Journal list menu
Export Citations
Download PDFs
research communications
High-throughput protein crystallography to empower natural product-based drug discovery
- Pages: 179-192
- First Published: 17 April 2025
Structures of Mycobacterium tuberculosis isoprenyl diphosphate synthase Rv2173 in substrate-bound forms
- Pages: 193-200
- First Published: 16 April 2025
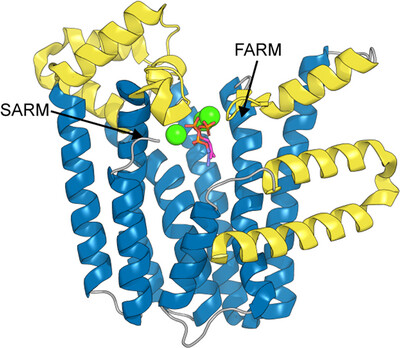
We report the structure of M. tuberculosis isoprenyl diphosphate synthase Rv2173 in three forms, including two with substrate (isoprenyl diphosphate and dimethylallyl diphosphate) occupying the allylic substrate site in different binding poses, with different numbers of metal ions bound. The homodimeric structures possess a canonical all-α-helical trans-isoprenyl diphosphate synthase fold, which supports small but significant differences, notably in the ordering of the C-terminus that closes the active site.
Molecular interactions between piperine and peroxisome proliferator-activated receptor gamma ligand-binding domain revealed using co-crystallization studies
- Pages: 201-206
- First Published: 16 April 2025
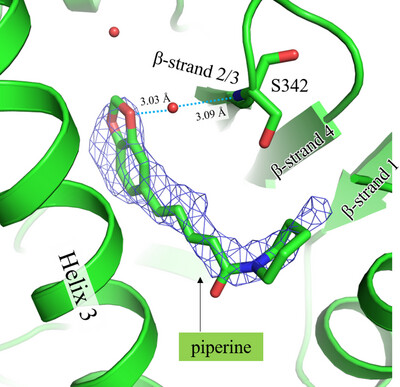
This study reports the co-crystallization of piperine with the peroxisome proliferator-activated receptor gamma (PPARγ) ligand-binding domain, providing detailed insights into the piperine binding position and its interactions with specific amino acids within the receptor. X-ray crystallographic analysis of the co-crystal structure revealed that piperine binds in the ligand-binding pocket of PPARγ via hydrogen-bonding and hydrophobic interactions, suggesting that it plays a role as a partial agonist or antagonist and thereby holds promise as a natural alternative to synthetic PPARγ modulators.
Structure of the Saccharolobus solfataricus GINS tetramer
- Pages: 207-215
- First Published: 16 April 2025
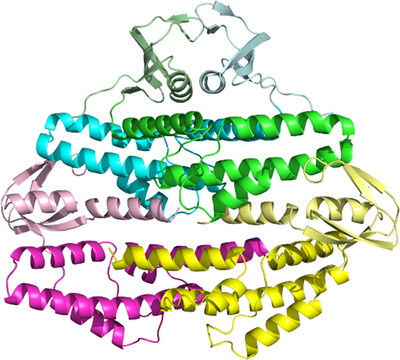
Archaeal organisms possess Cdc45 and GINS homologs that are likely to serve similar functional roles, but their structural interactions with the MCM helicase and hence their mechanism of MCM activation are not as well understood as for their eukaryotic counterparts. We present the crystal structure of the S. solfataricus GINS tetrameric complex and illustrate that a subdomain would need to move to accommodate known archaeal GINS-complex interactions and to generate an S. solfataricus CMG complex analogous to that of eukaryotes.
methods communications
Crystallization and initial X-ray crystallographic analysis of a de novo-designed protein with left-handed βαβ units
- Pages: 216-220
- First Published: 16 April 2025
addenda and errata
Purification, crystallization and preliminary crystallographic studies of a Kunitz-type proteinase inhibitor from tamarind (Tamarindus indica) seeds. Corrigendum
- Page: 221
- First Published: 16 April 2025
The article by Patil et al.[(2009), Acta Cryst. F65, 736–738] is corrected.