotin to load with ICA and Cur. The prepared nanoparticles had a pH-sensitive hydrazone bond group that enabled ICA and Cur to accumulate in the tumor tissue and cancer stem cells. In this work, HA was multifunctional modified with carboxyl groups to form pH-sensitive groups and realize tumor environment response for cancer therapy. Similarly, Yang et al.110 designed GSH-responsive nanomicelles (IR780/PTX/FHSV) to encapsulate PTX and the photosensitizer IR780 iodide. The synthetic amphiphilic HA derivative (FHSV) were self-assembled into nanomicelles in an aqueous medium. Then, PTX and IR780 were loaded into the nanomicelles to form IR780/PTX/FHSV micelles. The prepared micelles could effectively enter tumor cells and kill cancer cells by producing reactive oxygen species (ROS) and IR780-guided PTT.
2.1.2 Liposomes
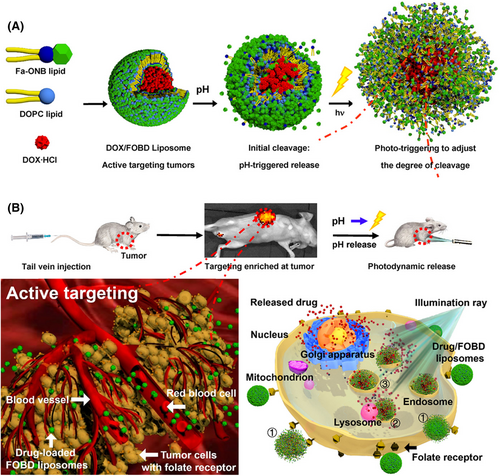
Liposomes are spherical vesicles with a bilayer structure composed of phospholipids.111 The structure of phospholipids contains a hydrophilic head consisting of a phosphate group and a quaternary ammonium salt group, as well as a lipophilic tail consisting of two longer hydrocarbon groups.112, 113 Liposomes have excellent compatibility with cells and are widely applied in drug delivery systems, and PEGylated liposomes are approved by the US FDA.114, 115 The components of multifunctional liposomes commonly include cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), dioleoylphosphatydic acid (DOPA), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and so on. These phospholipids are used in combination to increase stability of liposomes for multifunctional nanoparticle platforms, such as cholesterol/DSPC, cholesterol/DOPA, and DSPE/DOPC/DPPC.116-118 Due to the amphipathic structure of phospholipids, the multifunctional modification strategies based on liposomes mainly include physical encapsulation, chemical conjugation, and changes in the type of phospholipid head.119-122 Liposomes can encapsulate drugs in the inner water cavity or insert the drugs into a bilayer, forming a nanoparticle by self-assembly, such as a photosensitizer. For example, Liu et al.123 designed a biomimetic liposomal system (nano-Pt/VP@MLipo) for cancer treatment in which they loaded Pt nanoparticles in the liposome core, and encapsulated the photosensitizer Vitipophan (VP) in the liposome bilayer. These nanoparticles were coated with the RAW264.7 cell membrane, which could enhance the anticancer effect through VP-mediated PDT. Taking the advantage of the biocompatibility of liposomes, the multifunctional modification of cell membranes endows liposomes with biomimetic properties and tumor targeting, while encapsulates photosensitizers to achieve PDT of cancer. Additionally, Ding et al.124 prepared a multifunctional nanoparticle (LIC) to inhibit the PI3Kγ–AKT signaling pathway and function as a PDT under light irradiation by inserting an inhibitor and photosensitizer in the liposome bilayer. Additionally, liposomes can be grafted with other polymeric nanoparticles forming block copolymers to prolong the circulation time and coupling with other bioactive agents, such as DSPE–PEG–COOH. Gu et al.125 developed a multifunctional nanoparticle (CP@NP-cRGD) based on the DSPE backbone for codelivery with calcium chloroquine (CQ) and PD173074. DSPE–PEG2K–COOH was modified by cRGD by an amidation reaction to codeliver CQ and PD173074 to target pH-sensitive cancer. Similarly, Zhao et al.126 used PLGA12K–hyd–mPEG2K and DSPE–PEG1K–MAL to encapsulate metformin (MET), chlorin e6 (Ce6), and perfluorhexane (PFH) to effectively target cancer cells and suppress tumor growth by combining PDT and immunotherapy. Moreover, liposomes can also be synthesized with hydrophilic head and functional groups with environmentally responsive ability, such as o-nitro-benzyl ester. Indeed, Liu et al.127 developed a functional lipid (Fa–ONB) with FA and o-nitro-benzyl ester lipids (Figure 2). They conjugated activated FA to a lipid scaffold prepared from 4-(bromomethyl)-3-nitro-benzoic acid and didodecylamine by an amidation reaction to obtain the Fa–ONB lipid. The o-nitro-benzyl ester lipid enabled dual (pH and light) triggering reactions of drug release, while the FA could actively target the tumor tissue. However, the solubility and stability of liposomes are poor, which is not conducive to multifunctional modification. In addition, drugs encapsulated in liposomes are prone to leakage, and the preparation cost of liposomes are relatively high.
2.1.3 Lipid–polymer hybrid nanoparticles
Lipid–polymer hybrid nanoparticles mainly consist of a polymer core and lipid shell, forming a nanoparticle complex.128, 129 The polymeric nanoparticles can encapsulate active agents forming the core, with the lipid coated as a shell to increase the drug loading capacity.130 Lipid–polymer hybrid nanoparticles have been widely used in drug delivery for cancer therapy because of their stability and biocompatibility.130, 131 Due to the structural diversity of polymeric nanoparticles and liposomes, multifunctional modification strategies based on lipid–polymer hybrid nanoparticles can be used to deliver bioactive agents by physical capsulation forming a core–shell structure.132 For example, the hydrophobicity of a photosensitizer or sonosensitizer is encapsulated in a polymer core and coated with a target peptide-modified lipid shell. Chen et al.133 prepared folate-targeted lipid–polymer hybrid nanoparticles (LPH nanoparticles) loaded with ICG and perfluoropentane (PFP) for photo-sonodynamic therapy (SDT). The lipid film was prepared by DPPC, DPPG, DSPE–PEG-(2000)–FA, and cholesterol to form a lipid shell, while the PLGA polymer core was added through solution evaporation. LPH nanoparticles can target tumor cells with PA imaging guidance. LPH nanoparticles utilize the complementary characteristics of polymer and liposome to codeliver photosensitizers and sonosensitizers, while being modified with targeting receptors. These modifications achieve multifunctional lipid–polymer hybrid nanoparticles tumor targeting and dual-mode imaging guidance for cancer. Additionally, NIR dye and drugs can be encapsulated in a polymer core coated with multiple mixed lipid thin film. Zhang et al.134 reported the use of a B-PDEAEA/DNA@IR780-liposome to achieve efficient gene delivery and antitumor effects in pancreatic cancer via the binding of ROS-response polymer core and IR780-loaded liposome coating. Additionally, the polymer core can be designed with environment-response materials, while the lipid shell may also be modified by PEG to increase the stability and prolong the circulation time of drug release and loading with a sonosensitizer for cancer phototherapy.135, 136
2.1.4 Peptides and protein nanoparticles
Peptide nanoparticles consist of natural or synthetic amino acids the size of nanoparticles.137 Peptide nanoparticles are widely applied in cancer therapy, gene delivery, and target drug delivery because of the biodegradation capability of peptides.138, 139 Due to the active groups on the side chain of peptides, multifunctional design strategies based on peptide nanoparticles include physical adsorption and chemical covalent coupling.140, 141 For example, peptides contain benzene or macrocyclic structures that allow peptide nanoparticles to interact with various metals through π–π stacking or electrostatic interactions. Fan et al.142 assembled fluorescent peptide nanoparticles (RGD–f-PNPs/EPI) by cyclic peptides and modified them with RGD to encapsulate epirubicin (EPI) forming a multifunctional RGD–f-PNPs/EPI complex through π–π stacking and electrostatic interactions. Due to the surface modification of RGD, the prepared nanoparticles selectively targeted EC cells and promoted EPI internalization. These nanoparticles could be monitored by near-infrared fluorescence and were shown to have therapeutic effects against esophageal cancer.
RGD–f-PNPs/EPI nanoparticles utilized fluorescent peptide with cyclo[-(D-Ala-L-Glu-D-Ala-L-Trp)2-] sequence to creatively realize the potential of visible light and near-infrared molecular imaging, which provided a new idea for the construction of multifunctional peptide nanoparticles. Additionally, peptide side chains comprise numerous free carboxyl groups that can be covalently conjugated with amino groups. Guo et al.143 synthesized multifunctional dipeptide nanoparticles (DNPs nanoparticles) chelating Trp–Phe dipeptide with zinc ions. The carboxyl groups of DNPs nanoparticles were conjugated with clofarabine, aptamers AS1411, and influenza hemagglutinin peptide (HA) to codeliver siRNA and DOX to improve endosomal escape and biological imaging.
Proteins also have ideal multifunctional backbones with modification strategies that have been widely used in drug delivery for various cancer treatments because of their biocompatibility and biodegradability.144 As various amino acids consist of protein, multifunctional modification strategies based on protein nanoparticles include physical capsulation and chemical covalent conjugation.145 Albumin is a commonly used protein nanoparticle for cancer therapy due to its high drug loading efficiency, which bovine serum albumin (BSA) and human serum albumin (HSA) are commonly used in drug delivery system.146 There are multiple domains in the structure of albumin and different domains can bind with the different properties of drugs. Both BSA and HSA have three identical domains, and HSA has two major albumin binding sites, known as Sudlow site I and II, which can efficiently bind with hydrophobic drugs and deliver to the cancer site.147 Due to the functional groups of the side chain, multifunctional albumin nanoparticles could include modified amine groups with carboxyl groups.139 For example, Ding et al.148 synthesized a multifunctional nanoplatform (BITT@BSA–DSP nanoparticles) based on albumin to encapsulate the cisplatin (IV) prodrug. They oxidized cisplatin (IV) with H2O2 and reacted it with succinic anhydride to produce Pt(IV)–COOH (DSP). The carboxyl groups of DSP covalently reacted with BSA and were guided by AIEgen (BITT) to self-assemble, forming BITT@BSA–DSP nanoparticles that enhanced the chemotherapy effect of cisplatin and inhibited the growth of bladder cancer cells. Additionally, the amine groups in albumin nanoparticles can be functional modified by NHS ester. Zhang et al.149 designed a multifunctional nanosystem (MnAs/ICG/HSA–RGD) based on HSA. After the coordination reaction between cRGD-labeled HSA and manganese (Mn) chloride, ICG and arsenite were encapsulated to form MnAs/ICG/HSA–RGD; these nanoparticles released arsenite in a pH-responsive manner and significant inhibited the growth of liver tumor cells under the combination of ICG-guided-PTT and chemotherapy. However, protein nanoparticles are still worthy of improvement in maintaining protein activity, reducing immunogenicity, and improving stability.
2.2 Inorganic backbone
2.2.1 Metal nanoparticles
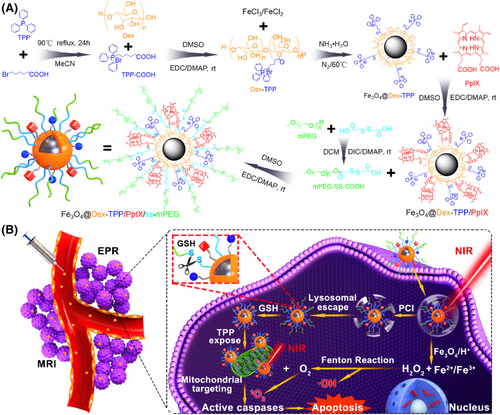
Fe3O4: Fe3O4 is a form of iron oxide with low toxicity, high biocompatibility, and magnetic properties that has been approved by the US FDA for biomedicine and widely applied in targeted drug delivery and MRI guidance for cancer.150-152 Due to the magneto-thermal energy conversion and delivery ability of Fe3O4, multifunctional modification strategies based on Fe3O4 nanoparticles mainly include codelivery, physical coating, and covalent coupling.153-156 Initially, Fe3O4 nanoparticles could be codelivered with polymeric nanoparticles and be used as the medium of magneto-thermal energy conversion for magnetic hyperthermia.157 For example, Liang et al.158 reported a multifunctional bone cement (DOX/Fe3O4@PMMA), in which DOX and Fe3O4 nanoparticles were interfused into poly (methyl methacrylate) (PMMA) powders by a mechanical vibration method. These nanoparticles could enhance the release of DOX and realize synergistic magnetic hyperthermia ablation and chemotherapy of osteosarcoma (OS). Based on Fe3O4 nanoparticles, different polymeric nanoparticles are introduced for multifunctional modification, which could not only increase the stability of Fe3O4, but also realize the magneto-thermal energy conversion for cancer therapy. Additionally, Fe3O4 nanoparticles can be coated with other photothermal polymers, forming a core–shell structure for PTT and magnetic targeting, such as PDA. Moreover, Wang et al.159 reported a type of multifunctional core–shell–corona nanohybrid (Fe3O4@PDA@anti-miRNA/DNA), in which dopamine was in situ polymerized on the Fe3O4 NP surface to form the core for PTT to provide magnetic targeting to tumor tissue. They also grafted anti-miRNA-21 oligonucleotides (anti-miRNA) onto its surface stranding as the shell, followed by base pairing with DOX-conjugated DNA-8pb corona, which could achieve consumption of tumorigenic miRNA-21 in tumor cells and the release of DOX–DNA-8bp for chemotherapy. Moreover, some organic materials (such as oleic acid) have the potential to be used to coat the surface of Fe3O4 nanoparticles to prevent their aggregation and improve their dispersion.160 Simultaneously, these organic materials can introduce various functional groups on the surface of Fe3O4 nanoparticles for further multifunctional modifications, such as oleic acid and PEG. Ren et al.161 developed a multimodified Fe3O4 nanoplatform (Fe3O4–PEG–Cy7–EMO), in which the Fe3O4 coated with oleic acid was conjugated with PEG-phospholipid and covalently grafted to GFLG–EMO and Cy7–NHS. These nanoparticles were shown to kill pancreatic cancer cells by FI/MRI dual-mode imaging in combination with chemotherapy. Similarly, Hou et al.162 used mPEG-ss-COOH to coat Fe3O4 nanoparticles and a delivery photosensitizer (protoporphyrin IX) for Fenton reaction-assisted PDT of cancer (Figure 3). Furthermore, the structure of Fe3O4 nanoparticles can also change the size and specific surface area, forming a mesoporous structure, which can increase the drug loading or dispersion by adsorption. Lu et al.163 designed a multifunction nanoparticle based on mesoporous Fe3O4 (PFP–m–Fe3O4@PGTTCs). In this case, magnetic mesoporous Fe3O4 was loaded with PFP by adsorption to form a PFP–m–Fe3O4 complex, which induced a magnetothermal effect to improve thermal ablation and achieve MRI and ultrasound imaging. Additionally, Deng et al.164 used the glypican-3 (GPC3) peptide to modify mesoporous Fe3O4 for specifically target hepatocellular carcinoma tumor cells and achieving combined ultrasound/photoacoustic imaging (PAI).
