Journal list menu
Export Citations
Download PDFs
ISSUE INFORMATION
RESEARCH ARTICLES
Advantages of a synchrotron light source for fluorescence-detected linear dichroism
- First Published: 12 April 2024
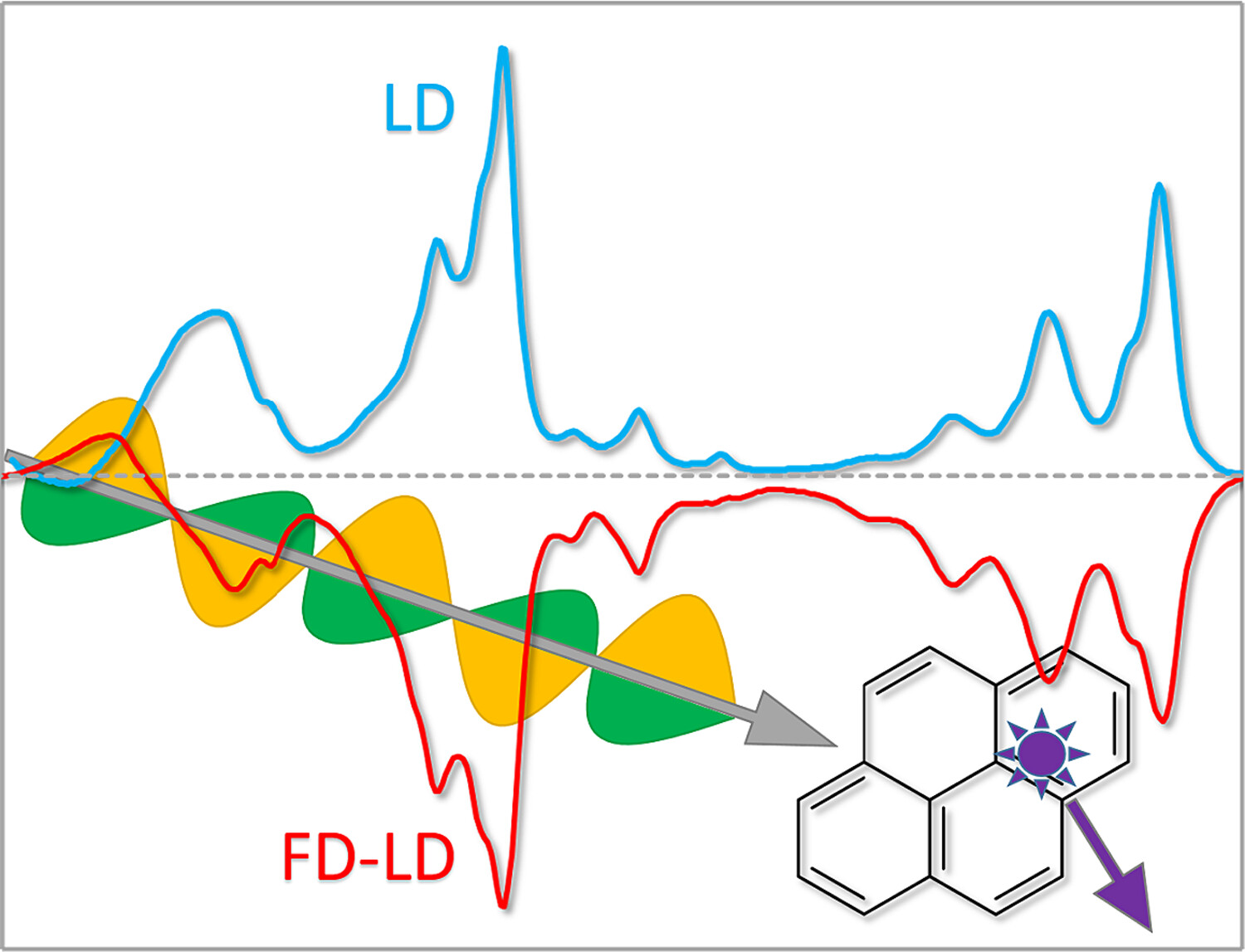
Fluorescence-detected linear dichroism (FD-LD) enables one to collect linear dichroism spectra for oriented fluorophores in the presence of other absorbing species and light scattering. This work uses a synchrotron radiation light source and fluorescence-detection to collect film and flow-oriented FD-LD spectra down to 170 nm.
