Journal list menu
Export Citations
Download PDFs
ISSUE INFORMATION
META-ANALYSIS
Meta-analysis: prevalence of, and risk factors for, non-alcoholic fatty liver disease in patients with inflammatory bowel disease
- Pages: 894-907
- First Published: 11 March 2022
Meta-analysis: hepatitis B vaccination in inflammatory bowel disease
- Pages: 908-920
- First Published: 08 March 2022
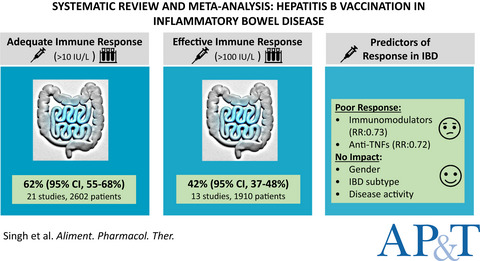
Pooled adequate immune response (AIR, >10 IU/L) and effective immune response (EIR, >100 IUL) rates after HBV vaccination in inflammatory bowel disease (IBD) patients were 62% (95% CI, 55–68) and 42% (95% CI, 37–48), respectively. Gender, IBD subtype, and disease activity did not affect the response rate. Use of immunosuppression [immunomodulators (RR: 0.73, 95% CI, 0.62–0.87) and anti-TNFs (RR: 0.72, 95% CI, 0.60–0.87)] was a predictor of poor immune response compared to no immunosuppressive therapy.
REVIEW ARTICLES
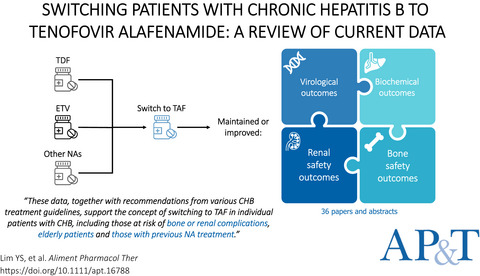
Review article: switching patients with chronic hepatitis B to tenofovir alafenamide—a review of current data
- Pages: 921-943
- First Published: 17 February 2022
Review article: role of glucagon-like peptide-1 receptor agonists in non-alcoholic steatohepatitis, obesity and diabetes—what hepatologists need to know
- Pages: 944-959
- First Published: 09 March 2022
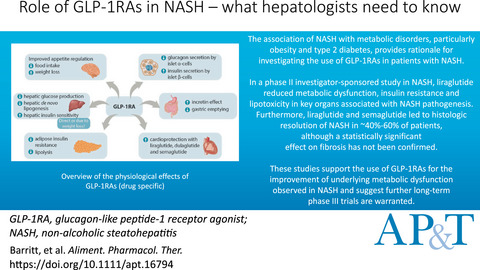
GLP-1RAs have a variety of hepatic and extra-hepatic physiological effects which may impact the natural history of nonalcoholic fatty liver disease and serve as the rational for potential therapeutic use in this population. GLP-1RA, glucagon-like peptide-1 receptor agonist; NASH, non-alcoholic steatohepatitis.
Review article: Lynch Syndrome—a mechanistic and clinical management update
- Pages: 960-977
- First Published: 21 March 2022
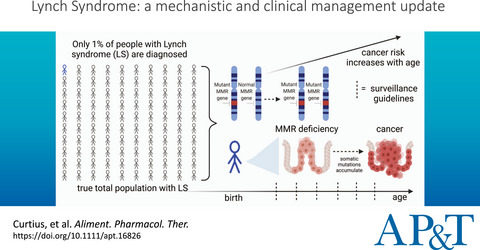
Surveillance guidelines only benefit the small proportion of patients with Lynch syndrome who are diagnosed after positive testing before potential cancer has developed. For those patients diagnosed, surveillance guidelines should consider pathogenic variant type, underlying mechanisms of mismatch repair deficiency, rates of mutation accumulation, and increasing cancer risk with age.