Superparamagnetic iron oxide nanoparticles (SPIONs) are synthesized by decreasing the particle size of Fe3O4. Due to their large specific area and superparamagnetism, SPIONs are regarded as multifunctional nanoparticles to directly deliver drugs and are widely used in MRI and drug delivery in cancer.165, 166 For example, Chen et al.167 prepared gelatin microspheres dual-loaded adriamycin (ADM) and Fe3O4 nanoparticles (ADM/Fe3O4 MS), in which the ADM/Fe3O4 MS were obtained using gelatin, ADM hydrochloride, and Fe3O4 nanoparticles via the high-voltage electrospray method. The introduction of SPIONs based on Fe3O4 enabled microspheres to possess the ability to induce hyperthermia and excellent T2-weighted MRI properties.
Au: Au nanoparticles consist of small Au particles forming nanoparticles in the shape of nanospheres, nanorods (NRs), nanocones, and nanomushrooms.168-171 Au nanoparticles have a large specific surface area and optical properties that allow drugs or bioactive agents to aggregate on the surface, and are widely used in cancer therapy.172, 173 Due to the large specific surface area for drug delivery, multifunctional modification strategies based on Au nanoparticles can be chemically or physically conjugated.174, 175 Initially, Au nanospheres were used to drugs or bioactive agents by noncovalent coupling through disulfide bonds (Au─S bond). Liu et al.176 used thiolated multidrug-resistance protein 1, multidrug resistance-associated protein 1, and breast cancer resistance protein combined with AuNP via Au─S bonds, forming MBs–AuNP. These nanoparticles were hybridized with three target mRNAs to reduce protein expression and restore the quenching fluorescence for in situ imaging. Au nanoparticles could combine with thiol groups to form the stability Au─S bond, which provide the great potential to prepare multifunctional Au nanoparticles. Similarly, Kim et al.177 described a nanoconjugate composed of Au nanosphere (Au nanoparticles) modified by photosensitizers by Au─S bonds for the treatment of glioblastoma multiforme with PTT and PDT. Zhang et al.178 also used cysteamine hydrochloride to modify Au nanoparticles using Au─S bonds for a cell fluorescence imaging (FI) probe. Additionally, Au nanospheres elongated along one direction could form Au nanorods (AuNRs), which have the same particle size as Au nanospheres, but a larger specific surface area for enhancing drug loading. Indeed, Pulagam et al.169 constructed multifunctional AuNRs, which were grafted with PEG methyl (mPEG) and modified by cobalt (III) bis (dicarbollide) (COSAN) via Au─S bonds. The high density of boron atoms on the AUNR surface enabled them to increase the local temperature under near-infrared light irradiation and realize the combination therapy of boron neutron capture therapies and PTT. Moreover, multifunctional Au nanoparticles could be modified by other metal materials by electrostatic interaction and used as a biosensor for monitoring cancer therapy, such as MoS2. Indeed, Hu et al.179 prepared an electrochemical cytokeratin fragment antigen 21-1 (CYFRA21-1) biosensor based on Au nanoparticles. To achieve this, they used HCl and LiF as etchant to react with Ti3AlC2 forming accordion-like Ti3C2Tx, and the MoS2 was embedded on the surface and crevices of Ti3C2Tx. The HAuCl4 was redox on the surface of MoS2 nanosheets forming AuNPs@MoS2@Ti3C2Tx. These prepared nanoparticles were combined with Nafion–Au nanoparticles loaded with Ab1, BSA, and CYFRA21-1 to obtain a biosensor, which could monitor nonsmall cell lung cancer because of its sensitive detection of CYFRA21-1.
Au nanoparticles can be combined with Fe3O4 to form Fe3O4/Au hybrid nanoparticles, which may have spherical, heterodimer, or core–shell structures.180-182 Due to the magnetic effect of Fe3O4 and the optical properties of Au, multifunctional modification strategies based on Fe3O4/Au hybrid nanoparticles primarily involve coating of the Fe3O4 surface with an Au forming core–shell structure, followed by covalent conjugation with other agents via the Au─S bond.183 The Fe3O4/Au hybrid nanoparticles are synthesized with a core–shell structure, and the Au surface could be modified by an Au─S bond, such as NHS-containing agents. Indeed, Rajkumar et al.184 synthesized a multifunctional core–shell Fe3O4@Au hybrid nanoparticle (Fe3O4@Au–DOX–mPEG/PEG–FA nanoparticles) to load DOX. HAuCl4 was reduced at the surface of Fe3O4 nanoparticles, forming Fe3O4@Au nanoparticles. l-Cysteine methyl ester was conjugated to the surface of Fe3O4@Au nanoparticles by forming an Au─S bond and then connected with NHS–mPEG and NHS–PEG–FA by forming amide bonds to obtain Fe3O4@Au–mPEG/PEG–FA nanoparticles, which could be used to load DOX for MR/CT imaging and synergistic chemotherapy/PTT for cancer. However, compared with organic nanoparticles, the disadvantages of multifunctional metal nanoparticles (Fe, Au) are the safety of in vivo metabolism, high cost synthesis materinals, and biocompatibility in clinical translation.
Cu: Copper (Cu) is a transition metal with excellent thermal and electrical conductivity, which can be used as a heat sink and a high thermal conductivity material.185, 186 Due to its large specific surface area and strong adsorption capacity for drug delivery, Cu nanoparticles can be used as multifunctional nanoparticles and hybridize with other nanoparticles to form nanocomplexes. According to the form of Cu, there are three main strategies based on the Cu multifunctional nanoplatform.187 Initially, due to their adsorption and redox reaction ability, the Cu ion could redox by glutathione (GSH) in cellular and chelate with other elements by forming coordination bonds, such via oxygen and sulfur atoms.188, 189 Chen et al.190 prepared a GSH-responsive nanoparticle (Cu–IXZ@DSF) based on Cu, in which the Cu was chelated with the ortho-hydroxy groups of ixazomib (IXZ) and was encapsulated with liposomes to codeliver disulfiram (DSF). The loaded DSF could be reduced to diethyldithiocarbamate by GSH, and then the sulfur atom could compete Cu2+ in Cu–IXZ to enhance drug release and tumor chemotherapy. They used the adsorption and redox reaction ability of Cu to modify other bioactive agents, and achieved Cu-based multifunctional nanoparticles for cancer treatment in GSH response. Additionally, copper sulfide (CuS) nanoparticles have excellent photothermal conversion and can be functionally modified by a Cu─SH bond, which is widely used in tumor combination therapy based on NIR-responsive PTT.191, 192 For example, Chen et al.193 developed an NIR light-triggered thermo-responsive multifunctional nanoparticle based on CuS (CuS–RNP/DOX@PEI) for controlled clustered regularly interspaced short palindromic repeat-associated Cas9 nuclease (CRISPR–Cas9) ribonucleoprotein delivery. DNA-SH fragments were combined with CuS nanoparticles by Cu─SH binding and assembled with Cas9 ribonucleoprotein (Cas9 RNP) through the principle of base complementary pairing. The DOX was inserted into the GC-sites of the DNA-SH/sgRNA complementary double strand, and finally PEI was coated to obtain CuS RNP/DOX@PEI. The prepared multifunctional nanoparticles could improve the release of Cas RNP under NIR light irradiation and realized photothermal cancer therapy for controllable gene editing. Meanwhile, CuS could be synthesized with a hollow porous nanostructure, which could deliver drugs in the porous channels with high loading by physical absorption. Moreover, Hao et al.194 reported using hollow porous CuS nanoparticles to codeliver six drugs for preventing tumor recurrence. The CuS was loaded with oxaliplatin in the hollow core and then modified by FA–polymer, TLR7/8 agonist, mannose, and poly(acetone oxime acrylamide) to induce immunogenic cell death (ICD) along with PTT. Furthermore, copper oxide (CuO) nanoparticles have excellent physical adsorption capacity and codelivery with polymeric nanoparticles, which could be used to enhance cancer therapy with PTT.195, 196
TiO2: Titanium (Ti) is a metal element with excellent ductility that is easily oxidized into various oxides in the air, among which, titanium dioxide (TiO2) is the most important stable oxide.197 TiO2 nanoparticles are poorly soluble and have low toxicity, and have been approved by the US FDA as a food color additive.198-200 TiO2 nanoparticles can kill tumor cells through photocatalytic reaction and are widely used as a sensitizer in cancer SDT.201-203 Due to the high surface area for drug delivery ability, multifunctional design strategies based on TiO2 nanoparticles are modified by physical adsorption or chemical covalent coupling. The surface of TiO2 nanoparticles could be modified through ionic bonds and electrostatic adsorption, such as Chlorin e6 and immune adjuvant. Indeed, Lin et al.204 developed a multifunctional nano sonosensitizer (TiO2–Ce6–CpG) based on TiO2 for the codelivery of Chlorin e6 and CpG oligonucleotide. The hydrolyzed TiO2 nanoparticles were loaded with Ce6 and CpG ODN onto the surface based on ionic bonds and electrostatic adsorption, forming TiO2–Ce6–CpG, which inhibited tumor growth in combination with TiO2–Ce6-guided SDT and anti-PD-L1 antibody checkpoint blockade. They utilized the advantage of high surface area in TiO2 to combine with photosensitizers through physical absorption and delivered immune agonists at the same time, which realized the immune-PDT for cancer based on multifunctional nanoparticles of TiO2. Similarly, Wang et al.205 reported the immunogenicity and adjuvanticity of TiO2 microparticles decorated with nanospikes to deliver monophosphoryl lipid A for cancer treatment. Additionally, the hydrogen groups on the surfaces of TiO2 can adsorb other molecules with hydrogen bonds, such as PEG. For example, Geng et al.206 designed multifunctional ultrafine W-doped TiO2 NRs (W-TiO2–PEG) based on TiO2 nanostructures. TiCl4 and tungsten hexacarbonyl (W(CO)6) forming W-TiO2 NRs were generated using a one-step high-temperature organic-phase method and then modified by PEG-600 by forming hydrogen bonds on the surface to form W-TiO2–PEG NRs. The prepared NRs could improve ROS generation and kill OS cells under SDT–CDT combination therapy.