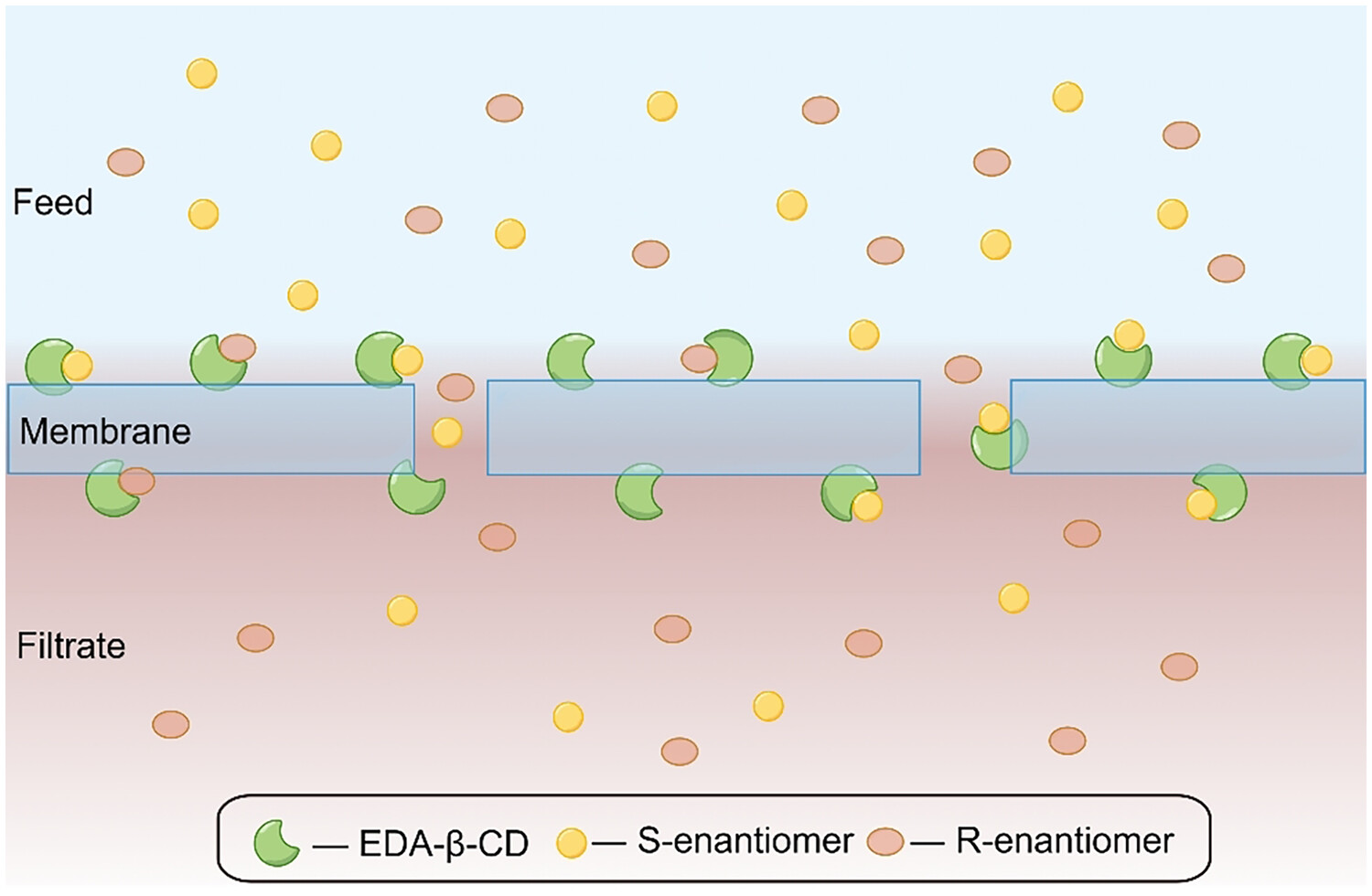
Novel ethylenediamine-β-cyclodextrin grafted membranes for the chiral separation of mandelic acid and its derivatives
- First Published: 04 April 2024
Preparation of amino acid chiral ionic liquid and visual chiral recognition of glutamine and phenylalanine enantiomers
- First Published: 03 April 2024
