Journal list menu
Export Citations
Download PDFs
Cover Picture
Macromol. Rapid Commun. 23/2012
- Page: 1973
- First Published: 05 December 2012

Cover: Multicompartment materials with hierarchical structures can be prepared by colloid-electrospinning. The image shows a superposition of one stimulated emission depletion (STED) microscopy image and several colored micrographs of nanofibers with polymer and silica nanoparticles. Further details can be found in the article by D. Crespy,* K. Friedemann, and A.-M. Popa* on page 1978.
Masthead
Contents
Review
Colloid-Electrospinning: Fabrication of Multicompartment Nanofibers by the Electrospinning of Organic or/and Inorganic Dispersions and Emulsions
- Pages: 1978-1995
- First Published: 06 November 2012
Feature Article
Anion Responsive Imidazolium-Based Polymers
- Pages: 1996-2014
- First Published: 21 September 2012
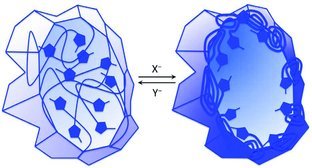
The anion or solvent stimuli responsiveness of imidazolium-based copolymers arises from dramatic solubility variations among particular anion–imidazolium ion pairs. These ion pairs can be tuned from solvophilic to solvophobic through anion exchange, and reversible poration, condensation, and stabilization are examples of concomitant properties.
Communications
Injectable Nanohybrid Scaffold for Biopharmaceuticals Delivery and Soft Tissue Engineering
- Pages: 2015-2022
- First Published: 03 September 2012
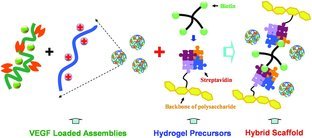
A nanofibrous hydrogel is produced via the biological conjugation of biotin-terminated star poly(ethylene glycol) and streptavidin-functionalized hyaluronic acid. Vascular endothelial growth factor (VEGF) is entrapped in low-molecular-weight heparin/N,N,N-trimethylchitosan chloride nanoparticles by affinity interactions.
Side-Chain Modification and “Grafting Onto” via Olefin Cross-Metathesis
- Pages: 2023-2028
- First Published: 05 September 2012
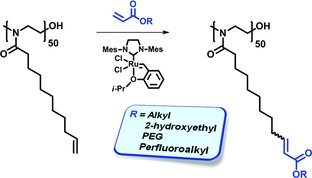
Olefin cross-metathesis (CM) enables an efficient side-chain functionalization of polymers having terminal alkenes as pendant groups. Acrylates and cis-2-butene-1,4-diacetate are used as CM partners to introduce a variety of functional groups. The scope and limitations of this polymer functionalization technique are discussed.
Arrangement of C60 via the Self-Assembly of Post-Functionalizable Polyisocyanate Block Copolymer
- Pages: 2029-2034
- First Published: 13 September 2012
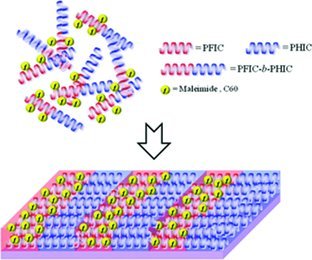
A well-defined post-functionalizable block copolymer, poly(furfuryl isocyanate)-b-poly (hexyl isocyanate) (PFIC-b-PHIC), is synthesized via living anionic polymerization. In addition, a very simple approach to produce highly arranged C60 involving the self-assembly behavior of the block copolymer is presented.
Toward Mass Producible Ordered Bulk Heterojunction Organic Photovoltaic Devices
- Pages: 2035-2040
- First Published: 19 September 2012
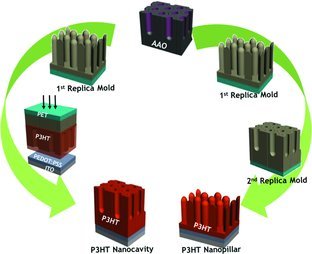
A new strategy to fabricate nanostructured P3HT films for organic solar cells by employing reusable soft replica molds is presented. With a UV-curable PFPE mold, P3HT nanopillars as well as P3HT nanocavity structures are obtained by exploiting the self-replication of replica molds. The method demonstrates the possibility to reproducibly prepare well-defined ordered bulk hetero-junction OPVs using reusable PFPE molds.
An Efficient Thiol-Ene Chemistry for the Preparation of Amphiphilic PHA-Based Graft Copolymers
- Pages: 2041-2045
- First Published: 07 September 2012
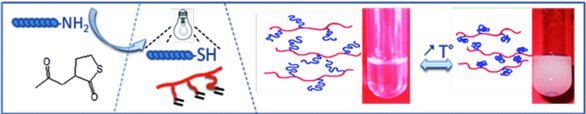
A simple and efficient method to prepare thermosensitive graft polyhydroxyalkaoate (PHA) is presented. Terminal amino groups of Jeffamine are turned into thiol groups by a reaction with N-acetylhomocysteine. Subsequent grafting of ω-thiol Jeffamine on unsaturated PHA is achieved via photoinduced thiol-ene addition, leading to amphiphilic copolymers.
Efficient Curing of Vinyl Carbonates by Thiol-Ene Polymerization
- Pages: 2046-2052
- First Published: 17 September 2012
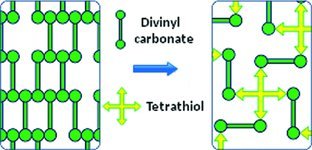
Vinyl carbonates exhibit low toxicity together with good mechanical properties, which qualifies them to be a potential alternative to state-of-the-art (meth)acrylates. A drawback of a certain class of them containing easily abstractable hydrogens is their moderate reactivity. With the help of thiol-ene polymerization, we are able to improve the reactivity enormously up to the level of acrylates.







