Journal list menu
Export Citations
Download PDFs
FRONT COVER
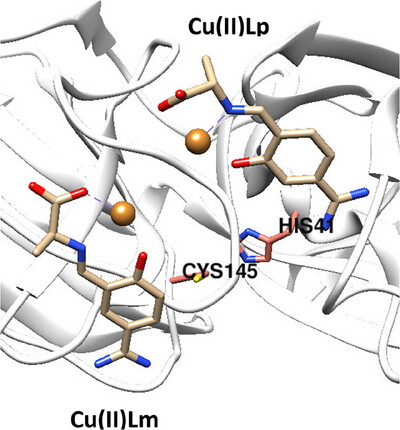
Front Cover: Copper(II) Inhibition of the SARS-CoV-2 Main Protease
- First Published: 22 July 2025
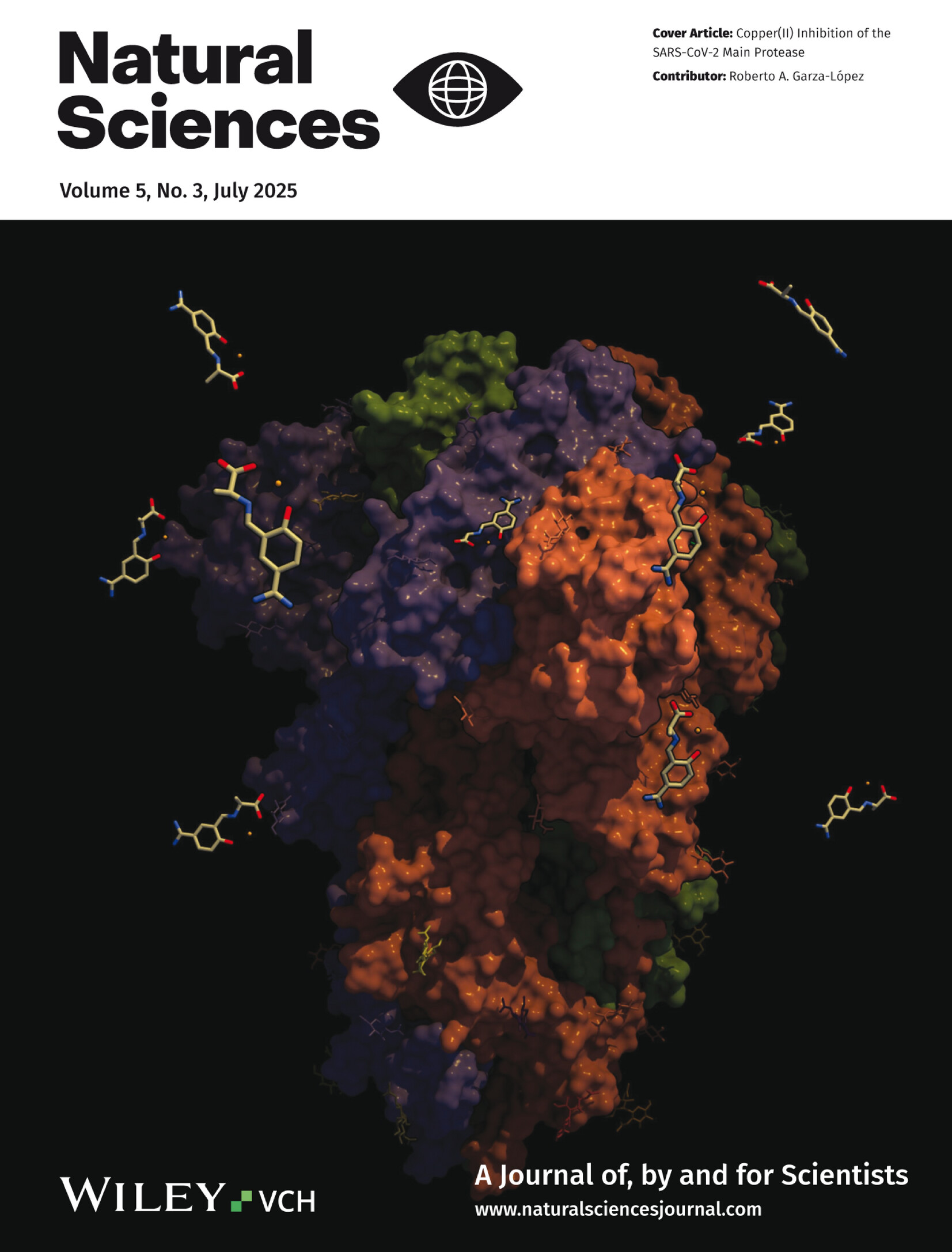
The cover image shows Cu(II) Schiff-base chelates, depicted as small molecular structures, just before binding to the SARS-CoV-2 spike glycoprotein, which is the multi-colored macromolecule. The research reveals that these chelates could disable this dangerous protein. Using induced-fit docking (IFD) and molecular dynamics (MD) simulations, the authors identified binding modes that likely would inhibit spike function. This work opens new avenues for developing broad-spectrum antiviral strategies, potentially offering a novel therapeutic approach against SARS-CoV-2 and other coronaviruses. More details can be found in article ntls.70012 by Roberto A. Garza-López and co-workers.