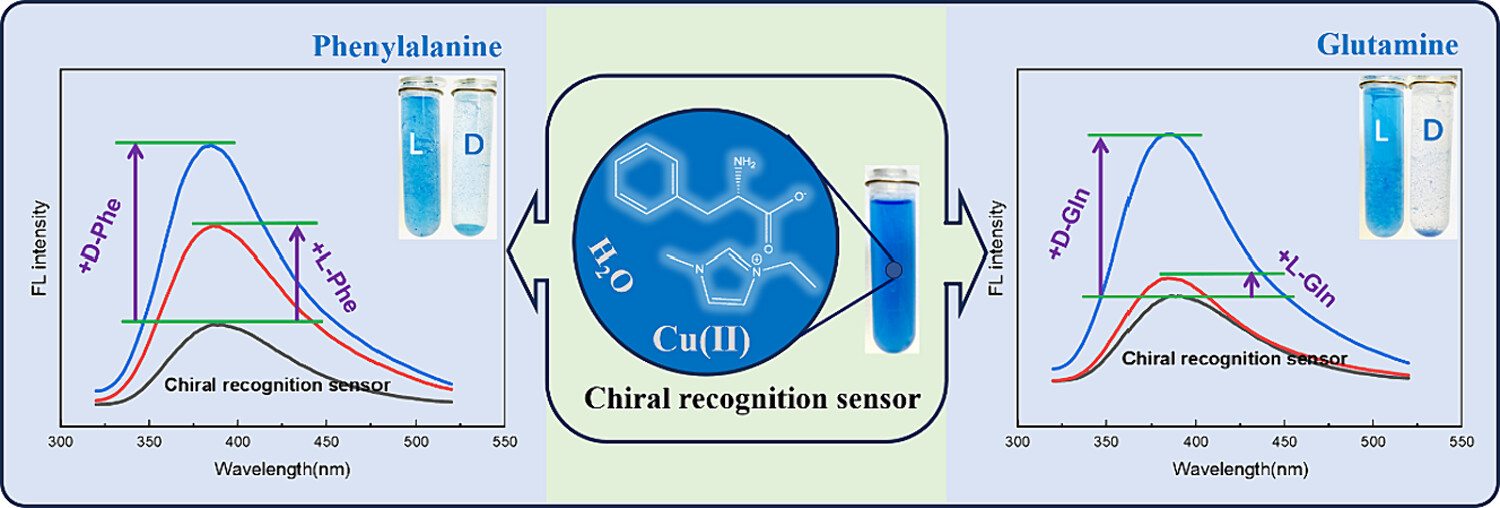
Amino acid chiral ionic liquid(AACIL) was successfully prepared and applied as a chiral recognition sensor. The sensor was applied for visual chiral recognition of the enantiomers of glutamine and phenylalanine, and the recognition conditions were optimized. Various methods are used to explore the mechanism of chiral recognition.