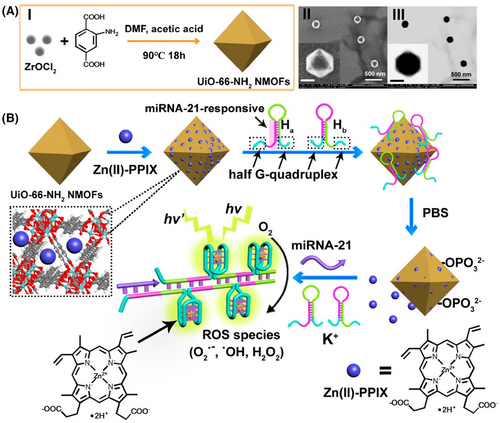
MOFs: Metal-organic frameworks (MOFs) are compounds formed with metals and organic ligands by organic linkers.207 Due to their cage-like structure, MOF can adsorb numerous drugs in the porous channels to realize codelivery.208, 209 Functional groups can be introduced in MOFs by different organic ligands, providing additional multifunctional reaction sites for cancer therapy. Due to the drug loading and delivery ability, multifunctional modification strategies based on MOFs include physical absorption and covalent coupling by the functional groups of organic ligands.210-213 According to the type of MOF, UiO-66 is a zirconium-based MOF, which was formed by terephthalic acid as the organic framework and connected with zirconium tetrachloride.214, 215 Due to the pores in UiO-66, drugs could be loaded in the pores by physical absorption. For example, Zhang et al.216 proposed a Zn (II)–PPIX-loaded nucleic acid-modified NMOF carrier for miRNA-guided imaging and PDT of cancer cells (Figure 4). The UiO-66–NH2 NMOFs were synthesized by the reaction of ZrOCl2 with aminoterphthalic acid ligand, and then loaded with Zn (II)–PPIX. Subsequently, nucleic acid hairpins Ha and Hb were bound to the vacant ligation sites of Zr4+-ions of NMOFs to obtain miRNA-21-responsive Zn (II)–PPIX-loaded Ha/Hb-locked NMOFs for selective imaging and PDT of cancer cells. The prepared NMOFs offered the high drug loading for miRNA, while ensured drug delivery and realized selective imaging and PDT treatment of cancer cells based on multifunctional nanoparticles MOFs. Additionally, UiO-66 could be introduced into functional groups (such as COOH or NH2) to conjugate with other agents, such as amine groups, by covalent coupling. Xiong et al.217 used 2COOH–UiO-66 to conjugate with the amine groups of antibody and PEI to coat Au nanoparticles to detect protein biomarkers in the early stages of cancer. Moreover, MOF-5 is a zinc-based MOF with an organic framework of terephthalic acid and a central metal of Zn2+. MOF-5 has a large specific surface area and could be substituted with other metal icons, such as Gd. Dai et al.218 reported a Ga-doped multifunctional nanoplatform based on MOF-5 (Gd–MOF-5). The terephthalic acid in MOF-5 was regarded as the ligand to conjugate Gd3+ forming Gd–MOF-5, which could achieve modulating immune stimulation signals and improve the efficacy of cancer immunotherapy. Moreover, MOF-74 is a MN-based MOF, which was formed by 2,5-dihydroxyterephthalate as the organic framework and the central metal with Mn2+. MOF-74 can be modified by metal nanoparticles by a metal─sulfur bond, such as Au─S or Ag─S. Liu et al.219 described a MnO@C nanocomposite based on Mn–MOF-74 for amplifying dual-signal electrochemical sensing signal of a sensitive cancer biomarker. Moreover, Mn–MOF-74 was synthesized to obtain MnO@C and conjugated with Au nanoparticles and Ag nanoparticles through Au─S and Ag─S bonds. The prepared nanocomposite prevented the agglomeration of MnO and improved its conductivity with metal modification. However, ZIF8220, 221, ZIF67222-224, and MIL100225, 226 were used as multifunctional nanoplatforms to enhance the treatment effect of cancer with PDT/PTT.
Pt: Platinum (Pt) is a transition metal that is commonly used as an alternative to Pt-based molecules in cancer therapy, such as cisplatin and oxiplatin (PtII).227 Pt(IV) can be oxidized to Pt2+ under slightly acidic conditions, which can trigger ICD in the context of tumor chemo-immunotherapy.228, 229 Due to the presence of empty orbitals in the valence electron shell of Pt, multifunctional modification strategies based on Pt nanoparticles form coordination bonds with other elements.230-232 Pt nanoparticles can be complexed with nonmetallic elements through coordination bonds, such as Pt-N3. Indeed, Lu et al.233 prepared dual-sensitive dual-prodrug nanoparticles (DD nanoparticles) based on Pt nanoparticles. The demethylcantharidin (DMC)–Pt (IV)–DMC prodrug was synthesized with DMC, AgNO3, and NaN3 to form a Pt─N3 bond, which was modified by mPEG2k─NH2 to obtain DDNP nanoparticles. The Pt─N3 bond of DD nanoparticles could be broken under UVA light irradiation and stability in the dark, imparting DD nanoparticles with an “on-off” effect to exert a greater anticancer effect. These modification strategies of Pt combined with other drugs through coordination bonds realized the dual-sensitive to light and pH of Pt-based multifunctional nanoparticles for cancer treatment. Additionally, Pt nanoparticles can be incorporated or coated with other polymers through physical absorption, such as PEG. Sun et al.234 prepared a multifunctional pH response nanoplatform (Pt@D nanoparticles) based on Pt nanoparticles. The PBA–PEG–PBA copolymer was mixed with H2PtCl6 to form PBA–Pt, and was BLZ-945 mixed with PLGA to form PNP. The PNP was modified by dextran, forming D nanoparticles, and covalent with PBA–Pt by a pH-responsive boronate bond to obtain Pt@D nanoparticles. The boronate bond could be broken under an acidic pH to release drugs to improve tumor cell killing in cancer chemo-immunotherapy. Moreover, Pt can be synthesized with a mesoporous structure to form mesoporous Pt nanoparticles, which possess the properties of large specific surface areas and adjustable apertures for enhancing high drug loading through electrostatic adsorption. Fu et al.235 reported a multifunctional nanoparticle (PEG@Pt/DOX) based on mesoporous Pt (mesoPt) nanoparticles. The mesoPt was synthesized using pluronic F127 surfactant as a structure-directing agent, and modified by SH–PEG–COOH on the surface, forming PEG@Pt nanoparticles. PEG@Pt was loaded with DOX by electrostatic adsorption forming PEG@Pt/DOX with high drug loading. Due to the Pt nanoparticles, PEG@Pt/DOX exhibited a strong photothermal transformation ability and CT imaging-guided PTT.
MoS2: Molybdenum disulfide (MoS2) is a two-dimensional transition metal dichalcogenide composed of molybdenum and sulfur.236, 237 MoS2 has a layered structure, in which the molybdenum atomic layer is sandwiched by the sulfide ion layer through van der Waals forces, forming a three-layer structure.238 Due to the sulfur atom being exposed on the surface of MoS2 nanosheets, forming defect vacancies, the multifunctional design strategies based on MoS2 nanosheets mainly include covalent or noncovalent combinations with other metals and organic small molecules by strong absorption.239, 240 In detail, the defect vacancies of MoS2 nanosheets can form a coordination bond with thiol, forming S─S bonds to enhance drug release with a GSH response.241 Liu et al.242 prepared a multifunctional nanoparticle (MoS2–SS–HA–CPT) based on MoS2, in which the MoS2 was bound with HA–SH though a disulfide bond, forming MoS2–SS–HA. Due to the modification of the disulfide bond, MoS2–SS–HA exhibited redox stimuli-responsiveness in the GSH environment. MoS2–SS–HA showed strong NIR absorbance and the capability of photothermal conversion. After loading with camptothecin (CPT), MoS2–SS–HA–CPT could target tumor cells for the synergetic chemo-PTT for cancer. They utilized the property of MoS2 to couple with thiol through forming S─S bonds and realized the redox/NIR dual response for cancer therapy based on multifunctional MoS2 nanoparticles. Additionally, the defect vacancies of MoS2 nanosheets can be encapsulated by polymers (such as HA, PEG, PEI, and CS), forming a C─S bond, which can enhance the drug release and be used in combination with photothermal and chemotherapy for cancer therapy.243-245 Dong et al.246 prepared MoS2–PEI–HA based on MoS2 nanoparticles. MoS2 nanosheets were coated with HA to introduce NH2 and COOH groups. The nanosheets could release drugs under acidic conditions, NIR laser lighting, and the enzyme HAase. Following conjugation with NOTA, the nanocomposite could chelate with 64Cu for PET imaging. After loading with DOX, MoS2–PEI–HA–DOX could release drugs in the tumor acidic microenvironment and be guided by PET imaging for cancer chemotherapy. Similarly, Liu et al.247 reported an MoS2 composite nanosheet material BBPL–MoS2–LP, in which the lipoic acid (LA)–PEG and LA–PEI-modified MoS2 was combined with biotin–BSA through amide bonds and loaded DOX to achieve synergistic targeted chemo-PTT.
Mn: Manganese (Mn) is a transition metal that is easily oxidized, with oxidation products including MnO, Mn2O3, MnO2, and Mn3O4. As Mn is a cofactor for many metabolic enzymes and has efficient redox properties, it can decompose H2O2 in tumors and generate highly toxic ROS, causing oxidative damage to tumor cells.248, 249 Due to their biocatalytic and redox abilities, multifunctional modification strategies based on Mn-based nanoparticles are modified by other agents by electrostatic attraction to form nanocomplexes.250-252 For example, Mn ions could be stored in oil phase and coated with pH-sensitive liposome to form a core–shell structure. Zhu et al.253 developed a multifunctional nanosystem (NanoMn–GOx–PTX) based on MN for the codelivery of the glucose oxidase (GOx), PTX. Na2HPO4 solution (mixed with GOx) was added to the configured oil phase and MnCl2 and DOPA were added to another oil phase, forming a monolayer lipid NanoMn–GOx core. PTX was loaded into a phospholipid bilayer shell to obtain NanoMn–GOx–PTX. These nanoparticles could generate ROS and enhance the anticancer effect. Additionally, Mn ions could be modified by CO32− ions by electrostatic attraction, forming MN ion carbonate with pH responsive abilities. Qi et al.254 constructed multifunctional nanotheranostics (BMC nanoparticles) based on pH-responsive MnCO3 nanocarriers to load DOX. They synthesized poly(ethylene glycol)-b-poly(l-aspartic acid) to conjugate with Mn2+ ions via electrostatic attraction and modified the complex with CO32− ions through an in situ mineralization approach to form biomineralized MnCO3 (BMC) nanoparticles. After loading DOX, the prepared nanoparticles effectively killed tumor cells by a chemodynamic therapy (CDT)/chemotherapy combination. Moreover, Mn ions could be redoxed by KMnO4, forming MnO2, which could be modified by polymers, such as polypyrrole. Li et al.255 reported multifunctional core-sheet polypyrrole@MnO2 nanocomposites (PPy@MnO2) to load methylene blue (MB). PPy@MnO2 was obtained using an in situ redox reaction between PPy and KMnO4. The mPEG–NH2 was conjugated on its surface by electrostatic attraction and then encapsulated MB through charge–charge attraction, forming PPy@MnO2–PEG–MB. The obtained nanocomposites showed high tumor inhibition effects under combined PPy-mediated PTT and PDT. Moreover, mesoporous MN trioxide (Mn2O3),256 MN phthalocyanine (MnPc),257 Mn3O4 nanoparticles,70, 258 and MnOx component259 were prepared based on Mn-based nanoparticles for enhancing cancer therapy with MRI, PTT, and SDT.
Bi2S3: Bismuth sulfide (Bi2S3) is an inorganic semiconductor material and is the main component of bismuthinite.260, 261 Due to its excellent biocompatibility and imaging performance, Bi2S3 was used as an imaging contrast agent and PTT agent in cancer therapy.262, 263 Regarding the imaging and delivery ability of Bi2S3, multifunctional design strategies based on Bi2S3 nanoparticles include codelivery with drugs or other agents to enhance drug delivery and cancer therapy with imaging guidance.264-267 Initially, multifunctional Bi2S3 nanoparticles could be coated on proteins by biomineralization and codelivery with metal nanoparticles,268 such as Au atoms. Nosrati et al.269 prepared a type of bimetallic theranostic nanoparticle, Bi2S3@BSA–Au–BSA–MTX–Cur, in which they used BSA to Bi2S3 nanoparticles by biomineralization and modified them with Au nanoparticles to deliver methotrexate (MTX) and Cur. The photothermal properties of Bi2S3 nanomaterials could be improved by incorporating Au atoms. Indeed, MTX conjugated to Bi2S3–Au nanoparticles as a targeted chemotherapeutic drug, and the loaded CUR could be used for both radiosensitizing and radioprotection. This platform served as a contrast agent, drug carrier, and nanoradiosensitizer, demonstrating effective anticancer effects. Additionally, multifunctional Bi2S3 nanoparticles could be capsulated by polymeric nanoparticles to realize codelivery, such as PLGA. Zhao et al.270 developed a multifunctional nanoparticle with targeted therapeutic function and dual-modal imaging guidance based on Bi2S3 for treating ovarian cancer. The Bi2S3 nanoparticles were encapsulated with PLGA–PEG–FA to deliver DOX and PFP, forming FBPD nanoparticles. These nanoparticles showed a good therapeutic effect of PTT combined with chemotherapy under the guidance of CT and PA imaging. Therefore, Bi2S3 nanoparticles could codeliver with multiple organic or inorganic nanoparticles to construct multifunctional nanoparticles, which realized imaging-guided photodynamic cancer therapy.
2.2.2 Nonmetallic nanoparticles
MSNs: Mesoporous silica nanoparticles (MSNs) are composed of the mesoporous form of silica with a pore size of 2–50 nm.271, 272 MSNs contain numerous silanol groups on the surface, which could be hydrolyzed under an acidic or alkaline environment, while silanol groups could be modified by various organic functional groups through covalent bonds or electrostatic interactions.273, 274 Drugs could be adsorbed in the pore channels of MSNs, which can protect drugs from being degraded and increase the solubility of drugs for cancer therapy.275 Based on the drug delivery ability of MSNs, the multifunctional design strategies of MSNs include coating with other compounds by electrostatic interactions or modifying with silanol groups by covalent coupling. Multifunctional MSNs could be coated with metal or organic materials by electrostatic interactions, such as MoS2, PDA, ICG, and CS.276 Wu et al.277 prepared a multifunctional PMOs–DOX@MoS2–PEI–BSA–FA complex based on periodic mesoporous organosilica nanoparticles (PMOs). PMOs were coated with MoS2 by electrostatic interactions and weak thiol reactions and modified by PEI–BSA–FA to deliver DOX to enhance drug release under laser irradiation to achieve synergistic chemo-photothermal targeted therapy. Similarly, Zhang et al.278 used PDA to coat mesoporous silica (MSNs) and modified it with ICG and RGD to absorb ammonium bicarbonate and DOX in the pores for combining cancer therapy with PTT/PDT. Park et al.279 also constructed multifunctional nanostructures (MONA) based on a mesoporous-silica nanosphere coated on an anti-EpCAM grafted Au layer for multifunctional cellular targeting and light-driven delivery of DOX to breast cancer. These modification strategies of coating realized cellular targeting and photodynamic cancer therapy based on multifunctional MSNs nanoparticles. Additionally, the silanol groups on the surface of MSNs could be modified by various organic functional groups through covalent bonds, such as NH2 and COOH.280, 281 Wagner et al.282 reported multifunctional nanocarriers (MSN–Phin–avidinout) based on core–shell MSNs. The core of the MSNs was functionalized with phenyl to accommodate hydrophobic R848, and the outer surface was functionalized with carboxyl groups to allow continuous modification to attach a pH-responsive acetal linker and a biotin-avidin complex for enhancing anticancer effects with immune reaction. Similarly, Yang et al.283 reported that carboxylated dendritic MSNs could be modified by PEI by forming amide groups and loaded with microRNA-125a for cancer immunotherapy. Lohiya et al.284 also conjugated carboxylated CS with the hydroxyl group on mesoporous silica to deliver DOX for targeted therapy for breast cancer.