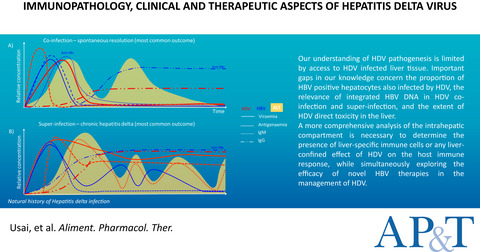
Review article: emerging insights into the immunopathology, clinical and therapeutic aspects of hepatitis delta virus
- Pages: 978-993
- First Published: 16 March 2022
FIB-4 FOLLOWED BY VCTE FOR DETECTION OF ADVANCED FIBROSIS
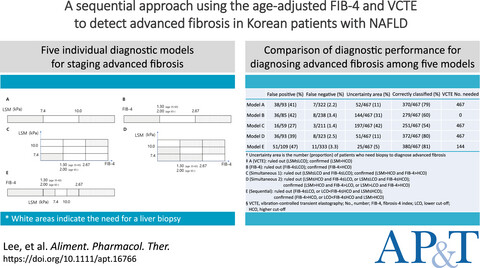
A sequential approach using the age-adjusted fibrosis-4 index and vibration-controlled transient elastography to detect advanced fibrosis in Korean patients with non-alcoholic fatty liver disease
- Pages: 994-1007
- First Published: 09 January 2022
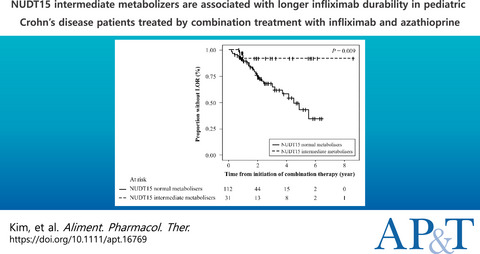
NUDT15 METABOLISM AND RESPONSE IN PAEDIATRIC CROHN'S DISEASE
HAEMOCHROMATOSIS AND IRON OVERLOAD IN FRANCE
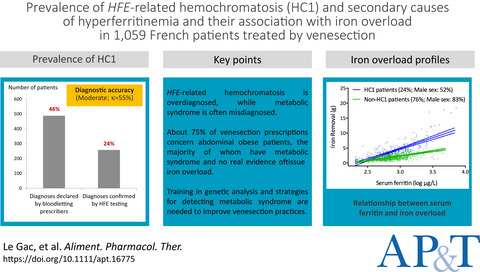
Prevalence of HFE-related haemochromatosis and secondary causes of hyperferritinaemia and their association with iron overload in 1059 French patients treated by venesection
- Pages: 1016-1027
- First Published: 04 February 2022
DILI-CAT - A NEW TOOL FOR DETECTION OF EARLY DRUG INDUCED LIVER INJURY
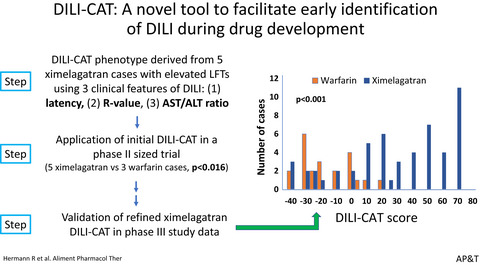
A novel phenotype-based drug-induced liver injury causality assessment tool (DILI-CAT) allows for signal confirmation in early drug development
- Pages: 1028-1037
- First Published: 09 March 2022
INVITED EDITORIALS
Editorial: is it not just PROs, but the most important individual PRO, that really matters in Crohn’s disease?
- Pages: 1038-1039
- First Published: 31 March 2022
This article is linked to Wong et al papers. To view these articles, visit https://doi.org/10.1111/apt.16805 and https://doi.org/10.1111/apt.16866
Editorial: is it not just PROs, but the most important individual PRO, that really matters in Crohn’s disease? Authors’ reply
- Pages: 1040-1041
- First Published: 31 March 2022
This article is linked to Wong et al papers. To view these articles, visit https://doi.org/10.1111/apt.16805 and https://doi.org/10.1111/apt.16843
Editorial: does TAF have a better or worse safety profile than TDF, to treat hepatitis B?
- Pages: 1042-1043
- First Published: 31 March 2022
This article is linked to Lim et al papers. To view these articles, visit https://doi.org/10.1111/apt.16788 and https://doi.org/10.1111/apt.16886
Editorial: does TAF have a better or worse safety profile than TDF, to treat hepatitis B? Authors' reply
- Pages: 1044-1045
- First Published: 31 March 2022
This article is linked to Lim et al papers. To view these articles, visit https://doi.org/10.1111/apt.16788 and https://doi.org/10.1111/apt.16854
Editorial: delayed gastric emptying as an independent predictor of mortality in gastroparesis—it is clinically relevant after all!
- Pages: 1046-1047
- First Published: 31 March 2022
This article is linked to Gourcerol et al papers. To view these articles, visit https://doi.org/10.1111/apt.16827 and https://doi.org/10.1111/apt.16885
Editorial: reply to delayed gastric emptying as an independent predictor of mortality in gastroparesis—it is clinically relevant after all! Authors' reply
- Page: 1048
- First Published: 31 March 2022
This article is linked to Gourcerol et al papers. To view these articles, visit https://doi.org/10.1111/apt.16827 and https://doi.org/10.1111/apt.16857
Editorial: is there a role for monitoring intermediate anti-TNF drug concentrations in IBD?