Separation of enantiomers of chiral fullerene derivatives through enantioselective encapsulation within an adaptable helical cavity of syndiotactic poly(methyl methacrylate) with helicity memory
- First Published: 01 April 2024
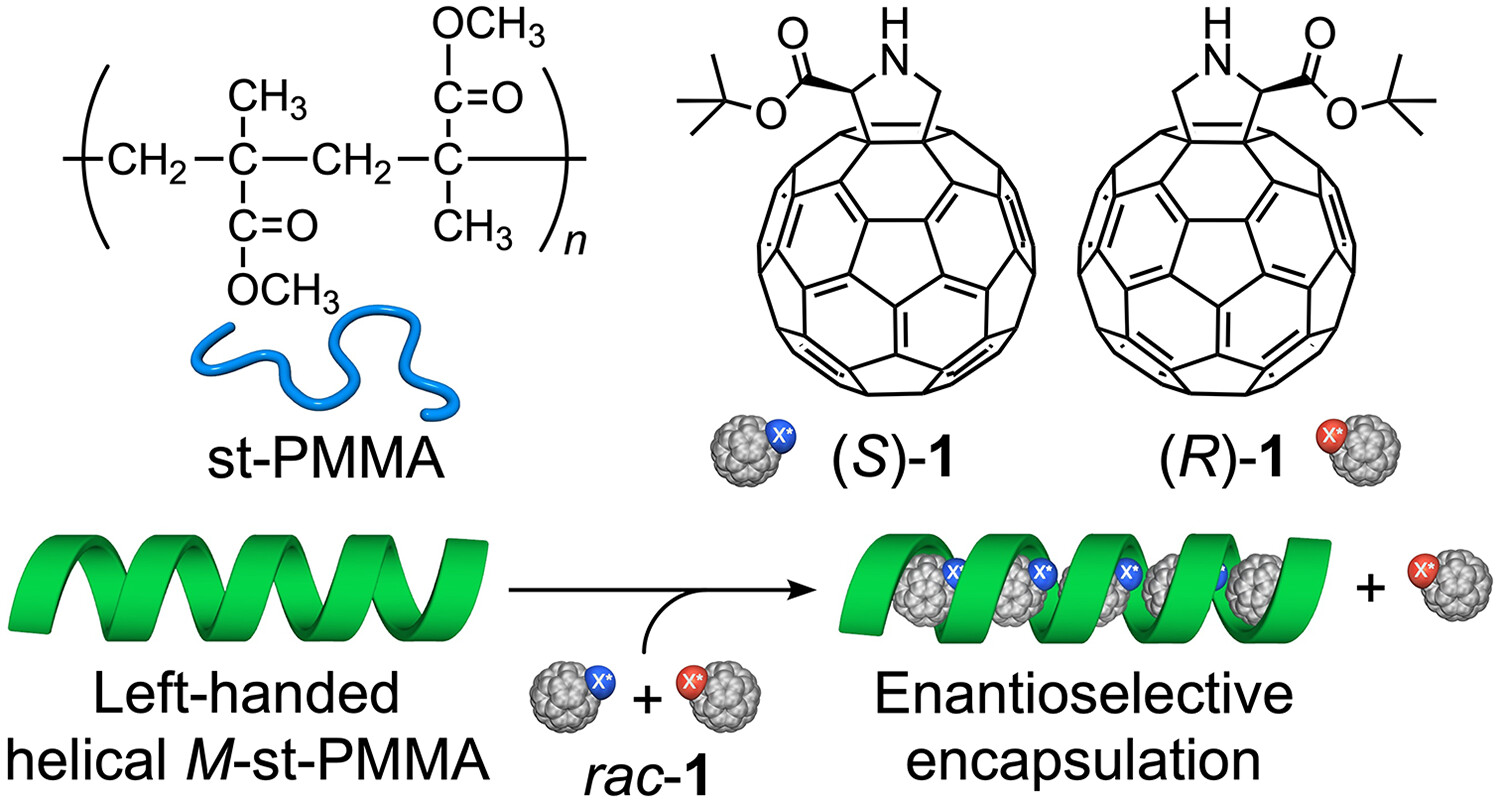
Optically active left (M)- and right (P)-handed helical syndiotactic poly(methyl methacrylate)s (M- and P-st-PMMAs) prepared by the “helicity induction and memory” strategy enantioselectively encapsulated the racemic fullerene derivatives within their helical cavities to form peapod-like inclusion complexes. Interestingly, the enantiomeric excess (ee) and separation factor (enantioselectivity) (α) of 3,4-fulleroproline tert-butyl ester (rac-1) used as the analyte encapsulated within the helical cavities of the M- and P-st-PMMAs were relatively higher (ee = 23%–25% and α = 2.35–2.50).
CONFERENCE REPORT
RESEARCH ARTICLES
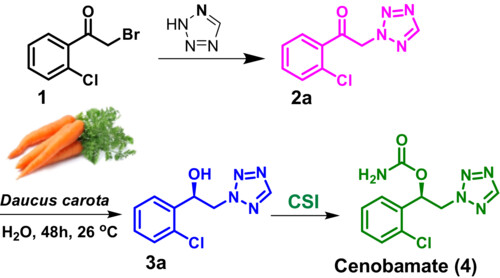
Biocatalytic enantioselective synthesis of cenobamate, an antiepileptic drug
- First Published: 21 March 2024