Hollow MSNs (HMSNs) are a derivative of MSNs with the dual advantages of being mesoporous and hollow.285 The regular pore structure and spherical hollow part increases the specific surface area and significantly improves the drug delivery ability.286 HMSNs could encapsulate hydrophilic and hydrophobic drugs, up to three drugs, which combine chemotherapy with various antitumor strategies.287 Due to the drug delivery of HMSNs, multifunctional modification strategies based on HMSNs include codelivery with multiple drugs by electrostatic adsorption or modification by silanol groups. For example, Goel et al.288 designed CuS-coated and [89Zr]-labeled hollow mesoporous silica nanoshell nanoparticles ([89Zr]CSNCs–PEG10k) to load porphyrin molecules (TCPP). Negatively charged CuS–Cit was grafted onto the positively charged HMSN–NH2 by electrostatic adsorption and conjugated branched PEG via a condensation reaction, encapsulating TCPP to form [89Zr]CSNCexosomes–PEG10k. The prepared nanoparticles could produce excellent anticancer effects under CuS-guided PTT and TCPP-mediated PDT.
Carbon-based nanoparticles: Carbon-based nanoparticles consist of carbon elements, mainly including graphene, graphene oxide (GO), carbon quantum dots (CQDs, C-dots or CDs), and carbon nanotubes.289 Carbon-based nanoparticles have a large specific surface area, strong drug absorption, and easy functional modification, and are commonly used for drug delivery.290 Due to the drug delivery ability of carbon nanoparticles, multifunctional modification strategy-based carbon-based nanoparticles can be covalently modified and physically absorbed.291-296 According to the different preparation and structure from carbon nanoparticles, graphene is a single layer formed by the close packing of sp2-bonded carbon atoms, and its diameter is the size of a single carbon atom. Due to the property of photothermal conversion under NIR, multifunctional construct strategies of graphene include drugs loaded through π–π stacking and coordinated with other metals by intercalating in the carbon structure.297, 298 For example, Chen et al.299 reported a Gd@GCNs nanoparticle based on gadolinium-encapsulated graphene carbon, in which Gd acted as a coordination center in the formation of crystalline carbon layers, which intercalated in the final carbon structure. Gd@GCNs showed strong fluorescence and high T1 relaxivity as a dual-modal imaging probe for cancer therapy.
GO is synthesized by the oxidation of graphite with a two-dimensional honeycomb network structure. Functional groups can be introduced in the synthesis of GO, and include hydroxyl groups, carboxyl groups, epoxy groups, and carbonyl groups. Due to the functional groups in GO, multifunctional modification strategies of GO are mainly via covalent coupling. For example, the epoxy groups of GO could be conjugated with amine groups by a ring-opening nucleophilic addition reaction.300, 301 Zeng et al.302 described a multifunctional nanocomposite based on GO for OS. The epoxy groups of GO were conjugated with the amine groups of PEG by a ring-opening nucleophilic addition reaction to load with ICG to enhance PDT/PTT and inhibit ATP production to induce tumor apoptosis. Additionally, the carboxyl groups in GO are modified by bioactive agents containing amine groups, forming an amide bond. Xing et al.303 prepared a multifunctional nanodrug GDYO–CDDP/DOX@DSPE–PEG–MTX (GCDM) based on graphdiyne oxide (GDYO) for targeted cancer photo-chemo synergetic therapy. They developed a three-dimensional framework GDYO–CDDP using the amide reaction of GDYO and cisplatin (CDDP). GCDM nanoparticles were then obtained using DSPE–PEG2000–MTX and GDYO–CDDP by self-assembly. The prepared nanoparticles could load DOX through π-π stacking and treatment cancer with photo-chemo synergetic therapy.
CQDs (C-dots, or CDs) are nanomaterials composed of carbon, hydrogen, oxygen, and nitrogen, with a particle size <10 nm.304-308 CDs not only possess excellent photostability and efficient catalytic ability but also have surface modification flexibility. Based on the fluorescence ability of CDs, multifunctional CDs are covalently modified the free amine and hydroxyl groups.309 In detail, the metal cations can be adsorbed on their surface by conjugating with amino and hydroxyl groups and cause fluorescence quenching of CDs. With the reduction reaction of metal cations, the fluorescence of CDs could be realized by FI. For example, Li et al.310 developed a TME stimuli-responsive nanomedicine (Fe–CD) with CDs and Fe (III). The amino and hydroxyl groups on the surface of the CDs were conjugated with Fe (III). The Fe was redoxed by GSH in the cellular and restored the strong fluorescence of CD, which could achieve FI-guided CDT. Additionally, CDs could be modified by magnet metals and modified by polymers through hydrophobic interactions. Jia et al.311 prepared a magnetofluorescent carbon dot (Mn–CD) based on MN (II) phthalocyanine (Mn–Pc). The Mn–CD prepared by Mn–Pc through a solvothermal method and modified by DSPE–PEG. The results showed that Mn–CD had efficacy as an H2O2-driven oxygenerator for bimodal fluorescence (FL)/MRI-guided PDT. However, compared with organic nanoparticles, multifunctional carbon-based nanoparticles are still poor in hydrophobicity, high cost in synthesis, and small production.
Black phosphorous: Black phosphorous nanosheets are exfoliated from black phosphorus (BP) with a flaky monolayer structure; as a result, black phosphorous nanosheets have large specific surface area, which could adsorb drugs with high drug loading capacity.312, 313 Black phosphorous nanosheets are widely used in cancer therapy with excellent photothermal conversion ability for PTT/PDT/SDT.314 Based on the drug delivery ability, multifunctional design strategies based on black phosphorous nanosheets include codelivery with other agents by electrostatic adsorption to enhance drug release with PTT/PDT/SDT.315 Black phosphorous nanosheets can be codelivered with polymeric nanoparticles by electrostatic adsorption, such as PAMAM and PEG. For example, Peng et al. constructed a multifunctional nanoplatform based on black phosphorous nanosheets (BP NS–PAMAM@DOX–HA) to deliver DOX.316 The PAMAM was grafted onto the surface of BP NSs by electrostatic adsorption and coupled with HA to deliver DOX, which could produce excellent anticancer effects under chemo-photothermal synergistic cancer therapy. Based on the large specific surface area of, black phosphorous nanosheets multifunctional modified with polymeric nanoparticles, and realized NIR thermal imaging-guided chemo-photothermal cancer therapy. Similarly, Zhang et al.317 also designed mPEGylated black phosphorous nanoparticles (BP) modified by HA through a condensation reaction to form HA–BP for targeted and multiple combination therapies of cancer. Additionally, black phosphorous nanosheets could be mixed with other metal materials by charge coupling and electrostatic adsorption, such as Zn and Fe. Kang et al.318 described a composite nanosheet (M-RP/BP@ZnFe2O4) based on red/BP (RP/BP). The ZnFe2O4 nanoparticles were synthesized in situ on the RP/BP NSs surface by electrostatic adsorption. The combination of BP and RP achieved photocatalytic water splitting to generate H2O2 and O2. ZnFe2O4 could promote PDT by catalyzing the Fenton reaction and regulating the intracellular redox level through GSH consumption. Similarly, Li et al.319 used Zn2+ to modify BP by charge coupling to deliver DOX for immunotherapy and PTT.
2.3 Biomimetic backbone
2.3.1 Cell membrane biomimetic nanoparticles
Cell membrane biomimetic nanoparticles are an emerging nanobiomimetic technology formed by encapsulating nanoparticles or drugs with the membranes of various cells.320 The main component of cell membranes are phospholipids with a bilayer structure, which can prolong the circulation time in the body and achieve homologous targeting delivery for cancer therapy.321, 322 Cell membranes can be coated on the drugs or other nanoparticles through a physical coextrusion approach, sonication, and microfluidic electroporation forming a core–shell structure.323 Due to the target delivery of cell membrane nanoparticles, multifunctional modification strategies based on cell membrane biomimetic nanoparticles include noncovalent coating and chemical modification.324-326 Due to the biocompatibility of the cell membrane, the cell membrane can be coated on the photothermal materials to form a core–shell structure, such as Pt, Au,327 IR780,328 and IR820329. For example, Zhai et al.330 prepared a traceable bioinspired nanovesicle (MPV) based on a cell membrane-derived shell. They synthesized gelatin nanogels (NGs) using a two-step desolvation method and loaded Pt and MB to form MPNGs, and then coated them with red blood cell membranes. The prepared bioinspired nanovesicles could be used in the treatment of 4T1 breast cancer with chemo-PDT, while the tumor cells could be monitored by multimodal imaging. The prepared MPV multifunctional nanoparticles realized the potential of cell membrane biomimetic nanoparticles for photodynamic cancer therapy. Additionally, the surface of cell membranes of biomimetic nanoparticles could be modified by peptides, such as angiopep-2. Indeed, Zou et al.331 described a multifunctional biomimetic nanomedicine Ang–RBCm@NM–(DOX/Lex) based on functionalized red blood cell membranes (RBCms). The RBCms were modified by angiopep-2 to encapsulate with adextran/DOX/lexiscan nanoparticle to enhance drug release by traversing the BBB and targeting glioblastoma multiforme. Additionally, the cell membranes can be functional modified with other groups due to the amine groups, thiol groups, and carboxyl groups existent on the surface of membrane proteins. For example, Yu et al.332 created 89Zr-labeled multicompartment membrane-derived liposomes (89Zr–Df–MCLs), which were prepared by a membrane reassembling method that incorporated Tween-80 into the 4T1 cell membranes. p-SCN-deferoxamine conjugated with amine groups of proteins in the bilayer and then radiolabeled 89Zr, forming 89Zr–Df–MCLs, which effectively killed cancer cells by effective PDT.
Moreover, the hybrid cell membrane is also a multifunctional cell membrane biomimetic nanoparticle strategy. A hybrid cell membrane is prepared by fusing various cell membranes through electrostatic interactions, which contain various surface membrane protein markers from different cells. Based on the delivery capability of a cell membrane, multifunctional hybrid cell membrane nanoparticles are codelivered with functional materials by electrostatic interaction.333-335 Initially, the cancer cell membrane could be fused with the bacteria membrane by electrostatic interaction to encapsulate photothermal nanoparticle. For example, Guo et al. developed a multifunctional nanoparticle Fe3O4/MnO2/MM/EM/D based on the MCF-7 breast cancer cell membrane (MM) and Escherichia coli membrane (EM).336 MM and EM were obtained using ultrasonic and gradient centrifugation with electrostatic interactions and encapsulated with Fe3O4/MnO2 nanoparticles, forming a core–shell structure. These nanoparticles possessed outstanding photothermal properties to achieve PTT and CDT. Additionally, the cancer cell membrane could be fused with the immune cell membrane using a physical ultrasound method. Hao et al. prepared a hybrid membrane-coated nanosuspensions (DNS-[C6&DC]m) based on a C6 cancer cell membrane and DC membrane to deliver drug and antigen for antiglioma therapy due to the high drug carrying capacity of nanosuspensions and the professional antigen presentation ability of DC membranes.337 Moreover, others used a red blood membrane hybrid with MCF-7 breast cancer cell membrane to deliver melanin nanoparticles for enhancing cancer therapy with PDT.334
2.3.2 Exosomesc
Exosomes, another type of biomimetic nanoparticle, are membranous vesicles actively secreted by cells with a double-layered membrane structure.338, 339 Because of stability and transmembrane transport properties, exosomes are used as delivery vectors for virous cancer therapy.340, 341 Based on the delivery ability of exosome, multifunctional modification strategies based on exosome are noncovalently and covalently modification. Given their high biocompatibility with lipids, exosomes can be codelivered with other functional nanoparticles, such as Fe3O4. For example, Pan et al.342 designed a multifunctional nanosystem (EXO–PMA/Fe–HSA@DOX) based on urinary exosomes for the delivery of DOX. HSA and DOX were conjugated to poly (isobutylene-alt-maleic anhydride)-modified Fe3O4 and encapsulated into urinary exosomes via electroporation method. Due to the homologous targeting, these nanoparticles could accumulate in prostate cancer cells, providing significant anticancer effects under chemo/CDT. They utilized exosomes multifunctional modified with Fe3O4, which realized the tumor-specific targeting and immune escape of exosomes and chemodynamic cancer therapy. Due to the protein in exosomes, there are many amine groups, thiol groups, and carboxyl groups that can be covalently modified by other functional groups, such as amino groups. Qian et al.343 designed multifunctional nanoparticles (131I-EM@ALA) based on 131I-labeled exosome mimetic (EM) to deliver 5-aminolevulinic acid (ALA). 4T1 cancer cell membranes squeezed together and modified by the amine groups in ALA to obtain EM@ALA.131I was radiolabeled EM@ALA by the chloramine T method, forming 131I-EM@ALA. The prepared nanoparticles produced ROS and killed tumor cells by inducing DNA damage and activating the lysosomal mitochondrial pathway. Additionally, other scholars used cell-penetrating peptide or magnet nanoparticles to modify exosomes in order to enhance their tumor targeting or dual-modal detection abilities.344, 345 However, compared with cationic nanoparticles, multifunctional biomimetic nanoparticles (cell membranes and exosomes) are poor in transfection efficiency, isolation, and purification process.