- Pages: 1049-1050
- First Published: 31 March 2022
This article is linked to Roblin et al papers. To view these articles, visit https://doi.org/10.1111/apt.16852 and https://doi.org/10.1111/apt.16893
Editorial: is there a role for monitoring intermediate anti-TNF drug concentrations in IBD? Authors’ reply
- Page: 1051
- First Published: 31 March 2022
This article is linked to Roblin et al papers. To view these articles, visit https://doi.org/10.1111/apt.16852 and https://doi.org/10.1111/apt.16889
Editorial: clinical impact of sofosbuvir renal toxicity—more light on the way
- Pages: 1052-1053
- First Published: 31 March 2022
This article is linked to Sulkowski et al papers. To view these articles, visit https://doi.org/10.1111/apt.16830
Editorial: some of the obstacles in managing mood disorders in IBD
- Pages: 1054-1055
- First Published: 31 March 2022
This article is linked to Jayasooriya et al papers. To view these articles, visit https://doi.org/10.1111/apt.16820
LETTERS TO THE EDITORS
Letter: sPD-1 as a predictor for HBsAg seroconversion—shed light on inactive carriers with chronic hepatsitis B
- Page: 1056
- First Published: 31 March 2022
This article is linked to Hu et al papers. To view these articles, visit https://doi.org/10.1111/apt.16752 and https://doi.org/10.1111/apt.16863
Letter: sPD-1 as a predictor for HBsAg seroclearance—shed light on inactive carriers with chronic hepatitis B. Authors' reply
- Pages: 1057-1058
- First Published: 31 March 2022
This article is linked to Hu et al papers. To view these articles, visit https://doi.org/10.1111/apt.16752 and https://doi.org/10.1111/apt.16833
Letter: serum growth differentiation factor 15 predicts hepatocellular carcinoma occurrence after hepatitis C virus elimination
- Pages: 1059-1060
- First Published: 31 March 2022
This article is linked to Myojin.et al's paper. To view this article, visit https://doi.org/10.1111/apt.16691 and https://doi.org/10.1111/apt.16862.
Letter: serum growth differentiation factor 15 predicts hepatocellular carcinoma occurrence after hepatitis C virus elimination—authors' reply
- Pages: 1061-1062
- First Published: 31 March 2022
This article is linked to Myojin.et al’s paper. To view this article, visithttps://doi.org/10.1111/apt.16691 and https://doi.org/10.1111/apt.16834
Letter: inflammatory bowel disease services during the Covid-19 pandemic
- Page: 1063
- First Published: 31 March 2022
This article is linked to Deputy et al papers. To view these articles, visit https://doi.org/10.1111/apt.16800 and https://doi.org/10.1111/apt.16884
Letter: inflammatory bowel disease services during the Covid-19 pandemic - authors' reply
- Page: 1064
- First Published: 31 March 2022
This article is linked to Deputy et al papers. To view these articles, visit https://doi.org/10.1111/apt.16800 and https://doi.org/10.1111/apt.16841
Letter: skipping breakfast is associated with an increased long-term cardiovascular mortality in metabolic dysfunction-associated fatty liver disease (MAFLD) but not MAFLD-free individuals
- Pages: 1065-1066
- First Published: 31 March 2022
This article is linked to Xie et al papers. To view these articles, visit https://doi.org/10.1111/apt.16727 and https://doi.org/10.1111/apt.16876
Letter: skipping breakfast is associated with an increased long-term cardiovascular mortality in metabolic dysfunction-associated fatty liver disease (MAFLD) but not in MAFLD-free individuals. Authors' reply
- Pages: 1067-1068
- First Published: 31 March 2022
This article is linked to Xie et al papers. To view these articles, visit https://doi.org/10.1111/apt.16727 and https://doi.org/10.1111/apt.16849
Letter: potential selection bias in the real-world comparison of ustekinumab versus vedolizumab as a second-line treatment for Crohn’s disease
- Page: 1069
- First Published: 31 March 2022
This article is linked to Lenti et al papers. To view these articles, visit https://doi.org/10.1111/apt.16742 and https://doi.org/10.1111/apt.16896
Letter: potential selection bias in real-world comparison of ustekinumab versus vedolizumab as a second-line treatment for Crohn’s disease—authors’ reply
- Pages: 1070-1071
- First Published: 31 March 2022
This article is linked to Lenti et al papers. To view these articles, visit https://doi.org/10.1111/apt.16742 and https://doi.org/10.1111/apt.16871
Letter: the value of quality liver biopsy before initiation of corticosteroids for severe alcoholic hepatitis-authors' reply
- Page: 1072
- First Published: 31 March 2022
This article is linked to Lanthier et al papers. To view these articles, visit https://doi.org/10.1111/apt.16835