Journal list menu
Export Citations
Download PDFs
research communications
The structure of the Gemella haemolysans M26 IgA1 protease trypsin-like domain
- Pages: 124-129
- First Published: 04 March 2025
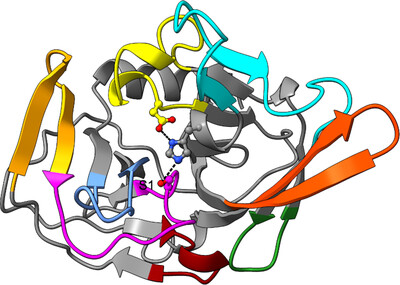
The 1.75 Å resolution structure of the G. haemolysans M26 IgA1 protease trypsin-like domain is presented. The structural data suggest that the domain exists in an inactive pro-enzyme-like state when in the context of the full-length protein. This putative pro-enzyme may be activated after being N-terminally excised from the larger M26 enzyme structure through the potential stabilization of its S1 pocket and rearrangement of adjacent surface loops.
The crystal structures of apo and tryptophan-bound tryptophanyl-tRNA synthetase from Neisseria gonorrhoeae
- Pages: 130-137
- First Published: 04 March 2025
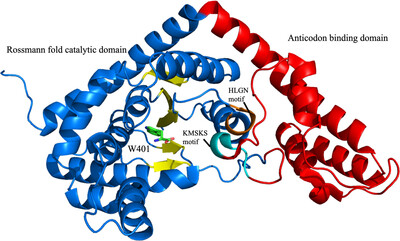
Crystal structures of tryptophanyl-tRNA synthetase from N. gonorrhoeae were solved in both the apo form and in complex with tryptophan to resolutions of 2.25 and 2.5 Å, respectively. These structures reveal conserved catalytic motifs and conformational changes at the active site upon ligand binding. Additionally, structural comparisons suggest that indolmycin may act as a competitive inhibitor, offering potential for antibiotic development.
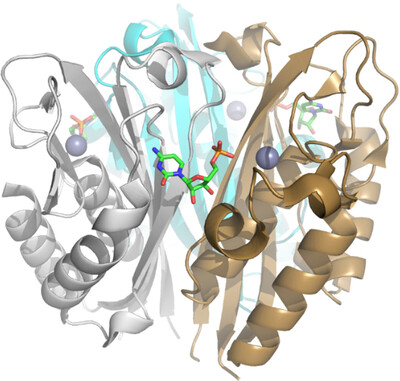
Analysis of Burkholderia pseudomallei IspF in complex with sulfapyridine, sulfamonomethoxine, ethoxzolamide and acetazolamide
- Pages: 138-145
- First Published: 04 March 2025
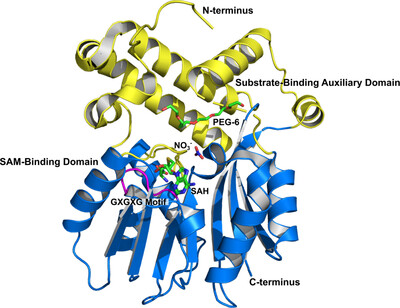
Crystal structure of the S-adenosylmethionine-dependent mycolic acid synthase UmaA from Mycobacterium tuberculosis
- Pages: 146-154
- First Published: 10 March 2025
Structural characterization of dUTPase from Legionella pneumophila
- Pages: 155-162
- First Published: 17 March 2025
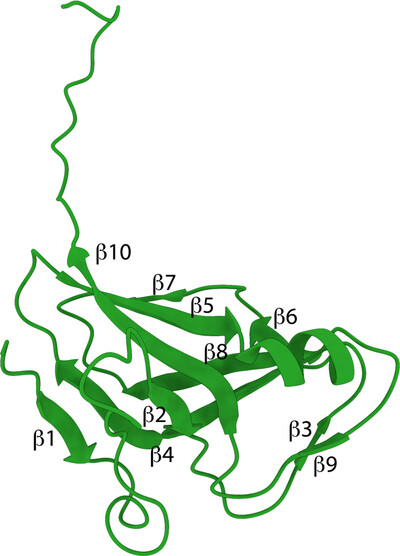
L. pneumophila serogroup 1 is the causative agent of Legionnaires' disease. Two crystal structures of apo and dUMP-bound deoxyuridine 5′-triphosphate nucleotidohydrolase (dUTPase) were determined to 1.80 and 1.95 Å resolution, respectively. dUTPases have been investigated as a potential druggable target in several pathogens.
Structures of Legionella pneumophila serogroup 1 peptide deformylase bound to nickel(II) and actinonin
- Pages: 163-170
- First Published: 17 March 2025
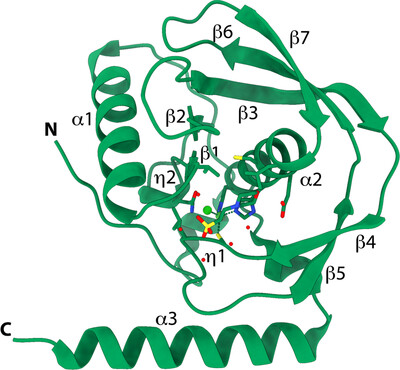
Peptide deformylases (PDFs) are of interest as viable drug targets for the development of new antimicrobials. Two crystal structures of PDF from L. pneumophila serogroup 1, the causative agent of Legionnaires' disease, bound to Ni2+ or to actinonin and Zn2+, were solved at 1.5 and 1.65 Å resolution, respectively.
Crystal structure of cyclophilin 37 from Arabidopsis thaliana
- Pages: 171-176
- First Published: 17 March 2025
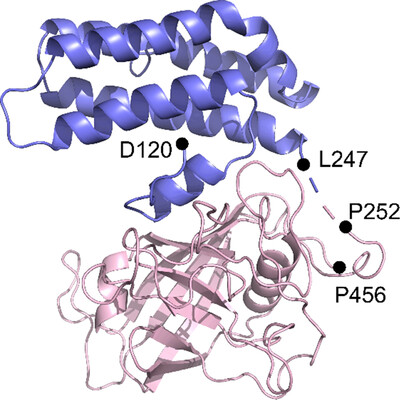
The purification, crystallization and determination of a 1.95 Å resolution structure of cyclophilin 37 from A. thaliana (AtCYP37) is reported. The structure, which is similar to those of Anabaena sp. CYPA and A. thaliana CYP38, is crucial for understanding how AtCYP37 interacts with the PetA subunit of cytochrome b6f, which is involved in the photoprotective mechanism of plants under high light conditions.
addenda and errata
The first report of structural analysis of a nucleic acid using crystals grown in space. Corrigendum
- Pages: 177-178
- First Published: 02 April 2025
The article by Ando et al.[(2025), Acta Cryst. F81, 95–100] is corrected.