3 APPLICATION STRATEGIES OF MULTIFUNCTIONAL NANOPARTICLES
Due to the variability and strong adaptability of cancer cells, they could adjust their structure or cell properties to adapt to the surrounding environment and continue to survive, which undoubtedly brings huge obstacles to treatment. However, a single functional nanoparticle drug delivery system seems not to be sufficient for diversified tumor treatment strategies. In order to monitor and treat the occurrence and development of tumor cells, multifunctional nanoparticles integrate different functions to further expand the carrier's application, thus achieving two or more capacities. Here, we highlight the delivery, therapy, and imaging function of multifunctional nanoparticles. The summary of functions associated with multifunction nanoparticles are in Table 2.
| Introduced function | Category | Functional groups/bonds/agents | Application | References |
|---|---|---|---|---|
| Delivery | Target | Folic acid | Chemotherapy with epithelial carcinoma tumor targeting | 127 |
| cRGD | Chemotherapy with nonsmall cell lung cancer targeting | 125 | ||
| Tumor cell membranes | Chemotherapy and immune stimulation with glioma targeting | 337 | ||
| Mucin-1 aptamer and T22-NLS peptide | Gene editing with tumor targeting | 360 | ||
| Cell-type-specific aptamer and TAT peptide | Targeting gene editing and photothermal therapy | 361 | ||
| AS1411 aptamer and TAT peptide | Gene editing with tumor targeting | 362 | ||
| pH sensitive | Imidazole | Chemotherapy with pH-sensitive promoting cell uptake | 372 | |
| Hydrazone bond | Chemotherapy with pH-sensitive promoting drug release | 109 | ||
| Amide bond | Chemotherapy with pH-sensitive promoting drug release | 34 | ||
| Zinc–ligand bonds | Cancer therapy with pH-sensitive enhancing mRNA delivery | 373 | ||
| Cyclic benzylidene acetal | Cancer therapy with pH-sensitive enhancing mRNA delivery | 374 | ||
| Added anilines to tune the pKa | Cancer therapy with pH-sensitive enhancing mRNA delivery | 375 | ||
| Redox sensitive | Diselenide bond | Protein therapy with redox-sensitive promoting drug release | 359 | |
| Disulfide bond | Gene therapy and chemotherapy with redox-sensitive promoting drug release | 80 | ||
| Thioketals bond | Chemotherapy with redox-sensitive promoting drug release | 387 | ||
| Therapy | Immunotherapy | Toll-like receptor 7/8 agonist | pH-sensitive promoting drug release and immune stimulation | 282 |
| PD-L1 inhibitory peptide | MRI-guided tumor targeting and immune stimulation | 324 | ||
| Ovalbumin | PTT collaborated with immune stimulation | 397 | ||
| Photodynamic (PDT) | IR780 | PDT collaborated with chemotherapy | 325 | |
| Quantum dots-chlorin e6 | PDT collaborated with cell therapy | 409 | ||
| Pyropheophorbide a | PDT collaborated with cancer therapy | 65 | ||
| Photothermal (PTT) | PDA | PTT collaborated with gene therapy | 42 | |
| Au | PTT collaborated with boron neutron capture therapy | 169 | ||
| Indocyanine green (ICG) | PTT, PDT, and starvation therapy | 419 | ||
| Sonodynamic (SDT) | AIPH | PAI/FI/US imaging-guided SDT | 431 | |
| Hematoporphyrin monomethyl ether | PAI-guided SDT | 428 | ||
| TiO2 | SDT collaborated with immune stimulation | 429 | ||
| Chemodynamic (CDT) | Fe3O4 | MRI-guided CDT and immune stimulation | 440 | |
| MnO2 | MRI-guided CDT and chemotherapy | 251 | ||
| Cu | CDT collaborated with chemotherapy | 441 | ||
| PDT/PTT | Au/Chlorin e6 | PDT collaborated with PTT | 177 | |
| Black phosphorus | FI/PAI-guided PDT, PTT and immune stimulation | 317 | ||
| PDT/CDT | Chlorin e6/hemin | PDT, CDT and ferroptosis | 451 | |
| PTT/CDT | Cu/black phosphorus quantum dots | PTT collaborated with CDT | 188 | |
| Therapy | CDT/PTT/PDT | MnO2/ICG/Au | MRI/FI/thermal imaging-guided PDT, CDT and PTT | 458 |
| Imaging | Magnetic resonance imaging (MRI) | SPIONs | MRI-guided hyperthermia and chemotherapy | 26 |
| ZnxFe3-xO4 NPs | MRI-guided immune stimulation | 468 | ||
| Mn2+ | MRI-guided PDT and immune stimulation | 470 | ||
| Fluorescence imaging (FI) | NCBS | FI-guided ROS-sensitive drug delivery | 478 | |
| Cr3+/Sn4+ | FI-guided chemotherapy | 479 | ||
| Fluorescent cyclic peptide nanoparticles | FI-guided chemotherapy | 142 | ||
| Photoacoustic imaging (PAI) | Black phosphorus quantum dots | PAI-guided CDT and PTT | 188 | |
| PDA | PAI-guided PTT and chemotherapy | 486 | ||
| Molybdenum | PAI-guided CDT and PTT | 487 | ||
| X-ray computerized tomography (CT) imaging | Cu3BiS3 | MRI /CT imaging-guided PTT and PDT | 445 | |
| Bi2S3 | PAI/CT imaging-guided PTT and chemotherapy | 270 | ||
| I2 | PAI/CT imaging-guided PTT | 492 | ||
| Multimodal imaging | Fe3O4/MnO2/Ce6 | MRI/FI/PAI-guided chemotherapy and PDT | 497 | |
| Fe3O4/perfluoropentane | MRI/PAI/US imaging-guided hyperthermia | 498 | ||
| 68Ga/Gd3+/Cr3+ | FI/MRI/PET imaging-guided chemotherapy and PDT | 499 |
- AIPH, Zr-MOF/2,2-azobis[2-(2-imidazolin-2-yl) propane] dihydrochloride; NCBS, silicon 2,3-naphthalocyanine bis(trihexylsilyloxide).
3.1 Multifunction in drug delivery
3.1.1 Targeted delivery
Targeting drug delivery systems refer to the selective delivery of drugs to target tissues or target organs by vectors and the mechanism of target delivery mainly includes passive targeting and active targeting.346 The advantage of targeted delivery is that the drug can be delivered to the specific treatment site to enhance the drug efficacy, reduce side effects, and achieve the purpose of effective cancer therapy.347, 348 To realize targeted drug delivery, multifunctional nanoparticles are modified by target function to enhance the activity of the drug at the target site and reducing the toxic and side effects at the nontarget site.349-351 One of the commonly target modifications is a small molecule compound, such as FA. FA is an essential substance in the process of tumor proliferation, and the folate receptor is highly expressed in most tumor cells as a mediator for tumor cells to absorb FA.352, 353 Liu et al.127 designed a multifunctional nanoplatform (FOBD) using FA-modified o-nitro-benzyl ester lipid for delivery of DOX-targeted therapy in cancer. Due to the high affinity of FA to folate receptors (FRs), the prepared nanoparticles not only achieved liposomal -based passive targeting (EPR) delivery, but also actively delivered DOX to tumor cells. This resulted in the enrichment of tumor cell activity after injection into tumor mouse models, achieving significant inhibition of tumor growth. Due to the high expression of integrin receptors on tumor cell membranes, cell penetrating peptides with RGD sequences (Arg–Gly–Asp) can bind to integrin receptors with high affinity, which is beneficial for nanoparticle tumor targeting.354-356 Gu et al.125 prepared a type of targeted ligand c(RGDfC)-modified multifunctional nanoparticle (CP@NP–cRGD) to deliver CQ and selective FGFR1 inhibitor for targeted therapy for nonsmall cell lung cancer. These nanoparticles could deliver drugs to tumor tissues with high delivery efficiency, and actively target tumor cells through cRGD peptides on the shell. Their results demonstrated that cRGD-nanoparticles showed more targeted distribution in vivo and effectively prevented the degradation of drugs during the circulation process to maximize the anticancer effect. Additionally, multifunctional nanoparticles coated with tumor cell membranes have the similar antigens and compositions to tumor cells, which show excellent tumor homologous targeting ability.357, 358 Hao et al.337 designed a hybrid membrane coated nanodelivery system (DNS–[C6&DC]m) based on a C6 cell membrane and dendritic cell (DC) membrane to load DTX for targeting glioma. The prepared nanoparticles had high drug loading ability to significantly increase the drug concentration, and due to the homologous targeting ability of the C6 cell membrane, these nanoparticles could more effectively inhibit the rapid growth of glioma by precise delivery of DTX to the brain tumor site. Additionally, Shao et al.359 also developed a bioinspired nanosystem (MSN@RNase A@CM) based on mesoporous silica coated cancer cell membrane (CM) for the delivery of RNase A targeted cancer therapy to show outstanding anticancer effects.
Moreover, due to the capability of precisely regulating the expression of abnormal genes, gene editing technology has a wide range of applications in various cancer. However, there are still challenges in efficient delivery the CRISPR–Cas9 systems. Except from the commonly used physical methods (electroporation and microfluidic membrane deformation methods) and viral vectors, multifunctional nanoparticles with ideal biocompatibility have shown promising applications in delivery CRISPR–Cas9 systems. These multifunctional nanoparticles commonly employ targeted modification strategies to increase the capability of affinity and precisely targeting to cancer cells. For example, Ren et al.360 used mucin-1 (MUC1) aptamer to modify alginate and added with T22-NLS peptide for multifunctional leukemia targeting CRISPR–Cas9 plasmid delivery system (P@PPM). In this work, MUC1 aptamer could specifically target MUC1 protein overexpressed on cancer cell membranes and the sequence of T22-NLS peptide could bind affinity with CXC chemokine receptor (CXCR4). These functional modifications endowed P@PPM a dual-targeting capability for leukemia cells. The prepared P@PPM could enhance CRISPR–Cas9 plasmid delivery into leukemia cell nuclei and obviously reduced the expression of CXCR4 mRNA after gene editing. In addition, the aptamer could also integrate with cell penetrating peptides, such as TAT peptide. Tang et al. developed multifunctional nanoparticles (GTLARC) based on Au NRs modified with TAT peptide and cell-type-specific aptamer (Linker-Apt) for targeted gene editing and cancer therapy.361 In this work, due to the specific recognition function of TAT peptide and Linker-Apt, GTLARC could target delivery sgRNA/Cas9 complex. The Au NRs in GTLARC showed strong photothermal conversion ability for PTT. These results showed that GTLARC could induce efficient cellular uptake by MCF-7 cells and inhibited the proliferation of tumor cells through combining targeting gene editing and PTT. Similarity, He et al.362 applied AS1411 aptamer and TAT peptide to multifunctional-modified carboxymethyl CS to delivery CRISPR–Cas9 plasmid for downregulation the β-catenin expression of tumor cells by targeting gene editing.
3.1.2 Environment-responsive delivery
The tumor microenvironment (TME) refers to the internal environment of tumor cells, including interstitial cells, microvessels, extracellular matrix, and many secreted factors.363, 364 Compared with normal tissues, the TME has abnormal physiological and biochemical characteristics, such as acidic extracellular pH, hypoxia, and excess GSH. To realize the target TME, multifunctional nanoparticles are designed with sensitive functional groups or bonds to enhance drug release for cancer therapy, such as pH sensitive and redox sensitive.365-367
pH sensitive: The pH-sensitive drug delivery of multifunctional nanoparticles is widely used in cancer therapy.368-370 Due to the hypoxia, lactic acid accumulation, and poor perfusion of the TME, the pH value of solid tumors is acidic. The extracellular pH of normal tissue is maintained at 7.4, while the pH of the TME is maintained at 6.5–6.8.371 To improve the acidic TME, multifunctional nanoparticles with pH-responsive bonds can be made to be cleavable in the TME and release drugs with tumor targeting. Multifunctional nanoparticles with the imine bond (C═N─) are prone to hydrolysis under acidic conditions, such as in the presence of amide bonds. For example, Jing et al.34 established a pH-responsive multifunctional nanoplatform (PECL/DA-Tat-M) composed of poly(ethylene glycol)–poly(ε-caprolactone) with a DA-Tat decoration to deliver 10-hydroxycamptothecin for improved anticancer efficacy. In the TME, the coupling of amide bond hydrolysis exposes positively charged Tat peptides. PECL/DA-Tat-M nanoparticles could promote cell uptake and show high antitumor efficacy, with inhibitory effects on lung metastasis of 4T1 breast tumor. Additionally, the hydrazone bond is similar to the amide bond with a large electron cloud density of the amino group, which is easier to cleave in the TME. Liu et al.109 constructed a type of multifunctional nanocarrier (Bio–oHA–Hyd–FA, nanoactiniaes) based on FA and biotin-conjugated HA (Bio-oHA) to codeliver icariin (ICA) and curcumin (Cur) for multitargeted therapy for breast cancer. This nanosystem not only enabled targeted drug delivery, but also allowed ICA and Cur to accumulate in tumor tissues through the formation of hydrazine bonds between Bio–OHA and FA. Their results showed that these nanoparticles could enhance the drug release at the TME and effectively suppress tumor volume with pH-sensitive properties. Due to the amphipathic nature of the imidazole compound, multifunctional nanoparticles modified by imidazole functional groups could be broken in the acidic TME. Indeed, Xu et al.372 designed a pH-responsive multifunctional nanosystem (DOX/IHC nanoparticles) based on imidazole and cholesterol conjugated hydroxyethyl starch (HES) for the delivery of DOX for cancer treatment. Due to the presence of amphiphilic imidazole on the surface, the IHC nanoparticles can effectively load DOX, showing pH-sensitive release behavior and the sustained drug release of nanoparticles. Their results also showed that the cumulative release of DOX/IHC nanoparticles increased significantly after 100 h with decreasing pH, reaching approximately 50.2 and 67.8% at pH 6.5 and 5.0, respectively.
