Journal list menu
Export Citations
Download PDFs
Cover Picture
Macromol. Rapid Commun. 13/2010
- First Published: 30 June 2010

Front Cover: The cover page presents the integration of an endosome-escaping function into PLys polyplex via electrostatic interaction with a charge-conversion polymer. The charge-conversion polymer converts its charge from negative to positive at acidic endosomal pH to induce endosome disruption, facilitating endosome escape of the PLys polyplex for safe and efficient transfection. Further details can be found in the article by M. Sanjoh, S. Hiki, Y. Lee, M. Oba, K. Miyata, T. Ishii, and K. Kataoka* on page 1181.
Contents
Editorial
Macromolecular Synthetic Biomaterials for Delivery of Therapeutics
- Page: 1121
- First Published: 30 June 2010
Reviews
pH-Responsive Polymers as Gene Carriers
- Pages: 1122-1133
- First Published: 30 June 2010
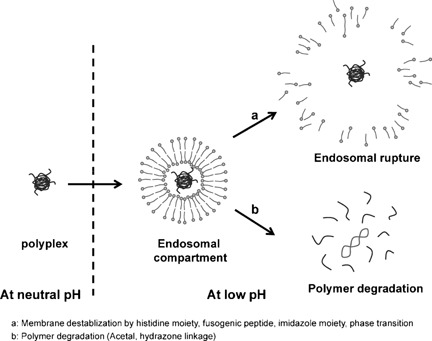
pH-responsive polymers are successfully designed and developed to achieve efficient gene delivery by exploiting the subtle pH differences within the cellular compartments. Installation of appropriate functional as well as structural attributes into the basic polymeric framework could trigger favorable pH-responsive transformations which provide enhanced gene delivery as well as higher biocompatibility.
Intracellular Protein Delivery Systems Formed by Noncovalent Bonding Interactions between Amphipathic Peptide Carriers and Protein Cargos
- Pages: 1134-1141
- First Published: 30 June 2010

Peptides are emerging as attractive alternatives to polymers and lipids for intracellular protein delivery. Amphipathic peptides are unique among peptide-based protein carriers in their ability to deliver protein cargos into cells without covalent conjugation. β-galactosidase proteins, after mixed with the amphipathic 16K-VTW peptide in solution, are delivered into cells in the presence of serum and able to catalyze the hydrolysis of its substrate X-gal, yielding blue color from the colorless substrate shown in the figure (scale bar = 100 µm).
Communications
Brush-Like Amphoteric Poly[isobutylene-alt-(maleic acid)-graft-oligoethyleneamine)]/DNA Complexes for Efficient Gene Transfection
- Pages: 1142-1147
- First Published: 30 June 2010
Injectable Biodegradable Poly(ethylene glycol)/RGD Peptide Hybrid Hydrogels for in vitro Chondrogenesis of Human Mesenchymal Stem Cells
- Pages: 1148-1154
- First Published: 30 June 2010
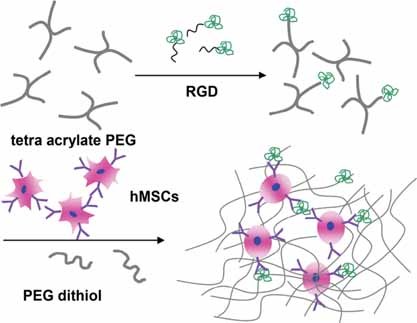
Injectable and biodegradable PEG/RGD peptide hybrid hydrogels are synthesized via Michael addition to study the effect of RGD content on chondrogenesis of hMSCs. The incorporation of RGD resulted in enhanced cell viability. Importantly, the presence of RGD at an optimal concentration promoted chondrogenesis of hMSCs.