ISSUE INFORMATION
CONTENTS
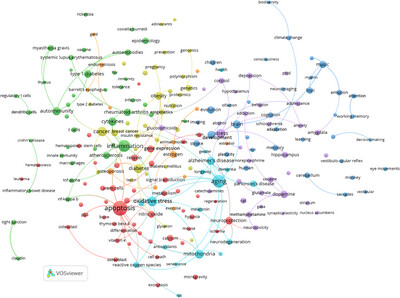
COMMENTARY
REVIEW ARTICLE
Two Hundred Years of the Annals of the New York Academy of Sciences: A Bibliometric Overview
- First Published: 19 May 2025
RESEARCH ARTICLE
Atomic Force Microscopy of Emulsions and Their Interfacial Nanoparticles
- First Published: 18 June 2025
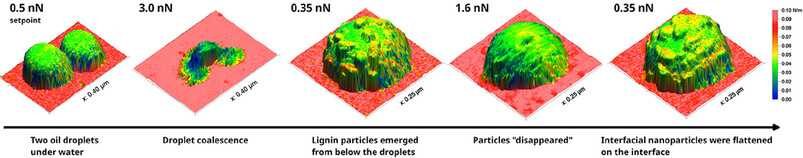
Emulsion droplets and their interfacial nanoparticles were imaged under water by atomic force microscopy. Changes in the sample were triggered by increasing the applied mechanical force. Mechanical data was overlaid on the topography data for an improved understanding of the properties and behavior of the droplets and particles.
Trikafta Rescues F508del-CFTR by Tightening Specific Phosphorylation-Dependent Interdomain Interactions
- First Published: 26 May 2025
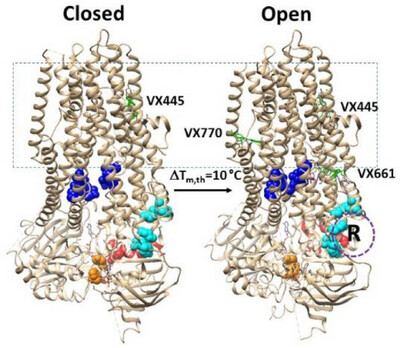
The most common cystic fibrosis mutation, F508del, destabilizes the first of two nucleotide-binding domains (NBD1 and NBD2) and compromises Mg/ATP-dependent gating at the interface between two transmembrane domains (TMD1 and TMD2) in the human cystic fibrosis transmembrane conductance regulator (CFTR). However, Trikafta can correct thermal and gating defects by enhancing TMD1–TMD2 (blue) and NBD1–NBD2 (orange) interactions and regulating the thermoring structures (red) of NBD1 through the specific binding of the dynamic phosphorylated regulatory domain (purple) to the TMD1/TMD2/NBD1 interfaces (cyan).
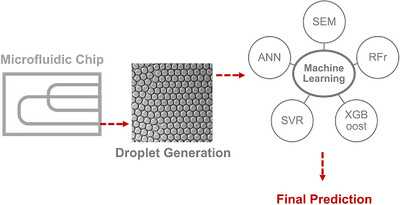
Designing Water-in-Oil Emulsion Using Microfluidic Systems Through Machine Learning
- First Published: 03 June 2025
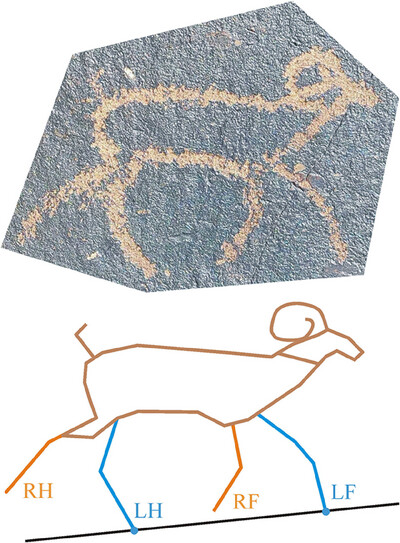
Biomechanical Analysis of Mongolian Bronze and Iron Age Four-Legged Walking Petroglyphs
- First Published: 23 June 2025