In addition, mRNA-based therapy has the advantages of not integrating into the genome and expressing the target protein in the cytoplasm, and has important application prospects in cancer therapy. Due to the instability of mRNA, the difficulty for mRNA applications lies in the development of delivery vectors. However, multifunctional nanoparticles provide a new idea for the application of mRNA. Based on the efficient delivery capability of mRNA, these multifunctional nanoplatforms add pH-sensitive bonds for enhancing mRNA release at tumor site, such as zinc─ligand bonds. For example, Wang et al.373 prepared a pH-sensitive mRNA delivery platform (DMONs–ZIF-8) based on zeolitic imidazolate framework-8 (ZIF-8) and dendritic mesoporous organosilica nanoparticles. In their design, the large pores of ZIF-8 were used for enhancing loading capacity of mRNA. The zinc─ligand bonds in DMONs-ZIF-8 would be cleaved by acidic pH and promoted endosomal escape. Their results suggested that the prepared nanoparticles enabled successful endosomal escape and higher mRNA transfection efficiency. Similarity, Li et al.374 used pH-sensitive cyclic benzylidene acetal groups to functional modify lipidoid nanoparticles for enhancing mRNA delivery. Moreover, multifunctional nanoparticles could achieve the purpose of pH-sensitive by chemical modification to adjust the acid dissociation constant (pKa value), such as increasing the pKa value to release the mRNA in tumor acidic environment. Xiong et al.375 designed a multifunctional dendrimer-based lipid nanoparticle platform (DLNPs) with pH-sensitive for mRNA delivery and NIR imaging-guided tumor therapy. In their design, they used tetramethyl-BODIPY (4,4′-difluoro-4-bora-3a,4a-diaza-s-indacene) as core and added anilines to tune the pKa. With the pH decreased during endosomal maturation, the protonation of aniline bonds in PBD lipid could not only expose the BODIPY core, but also ensure the effective release of mRNA in the tumor microenvironment. Their results demonstrated that DLNPs could efficiently release mRNA and produce 5 to 35-fold more functional proteins.
Redox sensitive: Redox-sensitive drug delivery systems rely on redox-sensitive chemical bonds that can be broken by GSH or ROS reduction for precise delivery and rapid drug release.371, 376, 377 Due to the abnormally expressed redox substances in the tumor environment, overexpressed GSH and ROS can act as stimulators and be used in the design of multifunctional nanoparticles with redox-sensitive bond to enhance drug release. Disulfide bonds are the most commonly used chemical bonds in multifunctional nanoparticle strategies, and following delivery to the TME, they are reduced to sulfhydryl groups at high concentrations of GSH.378, 379, 140 For example, Mousazadeh et al.80 developed a host-guest supramolecular nanoparticle (HGS nanoparticles) based on folate-appended-PEI-β-CD to deliver adamantane-conjugated DOX (Ad-DOX) and human telomerase reverse transcriptase-small interfering RNA (hTERT siRNA) for targeted cancer therapy. Due to the redox-sensitive disulfide bond in the system, the drugs could be released into cancer cells in response to their biological environment, showing the comprehensive antitumor properties of DOX-enhanced gene transfection. Additionally, selenide is a congener of sulfur and the diselenide bond could be oxidized by endogenous H2O2 to seleninic acid.380-383 Shao et al.359 used the cancer cell membrane-modified selenium-based MSNs to deliver RNase A (MSN@RNase A@CM) for cancer treatment. These diselenide-bridged MSNs can encapsulate RNase A into internal pores via electrostatic interactions and release RNase A upon exposure to oxidation or redox conditions in the TME. The results showed that selenium-based MSNs degraded rapidly after incubation with H2O2 and GSH conditions and exhibited excellent cancer cell inhibition efficiency. Moreover, thioketals (TK) bonds can be cleaved by ROS in the tumor site, forming a thiol-containing group and acetone.384-386 Liao et al.387 developed a nanomedicine (M-TDOX/Lap@TGC) composed of DOX conjugated MSN, β-Lapachone (Lap), and triphenylphosphonium-modified CS for improving synergistic oxidation-chemotherapy. DOX was conjugated with MSNs and formed TK bonds. The loaded Lap-induced increases in ROS through the quinone oxidoreductase 1 catalytic pathway. Their results showed that compared with the free DOX group and free Lap group, the M-TDOX/Lap@TGC group showed the most effective inhibitory effect, with a tumor inhibition rate of 78.49% in C666-1 tumor-bearing mice. Moreover, others used thioether bonds and Au─S bonds to modify nanoparticles to enhance drug release with a redox response.280, 388-390
3.2 Multifunction in therapy
3.2.1 Immunotherapy
Tumor immunotherapy is a treatment method that produces tumor-specific immune responses through active or passive methods to inhibit and kill tumors.391, 392 Tumor immunotherapy regulates the polarization of immune cells and the active immune signaling pathway for treatment of cancer with fewer side effects compared with conventional therapy. Based on the drug delivery ability of nanoparticles, the immune activators are codelivered by multifunctional nanoparticles to enhance cancer therapy through promoting an immune response. The small molecular Toll-like receptor (TLR) agonist is a natural immune system stimulator, which can activate DCs and effector T cells to release proinflammatory factors.393-395 For example, Wagner et al.282 designed a multifunctional nanoplatform (R848-loaded MSNs) based on MSNs to deliver the TLR7/8 agonist resiquimod (R848) for efficient cancer immunotherapy. In their design, R848 stimulated DC maturation, resulting in the expression of high levels of MHC and costimulatory molecules CD80 and CD86 on the surface of DCs, which promoted the secretion of proinflammatory cytokines leading to an immune response. The PD-L1 inhibitory peptide could block the combination of PD-1 on T cells and PD-L1 on tumor cells, to relieve the inhibition of T cells and resume normal defense function.396 Meng et al.324 constructed a multifunctional nanoplatform (SPIO NP@M-P) based on H460 lung cancer cell membranes coated with SPIONs for the targeted delivery of PD-L1 inhibitory peptide (TPP1) as a cancer immunotherapy. The results showed that the affinity between TPP1 and PD-L1 was higher than that between PD-1 and PD-L1, suggesting that TPP1 is an effective inhibitor of the PD-1/PD-L1 interaction to reactivate T cell function. The ability of TPP1 to interfere with PD-1/PD-L1 was then applied as a potential drug for tumor immunotherapy. Moreover, ovalbumin (OVA) is a protein antigen that can be taken up by DCs to present exogenous antigens to MHC I via lysosomal escape and further induce a cytotoxic T lymphocyte (CTL) response. Huang et al.397 used PDA coated MSNs to deliver model antigen OVA and ammonium bicarbonate ((MSN–ABC@PDA–OVA) for photothermal-immunotherapy against melanoma. OVA can be effectively delivered to the target site due to the load protection in these nanoparticles, which induces significant immune responses. The prepared nanoparticles significantly reduced tumor-associated immunosuppression and effectively eradicated primary tumors with a cure rate of 75% for B16-OVA melanoma in model mice. Additionally, others used multifunctional nanoparticles coated with a DC cell membrane to enhance tumor immune therapy337.
3.2.2 Photodynamic therapy
Photodynamic therapy (PDT) is a means of delivering photosensitizers (PS) by vectors to directly irradiate the site with a specific wavelength of laser to produce photochemical reactions.398 PDT needs to meet the conditions of visible light, oxygen, and the presence of PS.399 Several PS have been approved by the US FDA for cancer therapy, including Porfimer sodium (Photofrin) for bladder cancer treatment and 5-ALA for actinic keratosis treatment.400 When the PS receives light energy, it can transfer the energy to the surrounding oxygen and generate ROS or singlet oxygen (1O2)401. Due to the enhanced permeability and retention effect (EPR effect) of the multifunctional nanoparticles, PS can be delivered to the tumor site and enhance cancer therapy with light energy.402-404 IR780 is a small molecule of cyanine dye, containing two nitrogen-contained heterocycles connected by sparse bond.110 The nitrogen-containing heterocycles have charged chromophores that can generate electronic transitions and induce PDT under near-infrared light irradiation. For example, Zhang et al.325 developed a multifunctional nanoparticle (HA&RBCm-LC nanoparticles) composed of an HA-hybridized RBC membrane and lipid to deliver of PTX and IR780 for nonsmall cell lung cancer PDT. IR780 could quickly and efficiently produce toxic1O2 after being irradiated by NIR at 808 nm, and generated ROS for PDT-induced endogenous mitochondrial apoptosis. Their results showed that the apoptosis rate of A549 cells in the PTX/IR780-HA&RBCm-LCNP group treated by NIR was higher than that in the non-NIR-treated group, suggesting that IR870-induced PDT significantly increased tumor apoptosis. Chlorin e6 (Ce6) is obtained from the degradation of chlorophyll a, and has strong absorption in the near-infrared for deep tissue treatment.405-408 Dapkute et al. prepared photosensitizer Ce6-modified CdSe/ZnS photoluminescent quantum dots (QDs), which combined cancer diagnostic and PDT abilities.409 The energy from excited QDs could be efficiently transferred to Ce6 attached to its surface via the Förster resonance energy transfer mechanism under blue light irradiation, leading to the generation of 1O2 and improved PDT. Their results illustrated that QD-Ce6 complexes generated 10-fold and 19-fold higher singlet oxygen than Ce6 and QDs in PBS, respectively. Additionally, Ppa, a compound formed by the natural product chlorophyl a with an aromatic azalenene structure, has strong absorption spectra at 600—700 nm.410 Zheng et al.65 developed a self-stabilized supramolecular assembly (SSA) based on a PEG-dendritic peptide-Ppa (PDPP). Ppa possesses an aromatic azalenene structure, which could generate singlet oxygen and trigger cell death under 660 nm laser irradiation. Their results demonstrated that Ppa exhibited strong cytotoxicity to A549 cells under laser irradiation and was used for PDT. After laser irradiation, the tumor retention and PDT effect of Ppa-based SSA were significantly enhanced in two highly metastatic 4T1 breast cancer and A549 lung cancer animal models. Moreover, multifunctional nanoparticles with photo sensitivity could be used as photosensitizers to enhance cancer therapy with light energy, such as Pt,233 Mo2C,295 ICG,411 red/BP,318 and CQDs.309
3.2.3 Photothermal therapy
PTT refers to the use of photothermal agents (PTA) to absorb the energy of near-infrared light and convert it into thermal energy, causing thermal damage and ablation to tumor tissue or cells.412 In this process, PTA exhibits a thermal effect and the increased temperature could damage the tumor vasculature and lead to a lack of oxygen and nutrients, causing the change in tumor microenvironment and the death of tumor cells.334 The thermal energy is introduced in multifunctional nanoparticles for increasing drug release and cancer therapy with PTT.413 To realize PTT, multifunctional nanoparticles with photothermal conversion ability or codelivery with photosensitive molecules can be employed in this delivery system. Moreover, PDA is an organic polymer that can absorb NIR light and improve photothermal conversion ability, while increasing the drug release as ananoparticle vector.207, 414 Zhang et al.42 prepared FA-modified PDA nanoparticles for ROC1 siRNA delivery with PTT. The prepared nanoparticles not only had the ability to target tumor cells and pH response to effectively deliver ROC1 siRNA, but could also induce the killing of a vast number of cancer cells with the photothermal properties of PDA. Compared with control groups, their results showed that PDA nanoparticles improved drug release to 26% by thermal expansion efficacy. After knocking down the ROC1 gene, PDA nanoparticles could inhibit the growth of Huh7 cells increasing to 55%. Additionally, Au nanoparticles have the ability to localize surface plasmon resonance (LSPR) absorption, which can convert light energy into heat energy by nonradiative relaxation.415-417 Pulagam et al. designed multifunctional nanoparticles ([64Cu]AuNR–mPEG@[4]−) based on 64Cu-labeled Au NRs modified by boron-based anion and PEG (mPEG) for PTT for cancer.169 Due to LSPR absorption and scattering of AuNRs, light energy is converted into heat energy by nonradiative relaxation under NIR light, resulting in cell death. Their results showed that under the irradiation of 808 nm NIR laser for 48 h, the local temperature of the 3D cell model incubated with AuNRs significantly increased to 23°C and the cell viability was obviously decreased. Additionally, ICG is a near-infrared dye with an ethenyl indole structure that has been approved by the US FDA for clinical diagnostic imaging with good photothermal conversion efficiency.418 Zhou et al.419 designed a HA-modified multifunctional microneedle (ZIF–ICG@ZIF–GOx–Cat@HA MNs) based on a ZIF-8 to codeliver GOx, catalase (Cat), and ICG for cascade reaction-enhanced cancer therapy. The stability of GOx and Cat was improved due to the spatial limitations of ZIF-8. The results showed that tumor cells were more sensitive to ICG-guided PTT through the down-regulation of heat shock protein induced by Gox, while the local tumor temperature was rapidly increased to inhibit the growth of tumor cells following intravenous injection.