Efficient Liposomal Nanocarrier-mediated Oligodeoxynucleotide Delivery Involving Dual Use of a Cell-Penetrating Peptide as a Packaging and Intracellular Delivery Agent
- Pages: 1155-1162
- First Published: 30 June 2010
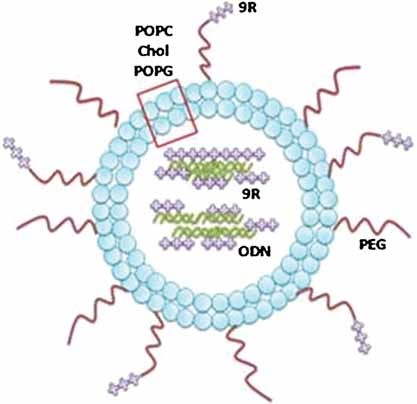
A novel liposomal nanocarrier system composing oligoarginine (nonaarginine) as both complexation reagent and as cell penetrating peptide is presented. Nonaarginine, when complexed to oligonucleotide (ODN), greatly increase the encapsulation efficiency of ODN into the liposome (i.e., almost 100% encapsulation). When nonaargine is conjugated to the liposome, it functions as cell penetrating peptide that greatly enhances the uptake of our liposomal formulation in vitro. This 9R-LS holds great promise for a myriad of applications including higher loading of gene or drugs, enhanced cell transfection efficacy and can simultaneously used for tumor-specific targeted delivery in vivo.
Folate-Conjugated Polymer Micelles with pH-Triggered Drug Release Properties
- Pages: 1163-1169
- First Published: 30 June 2010
Novel Triblock Oligopeptides as Efficient Nonviral Vectors: Characterisation and Further Insights
- Pages: 1170-1174
- First Published: 30 June 2010
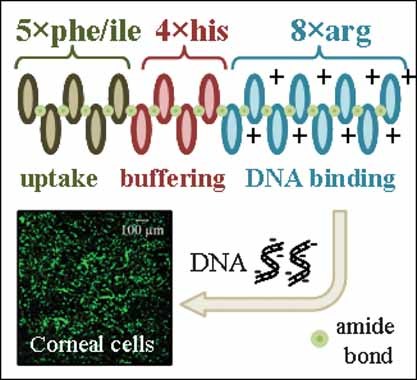
The ex vivo manipulation of corneas prior to transplantation is a promising strategy to prolong graft survival rates. Previously, we designed novel triblock oligopeptides and demonstrated their potential to mediate gene transfer into mouse corneal endothelial cells (MCEC). This study provides further insights into the structure–property relationship of this new class of nonviral vector.
Synthesis of Guanidinium-Modified Hyaluronic Acid Hydrogel
- Pages: 1175-1180
- First Published: 30 June 2010
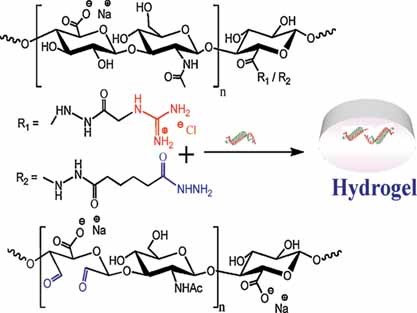
A new guanidinylating reagent is developed and employed to synthesise dually functionalised hyaluronan possessing guanidinium and crosslinkable hydrazide moieties. Hydrozone crosslinked hydrogel is formed within 50 s with improved mechanical properties. This derivative has minimal toxicity (∼80% cell viability at 2.5 × 10−3 M concentration), forms polyelectrolyte complex with plasmid DNA and shows DNA transfection to CD44 positive cells.
pDNA/poly(L-lysine) Polyplexes Functionalized with a pH-Sensitive Charge-Conversional Poly(aspartamide) Derivative for Controlled Gene Delivery to Human Umbilical Vein Endothelial Cells
- Pages: 1181-1186
- First Published: 30 June 2010
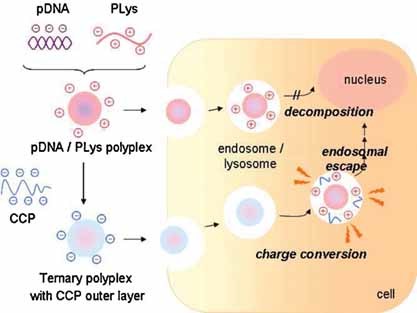
The enhanced gene expression of poly(L-lysine) polyplex was successfully achieved by integration of charge-conversional polymer (CCP). CCP showed charge-conversional property in response to endosomal pH, leading to outstanding endosomal escape of the polyplex to achieve high transfection efficiency against endothelial cells without toxicity.
