Journal list menu
Export Citations
Download PDFs
research papers
The crystal structure of an uncharacterized domain of P113 from Plasmodium falciparum
- Pages: 212-222
- First Published: 17 April 2025
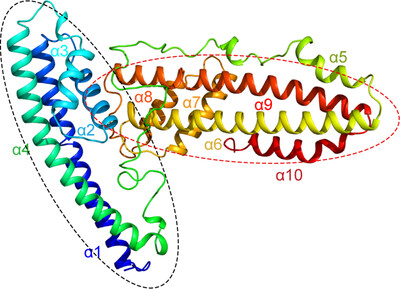
P113 is a membrane-anchored protein in Plasmodium falciparum that stabilizes the RH5 complex, facilitating erythrocyte invasion by interacting with the host receptor basigin. The helical-rich domain of P113 (residues 311–679) was crystallized, revealing a predominantly α-helical structure with two four-helix bundles and a disordered connecting region.
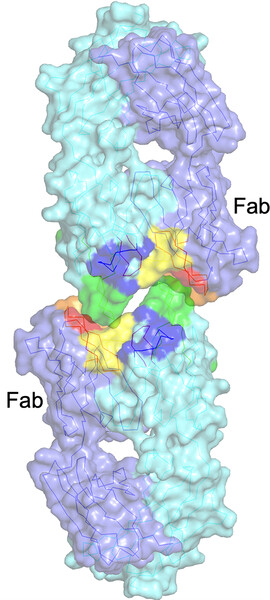
Structure of the Fab fragment of a humanized 5E5 antibody to a cancer-specific Tn-MUC1 epitope
- Pages: 223-233
- First Published: 17 April 2025
Structures of α-galactosaminidases from the CAZy GH114 family and homologs defining a new GH191 family of glycosidases
- Pages: 234-251
- First Published: 17 April 2025
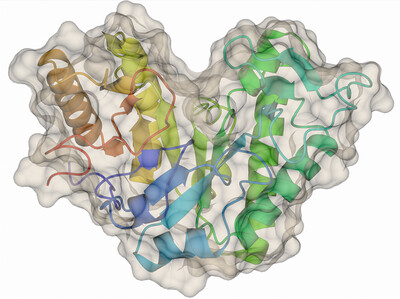
Structural and functional analysis of a new fungal GH114 endo-α-1,4-galactosaminidase, along with homologs that establish a new GH191 family of glycoside hydrolases, are reported. In addition, various crystal pathologies led to the development of a new refinement procedure for disordered structures.
Unique double-helical packing of protein molecules in the crystal of potassium-independent l-asparaginase from common bean
- Pages: 252-264
- First Published: 17 April 2025
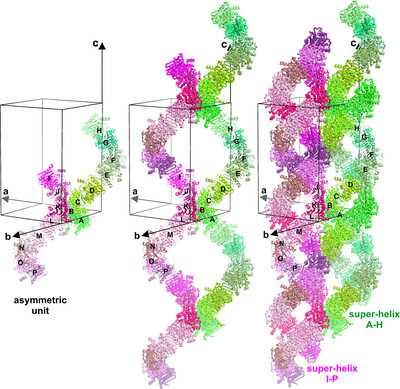
The crystal structure of the potassium-independent l-asparaginase PvAIII, with a rare P2 space-group symmetry, shows an unusual pseudosymmetric 41-like double-helical packing with 32 protein chains in the asymmetric unit. The packing is determined by an extended intermolecular β-sheet, by incomplete degradation of the interdomain linker. and by intermolecular `hydrogen-bond linchpins' that connect adjacent protein chains together.
Robust error calibration for serial crystallography
- Pages: 265-275
- First Published: 06 May 2025
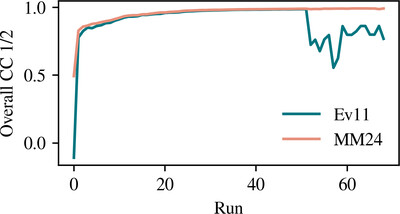
We present a new error-calibration algorithm for reflection intensities in serial crystallography. We reformulate the mathematical basis of the problem and apply different levels of uncertainty to each observed lattice corresponding to its measurement accuracy. These uncertainties are more consistent with theoretical expectations than the Ev11 error-calibration algorithm previously implemented in cctbx.xfel.merge.