3.2.4 Sonodynamic therapy
SDT is a type of PDT that uses low-frequency low-intensity ultrasound to irradiate a sonosensitizer to generate ROS to kill tumor cells.420 The treatment mechanism of SDT mainly includes generating ROS, acoustic cavitation, and inducing tumor apoptosis.421 SDT is a noninvasive treatment method that can deeply penetrate the tumor with fewer side effects, showing excellent development potential in the field of tumor treatment.422-424 Moreover, ultrasound has also been introduced in multifunctional nanoparticles to increase tumor apoptosis and enhance cancer therapy with SDT. Multifunctional nanoparticles could be codelivered with a sonosensitizer to realize SDT.425-427 Hematoporphyrin monomethyl ether (HMME) is a porphyrin derivative with a tetrapyrrole ring structure that could generate highly toxic singlet oxygen under ultrasound irradiation. Huang et al.428 prepared PLGA-encapsulated melanin nanoparticle and HMME nanoparticles with FA modification for PAI-guided SDT. The tetrapyrrole ring structure of HMME enables it to absorb energy and produce highly toxic singlet oxygen under ultrasound irradiation. After treatment with FHMP-nanoparticles for 15 days, the nanoparticles exhibited high tumor growth rate in MDA–MB-231 tumor-bearing mice and combined US irradiation with PAI for cancer therapy. Additionally, multifunctional nanoparticles could be codelivered with TiO2, in which the defect in the TiO2 lattice acts as an electron trap site to oxidize H2O to generate •OH and 1O2 under US irritation.202 Tao et al.429 reported a multifunctional cascade nanozyme (HABT-C) with TiO2 nanosphere, which was modified by Au nanoparticles and a carbon dot to enhance the effect of SDT, alleviate hypoxia, and reverse immunosuppression for cancer therapy. The defect in the TiO2 lattice acted as an electron trap site to oxidize H2O to generate •OH and 1O2 under US irradiation, resulting in oxidative damage to cancer cells. Their results showed that the tumor growth of the HABT-C group with US irradiation was effectively suppressed after 16 days of treatment, and no obvious tumor recurrence was observed in the tumor xenograft mouse model. Moreover, multifunctional nanoparticles could be codelivered with an MOF based on a porphyrin derivative as an organic ligand, which could convert the surrounding oxygen into 1O2 under US stimulation.430 Zhang et al.431 prepared a dual-sonosensitizer nanoparticle (Zr–MOF@AIPH nanoparticles) based on a zirconium MOF loaded with 2,2-azobis[2-(2-imidazolin-2-yl) propane] dihydrochloride (AIPH) for photoacoustic, fluorescence, and ultrasound imaging-guided SDT. As a porphyrin-based sonosensitizer, Zr–MOF could convert the surrounding oxygen to 1O2 under US stimulation. AIPH could generate alkyl radicals under hypoxia. The generation of singlet oxygen and oxygen-irrelevant radicals enabled strengthened SDT efficiency. Their results showed that the Zr–MOF@AIPH with US had an obvious inhibitory effect on tumor growth with a tumor inhibition rate of 41.9%.
3.2.5 Chemodynamic therapy
CDT is a new type of minimally invasive therapy for tumor treatment that is based on the acidic tumor environment, in which the overexpressed H2O2 is catalyzed by transition metals, causing a Fenton or Fenton-like reaction in tumor cells.432, 433 The Fenton reaction refers to a classical reaction in which H2O2 is catalyzed by ferrous ions (Fe2+) to generate hydroxyl radical (•OH). The Fenton-like reaction refers to a reaction process in ferric iron (Fe3+) or other transition metals (such as Cu, Mn, and Ag), which accelerate or replace Fe2+ to catalyze H2O2.434, 435 Multifunctional nanoparticles are introduced with CDT, which could enhance cancer therapy with Fenton or Fenton-like reactions.436 In detail, multifunctional nanoparticles could be codelivered with metal materials for achieving CDT, such as MnO2,437 CuAS,438 Fe3O4, and Zn–Mn–Ferrite439. For example, Zhao et al.440 designed a multifunctional yolk–shell nanosystem (Fe3O4@C/MnO2–PGEA, FCMP) composed of iron oxide (Fe3O4) cores, C/MnO2shells and polycationic cloak (CD–PGEA) to deliver the anticancer gene p53 for MRI-guided multiaugmented CDT and synergistic tumor treatment. In their design, the reduction of MnO2 was triggered by GSH to produce Mn2+, and the FCMP nanohybrid partially dissociated the Fe3O4 nucleus to release iron ions, which reacted with endogenous hydrogen peroxide (H2O2) to produce toxic hydroxyl radicals (·OH). Their results showed that under an external magnetic field, the most significant tumor suppressive effect was observed following intravenous injection of the FCMP/p53+NIR group after 10 days. Similarly, Lin et al.251 designed a multifunctional nanoplatform (MS@MnO2 nanoparticles) based on MN dioxide (MNO2)-coated mesoporous silica to deliver CPT for MRI-monitored chemo-chemodynamic combination cancer therapy. In their results, the tumor growth in U87MG tumor-bearing mice was significantly inhibited and showed higher rates of apoptosis than control groups after intravenous injection of MS@MnO2 nanoparticles. Moreover, Lee et al.441 reported using copper (II) arsenite (CuAS) coupled in the core domains of poly (ethylene glycol)-b-poly(3,4-dihydroxy-L-phenylalanine) to form CuAS-PMs for CDT treatment of cancer.
3.2.6 Synergistic therapy
Tumor cells are complex, diverse, and specific, leading to enormous challenges in treatment. At present, clinical anticancer research focuses on transforming monotherapy to a synergistic therapy of multiple treatment modality.442 The multifunctional nanoparticle delivery system integrates the treatment methods of monotherapy into a platform to obtain synergistic therapeutic effects, which greatly increases their anticancer ability. The complementary properties of these synergistic therapy strategies can compensate for the insufficiency of monotherapy.443
PDT/PTT: PDT requires high volumes of oxygen. However, the excessive consumption of oxygen will make the tumor excessively hypoxic, weakening the therapeutic effect of PDT. To improve the efficacy of PDT, multifunctional nanoparticles could be used to codeliver PTT photosensitizer to improve the ROS production capacity of the photosensitizer and increase the oxygen permeability, such as metal materials with photosensitizer and material with nonmetal material.444-448 Based on drug delivery ability, Au is an excellent PTT material that can be codelivered with PS, such as Ce6. For example, Kim et al. prepared a multifunctional nanoplatform (Ce6–AuNP–Lf) based on lactoferrin-coated Au nanoparticles to deliver Chlorin e6 (Ce6) for the treatment of glioblastoma multiforme with phototherapy.177 The PDT photosensitizer Ce6 was proximately conjugated on the surface of the PTT agent AuNP, leading to plasma crossover between Ce6 and AuNP that greatly improved the ROS production capacity of Ce6. Their results illustrated that Ce6–AuNP–Lf could effectively intervene in the progression of glioma and suppress the growth of tumors in a subcutaneous GBM mouse model by first applying PDT (671 nm), followed by PTT (532 nm). Additionally, multifunctional nanoparticles with dual roles of PTT and PDT could be used as PDT/PTT, such as black phosphorous nanosheets. Zhang et al.317 designed multifunctional nanoparticles (HA–BP) based on BP modified by HA for the combination therapy of cancer. With the modification of HA, HA–BP could target cancer cells and reverse TAMs to the M1 type. Due to the high photothermal conversion efficiency and ROS capacity of BP, the prepared nanoparticles could induce ICD-mediated antitumor immunity through phototherapy in vitro and in vivo.
PDT/CDT: As CDT can catalyze H2O2 to generate oxygen through Fenton and Fenton-like reactions, CDT can provide high volumes of oxygen for PDT and enhance the generation of hydroxyl radicals (•OH).449, 450 Multifunctional nanoparticles can catalyze H2O2 to enhance PDT with CDT and are multifunctional based on the fact that metal nanoparticles exhibit excellent catalysis for codelivery with photosensitizers, such as ICG and Ce6. For example, Chen et al.451 reported the self-assembly of Ce6 and hemin into multifunctional nanoparticles (HC nanoparticles) for PDT/CDT/Ferroptosis combination therapy. The PDT reagent Ce6 could convert O2 produced by hemin catalysis H2O2 into 1O2 to enhance PDT efficiency. Hemin induces GSH to release Fe3+ and redox to Fe2+ for effective CDT through the Fenton reaction. These results suggest that a low dose of HCNP nanoparticles may effectively destroy cancer cells and inhibit tumor growth under 660 nm laser irradiation.
PTT/CDT: Due to the limited transmission ability of laser light, PTT cannot penetrate into the deep layer of the organism and is still difficult to implement in monotherapy with PTT. Based on the photothermal conversion ability of PTT, multifunctional nanoparticles are introduced chemodynamics to enhance the photothermal performance by catalyzing H2O2 molecules.452-454 For example, Li et al.188 designed a multifunctional nanosystem (BCG) based on Cu-modified black phosphorous quantum dots (BPQDs) to load glucose oxidase (GOD). BPQDs released Cu2+ to induce GSH depletion, and transformed Cu2+ into cuprous ions for CDT. GOD consumes glucose to produce H2O2 and enhance CDT, and BPQDs capture Cu2+ to enhance the photothermal performance. In vitro and in vivo experiments showed that this combined PDT and CDT strategy had remarkable anticancer effects.
CDT/PTT/PDT: CDT/PTT/PDT can also be combined for trimodal synergistic therapy. Due to the tissue penetration ability of PTT, CDT can promote the production of oxygen and can generate singlet oxygen with strong activity with PDT, achieving effective tumor killing effect in deep tissues. The multifunctional nanoparticles are introduced with light/heat/physical dynamics for cancer therapy with synergistic therapy.455-457 For example, Sun et al.458 constructed a multifunctional nanoplatform (AMIT) based on Au nanoclusters (AuNCs) coated with MnO2 to codeliver ICG and the aptamer AS141 for targeting and image-guided PDT/CDT/PTT (Figure 5). The TME-responsive dissociation of MnO2 could produce O2 to enhance ICG-guided PDT and produce Mn2+ for Fenton-like reaction-mediated CDT. The treatment efficacy of PDT/PTT could be improved by codelivery with ICG and AuNCs, causing deep tumor penetration. Their results showed that the weight and size of the AMIT group was much smaller than those of the other groups after 21 days. Additionally, Wang et al.456 designed a multifunctional nanoplatform (MA) based on MNO2-coated Ag3SbS3 (PTT and PDT agent) for cancer treatment with PDT/CDT/PTT under a single NIR-II laser (Figure 6). Their results showed that the tumor growth was inhibited or even eliminated in the MA group under 1064 nm laser.

3.3 Multifunction in imaging
3.3.1 Magnetic resonance imaging
MRI uses hydrogen protons in tissue to form magnetic moments and excitation by a pulsed radiofrequency evacuation.459 Subsequently, the hydrogen proton will release the excitation energy received by radiofrequency coils and convert it to a grayscale image.460 MRI does not require injection of contrast agents, has no ionizing radiation, and has no adverse effects on the body, with excellent potential advantages for disease diagnosis.461-463 The multifunctional nanoparticles are introduced ferromagnetism or paramagnetic for enhancing tumor therapy with MRI-guiding.464-466 There are some MRI contrast agents that have been used in clinical or are undergoing clinical trials based on multifunctional backbone for treatment cancer. For example, SPIONS is injected to track the drugs for MRI cellular imaging of hepatic parenchyma (NCT04682847); Ferrotran® (a Fe3O4 preparation) is used to virtual histology of the bladder wall for bladder cancer staging (NCT04369560); and ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles are applied for measuring the activity of innate immune system within MS lesions (NCT05357833). Meanwhile, multifunctional nanoparticles containing SPIONs showed a typical darkening property under static magnetic fields because of the short transverse relaxation time (T2) of protons.165, 467 Thirunavukkarasu et al.26 prepared MIDEN with SPIONs (Fe3O4, SPIONs) and DOX for MRI-guided thermo-chemotherapy. In their design, SPIONs showed a typical darkening property under static magnetic fields due to the short transverse relaxation time (T2) of protons and demonstrated that MIDEN could be used in MRI. After intratumoral injection of MIDEN in CT26 tumor-bearing mice, MRI signals were homogenously observed in the tumor region to achieve tumor imaging. Due to the reduced antiferromagnetic interaction, SPION multifunctional nanoparticles can be substituted by nonmagnetic atoms to enhance the MRI signal. For example, Gondan et al. prepared mZnSPION–polyIC–R837 based on PEGylated phospholipid-encapsulated ZnxFe3-xO4 nanoparticles to deliver polyIC and the TLR7 agonist imiquimod (R837) for MRI and antitumor immunity effects.468 In their design, the overall magnetization and the spin–spin relaxation rate (R2) of SPION could be optimized and significantly increased by zinc (II) doping, resulting in the improved MRI detection sensitivity of SPION. They illustrated that mZnSPION exhibited a strong MRI contrast effect with a transverse relaxivity (r2) that was three times larger than mSPION. Due to the paramagnetic relaxed ability, multifunctional nanoparticles containing Mn2+ could be used for MRI with T1 signals.469 Moreover, Chen et al.470 reported a biomimetic virus-like nanocomplex (SDN) composed of photosensitizer Ce6-loaded nanostructured lipid carrier and poly (allylamine hydrochloride)-functionalized MnO2 nanoparticles. In their design, Mn2+ could be released quickly due to the presence of intracellular redox components. The SDN nanocomplex exhibited strong T1 MRI signals, which were enhanced by Mn2+.