Delivery of Anticancer Drugs Using Polymeric Micelles Stabilized by Hydrogen-Bonding Urea Groups
- Pages: 1187-1192
- First Published: 30 June 2010
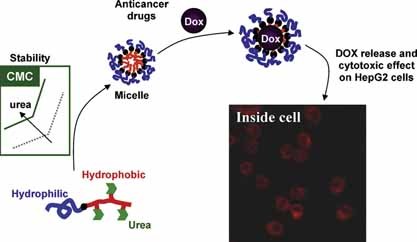
Hydrogen-bonding urea groups were implemented within the hydrophobic domain of amphiphilic micelles.This non-conventional approach improved micelle stability and doxirubicin loading capacity. The drug-loaded micelles were then shown to effectively kill all cell lines tested, while the polymeric carrier exhibited no cytotoxicity.
Formation and Degradation of Biodegradable Triple-Layered Microparticles
- Pages: 1193-1200
- First Published: 30 June 2010
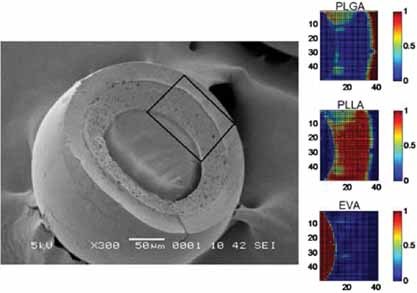
Triple-layered PLGA/PLLA/EVA (shell to core) microparticles are fabricated through a simple, economical and reliable one-step solvent evaporation technique. Compared to the single- and double-layered microparticles, triple-layered microparticles possess unique structural attributes and hydrolytic degradation characteristics. These triple-walled microparticles could then provide a means of controlling drug release.
Core–Shell–Corona Micelle Stabilized by Reversible Cross-Linkage for Intracellular Drug Delivery
- Pages: 1201-1206
- First Published: 30 June 2010
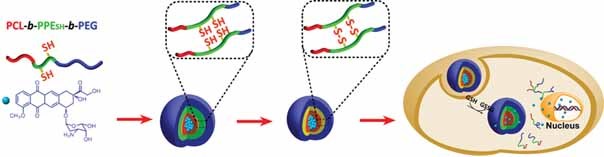
Biodegradable polymeric micelles with core–shell–corona structures are prepared. Selective cross-linking of the shell layer stabilizes the micelles against dilution and retards drug release in a non-reductive environment. However, the release is accelerated intracellularly, which results in enhanced cytotoxicity to A549 cancer cells.
Photocrosslinked DNA Nanospheres for Drug Delivery
- Pages: 1207-1211
- First Published: 30 June 2010
Design and Evaluation of Peptide Amphiphiles with Different Hydrophobic Blocks for Simultaneous Delivery of Drugs and Genes
- Pages: 1212-1217
- First Published: 30 June 2010
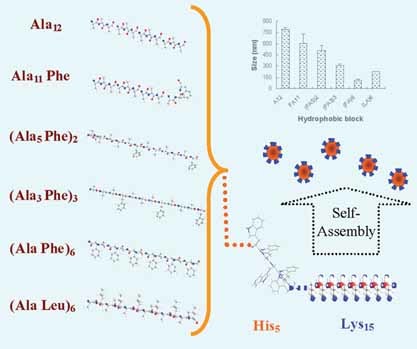
Cationic amphiphilic triblock oligopeptides with different amino acid compositions in the hydrophobic block have been designed and synthesized. Such compositions have a significant effect on micellization, drug loading and gene transfection. The peptide with the optimized composition results in nanosized micelles, high drug loading capacity and efficient gene transfection. Importantly, it can deliver drug and gene simultaneously, which further enhances gene transfection.
Back Cover
Macromol. Rapid Commun. 13/2010
- First Published: 30 June 2010

Back Cover: The figure shows a liposomal nanocarrier (9R-LS) encapsulating a nonaarginine oligodeoxynucleotide (9R/ODN) complex. After 9R has facilitated the uptake of 9R-LS, the 9R/ODN complex is dissociated in the cytoplasm. The ODN blocks the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) binding to the promoter region of target genes, thus shutting down transcription of proliferation-related genes. Further details can be found in the article by P. E. Saw, Y. T. Ko,* S. Jon* on page 1155.





![Brush-Like Amphoteric Poly[isobutylene-alt-(maleic acid)-graft-oligoethyleneamine)]/DNA Complexes for Efficient Gene Transfection](/cms/asset/37f2bbf1-3972-47da-bb26-9aa4fa6c0103/mcontent.jpg)