3.3.2 Fluorescence imaging
FI uses a specific wavelength of laser light to irradiate fluorophores with fluorescent emission properties, absorb the light energy, and emit light for biological imaging.471 FI can be performed in vivo without puncturing the skin, and has the advantages of less influence on biological tissue, strong tissue penetration, and a high signal-to-noise ratio.472 Multifunctional nanoparticles with fluorescent emission properties can be used in FI with real-time tracking of drug delivery and release.473-475 In detail, multifunctional nanoparticles can be codelivered with bioactive agents containing luminescent groups to form nano prodrugs, such as dioxetane.476 The dioxetane is unstable after being irradiated by NIR and forms two ketone structures located in the ground state and the excited state, respectively.477 When the excited ketone structure transitions back to the ground state, fluorescence is generated. For example, He et al.478 developed an organic afterglow protheranostic nanoassembly (APtN) for activating both pharmaceutical effects and diagnostic signals in the TME. The phenylboronic ester group of APtN could be cleaved by H2O2 to uncage the afterglow substrate and react with silicon 2,3-naphthalocyanine bis(trihexylsilyloxide) (NCBS) to form PEG–dioxetane. Due to the instability of PEG–dioxetane, fluorescence was generated under NIR. Their results showed that the afterglow of APtN in solution could be increased 820-fold after treatment with H2O2, causing permitting the real-time in vivo feedback for the status of prodrug activation. Additionally, multifunctional nanoparticles can be codelivered with persistent luminescence nanoprobes (such as rare-earth-doped nanoparticles), which can store photons in the traps by irradiating them with ultraviolet (UV) light and subsequently releasing the stored energy under thermal energy excitation. Kong et al.479 reported a persistent luminescence nanoprobe (TRZD) for the NIR imaging and therapy of glioma. Due to the Cr3+/Sn4+ codoped ZnGa2O4, TRZD could cause persistent luminescence in NIR imaging under thermal energy excitation. Their results demonstrated that the luminescence of TRZD could last longer than the 7200 s and still maintain comparable intensity, and luminescence could also be renewed by UV light. Additionally, multifunctional nanoparticles with aromatic amino acids are intrinsically fluorescent under near-infrared light, such as tryptophan, tyrosine, and phenylalanine. Fan et al.142 prepared an NIR fluorescent peptide nanoparticle (RGD–f-P nanoparticles/EPI) with RGD and EPI for cell FI and target drug delivery. In their design, the cyclo [-(D-Ala-L-Glu-D-Ala-L-Trp)2-] peptide structure of f-P nanoparticles enabled it to emit visible and NIR fluorescence under 370 and 760 nm laser irradiation. Zinc coordination-limited energy dissipation during thermal relaxation pathways to obtain better quantum yield and fluorescence intensity. Their results showed that drug delivery to tumor sites could be monitored in vivo by the NIR fluorescence of RGD–f-P nanoparticles/EPI.
3.3.3 Photoacoustic imaging
PAI uses the photoacoustic effects of biological tissue to perform imaging.480 The imaging principle is that when biological tissue is irradiated by a pulsed laser, absorbed light energy, slight local heating and rapid thermal expansion results. This region of the biological tissue expands outward and generates acoustic waves to form a photoacoustic signal.481, 482 The advantages of PAI include high resolution, strong contrast, and deep penetration, with excellent development potential in cancer therapy.483, 484 Multifunctional nanoparticles are added with ultrasound for tracking drug release with PAI-guided cancer therapy.485 In detail, multifunctional nanoparticles with light absorption and high photothermal conversion efficiency could achieve PAI, such as inorganic materials (BP quantum dots). Black phosphorous quantum dots with unique layered structures could absorb laser light to convert heat energy and store the energy for PAI. For example, Li et al. prepared a multifunctional Fenton nanocatalyst (BCG) composed of Cu-doped BPQDs and GOx for cancer therapy with PAI guidance.188 Due to its unique layered structure and layer-dependent bandgap under 808-nm laser NIR, the BCG in BPQDs possessed strong absorption in the NIR region and allowed for the PAI. Their results suggested that PAI intensity was concentration-dependent and the PA signal was improved in the BCG NP solution with a concentration of 1 mg/ml. Additionally, multifunctional nanoparticles with photothermal conversion capabilities can also be used for PAI due to their ability to induce thermoelastic and ablative generation of ultrasound, such as PDA. Zhang et al.486 proposed the use of acorn-like Janus nanoparticles (PAA–mCaP/PDA–PEG J nanoparticles) based on mesoporous calcium phosphate for the synergistic treatment of cancer with PAI-guided chemo-phototherapy. PDA possessed strong NIR absorption and high photothermal conversion efficiency under 808-nm laser illumination. Their results showed that the PA signal intensity increased gradually, reaching a peak after intravenous injection of these nanoparticles for 24 h in HepG-2 tumor-bearing mice. Moreover, multifunctional nanoparticles codelivered with metal materials can also induce PAI via photo-induced charge transfer, such as Mo (VI). Wang et al.487 reported a multifunctional nanoenzyme (Ox-POM@Cu) based on a polyoxometalate doping with Cu ions for NIR-II PAI-guided chemodynamical and PTT. The photo-induced charge transfer of Mo could realize PAI under 1064 nm laser illumination, leading to an increase in their absorbance in the NIR-II region. Their results showed that the PA signal was the strongest in tumor tissue after injection of these nanoparticles for 4 h in 4T1 tumors.
3.3.4 X-ray computerized tomography imaging
X-ray computerized tomography (CT) imaging is a medical imaging technique that uses the absorption properties of tissue under ray energy to realize tomographic imaging and visualize interior features of tissue.488 According to the structure and position of object, the intensity of X-ray energy could decrease and reflect as the grayscale images. CT imaging exhibits the great advantages of painless, noninvasive, and clear information. In addition, CT imaging is widely applied in cancer treatment and some iodinated contrast medium (ICM) are approved by US FDA, such as iohexol and iodixanol.489 Multifunctional nanoparticles modified with large X-ray attenuation coefficient could monitor drug effect and apply to CT imaging-guide cancer therapy. Due to the high electron density and atomic number, metallic nanoparticles show large X-ray attenuation coefficient used for CT contrast agents, such as Au, bismuth, barium, and gallium.490, 491 For example, Wang et al.445 proposed a multifunctional nanosystem (Cu3BiS3–PEG–(Ce6–Gd3+)–FA NPs) using Cu3BiS3 NPs to anchor FA and chlorin e6 with Gd3+ for dual-modal CT and MR imaging guided cancer treatment. Bismuth element possessed excellent near infrared photothermal conversion performance and large X-ray attenuation coefficient, which made the prepared nanoparticles have excellent CT imaging performance. Their results indicated that these nanoparticles had a high X-ray absorption coefficient of 17.7 HU mmol Bi per L and the contrast of CT signal in the tumor was significantly enhanced after 4 h. Similarly, Zhao et al.270 also designed a Bi2S3-based multifunctional nanoparticle (FBPD NPs) for CT/PA dual-mode imaging-guided collaborative therapy of ovarian cancer. Moreover, iodides are small molecular with strong attenuation of X-ray absorption and have great compatibility in the body, which is usually used as contrast agent, such as iodinated nanoparticles. Fu et al. prepared LC@I-PANi nanoparticles through the combination of iodic acid and aniline monomers for CT imaging and PA imaging-guided PTT.492 In their design, iodine has a high atomic number, and the introduction of iodinated materials could produce image contrast due to different photoelectric absorption for enabling CT imaging. Their results showed that a strong CT signal was generated at the tumor site after intratumoral injection of LC@I-PANi in 4T1 tumor-bearing mice and LC@I-PANi possessed good biocompatibility in the body.
3.3.5 Multimodal imaging
Multimodal imaging is the organic superposition of multiple modal image information, which is better used in clinical research and treatment. Multimodal imaging has high sensitivity and resolution, which solves the limitations of a single imaging method and expands tumor imaging methods.493-495 In this method, multifunctional nanoparticles are added with multiple imaging capabilities to track the process of cancer therapy by multimodal imaging.496 In detail, multifunctional nanoparticles with ferromagnetism or paramagnetic for MRI can be combined with photosensitizers for FI and PAI, such as Fe3O4 codelivery with MnO2 and Ce6. The Fe3+ and Mn2+ ions have the short transverse relaxation time (T2) of protons for MRI, and Ce6, with a tetrapyrrole ring structure, can absorb the NIR light for FI. Fan et al.497 developed a multifunctional nanoplatform (Fe3O4@MnO2–CSL/Ce6) composed of Fe3O4 nanoparticles with MnO2 coated on the surface, with celastrol and Ce6 for multimodal imaging-guided chemo-PDT. Due to the Fe3+/Mn2+ ions and Ce6, these nanoparticles can significantly improve the magnetic resonance signal and have been used in FI of Bel-7402 tumor-bearing mice. The LSPR of Fe3+ and Mn2+ enabled Fe3O4@MnO2–CSL/Ce6 for PAI under 850 nm wavelengths. Additionally, multifunctional nanoparticles could be codelivered with PFP, which could expand them from nanoscale nanobubbles to microbubbles to achieve the magnetic droplet vaporization (MDV) effect under alternating magnetic field (AMF) irradiation, resulting in further enhancement of USI. Wang et al.498 reported magnetic nanodroplets (Fe/Art-Lip@PFP) based on Fe3O4 and PFP encapsulated-liposomes and artesunate (Art) for enhancing the antitumor efficacy of ferroptosis with the guidance of multimodal imaging. The LSPR of Fe3O4 was useful for PA imaging under 690 nm wavelengths, and the results showed that the PA signal increased linearly with increasing concentrations of MNDs. MNDs filled with PFP could achieve a MDV effect under AMF irradiation to enhance USI. Moreover, multifunctional nanoparticles grafted with radionuclide that have paramagnetic properties and a photothermal conversion ability can be used for MRI/PET/FI. The Gd3+ radionuclides emitted positrons during its decay and could be used in PET imaging. Zou et al.499 developed multifunctional mesoporous nanoparticles (68Ga/DOX/Si-Pc-HMNPs) composed of Ga2O3 (Cr3+, Nd3+), Gd2O3, and 68Ga for multimodal imaging-guided PDT and chemotherapy (Figure 7). Their results showed that the longitudinal relaxivity (r1) of HM nanoparticles was estimated to be 8.908 mM−1 s−1, higher than that of a commercial positive contrast agent, indicating that HM nanoparticles are effective MRI-positive contrast agents. After intravenous injection of HM nanoparticles with UV lamp excitation, NIR-PL signals were detected in the whole LNCaP tumor-bearing mouse within 5 min. The 68Ga of HM nanoparticles emitted positrons during their decay and could therefore be used in PET imaging.
4 CONCLUSIONS AND PERSPECTIVES
Recently, researchers have made outstanding progress on the development of multifunctional nanoparticles for cancer therapy. The construction potential of numerous backbones, both organic and inorganic, has been thoroughly evaluated. Moreover, strategies for introducing functional groups or agents to the backbone have also been investigated. As a result, a growing number of anticancer nanoparticles with extensive and synergistic multifunctions have emerged. However, several issues not included in this review remain. As for construction strategies, existing nanomaterials remain insufficient to provide enough docking sites for extension of function. Backbones made from natural products such as proteins, cells, and microorganisms may be exploited in the future. More hybrid backbones are also expected to achieve optimized properties. For example, the organic nanoparticles are expected to be combinated with inorganic nanoparticles. The biodegradability and biocompatibility of organic nanoparticles could provide better delivery capability for inorganic nanoparticles, while inorganic nanoparticles might offer multiple bioimaging functions. Moreover, due to high drug loading (up to 100%) and excellent optical properties, carrier-free nanoparticles and semiconducting polymer nanoparticles have great potential in constructive multifunctional nanoparticles, and more attention should be paid.
Regarding function design, the application of multifunction nanocarriers should be further expanded rather than just limited to drug delivery or imaging. Promising biomimetic functions, such as recognition, repair, and biomanufacturing, are potential choices for functional enrichment. In addition, to further enhance their clinical potential, it is important to develop more advanced formulations with existing multifunctional nanoparticles. We also suggest paying extra attention to the potential drug production issues for further pharmaceutical development, including formulation, quality control, the availability of raw materials. It is also important to consider in vivo safety studies of multifunctional nanoparticles to obtain more evidence for further clinical application. Despite these limitations, multifunctional nanoparticles exhibit high potential in the context of cancer therapy. More advanced and exciting strategies are expected to be developed in the near future.
AUTHOR CONTRIBUTION
Y. G., K. Y. W., and J. Z. drafted this manuscript and prepared the figures. X. M. D., Q. S., and K. M. designed the framework for the entire manuscript, provided detailed guidance, and financial support. All authors have checked the manuscript and agree to be publication.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation (82003258), Medico-Engineering Cooperation Funds from University of Electronic Science and Technology of China (No. ZYGX2021YGLH225)
CONFLICT OF INTEREST
There is no conflict of interest to declare.
ETHICS STATEMENT
The authors declare that human ethics approval was not needed for this study.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.