Review article: role of glucagon-like peptide-1 receptor agonists in non-alcoholic steatohepatitis, obesity and diabetes—what hepatologists need to know
The Handling Editor for this article was Dr Rohit Loomba, and this uncommissioned review was accepted for publication after full peer-review.
Funding information: This article was supported by Novo Nordisk Inc., who performed a medical accuracy review. Writing support was provided by Emma Marshman, a contract writer working on behalf of Axis, a division of Spirit Medical Communications Group Limited, and was funded by Novo Nordisk Inc. in accordance with Good Publication Practice 3 (GPP3) guidelines (www.ismpp.org/gpp3). Emma Marshman and Laura Ward, a former employee of Axis, a division of Spirit Medical Communications Group Limited, performed the literature review, funded by Novo Nordisk Inc.
Summary
Background
Non-alcoholic steatohepatitis (NASH) is characterised by hepatic lipid accumulation, cell injury, inflammation and fibrosis. Insulin resistance, a hallmark of type 2 diabetes (T2D) and obesity, is a key pathogenic driver of NASH. Other than difficult-to-maintain lifestyle changes, there are no approved treatments for NASH. Due to their effects on multiple pathophysiological processes, glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been tested in disorders related to insulin resistance and metabolic defects.
Aims
To summarise studies of GLP-1RAs relevant to the treatment of NASH.
Methods
PubMed searches were performed and results were compiled.
Results
Large trials with GLP-1RAs in T2D demonstrate highly effective glucose lowering, with body weight loss, and in some cases, reduced cardiovascular events and improved liver transaminases. The GLP-1RAs, liraglutide and semaglutide, were associated with clinically relevant, sustained body weight reduction in individuals with overweight or obesity and without T2D. In a phase II trial in NASH, liraglutide reduced metabolic dysfunction, insulin resistance and lipotoxicity in key organs associated with NASH pathogenesis. Furthermore, liraglutide and semaglutide led to histological resolution of NASH in ~40% to 60% of patients, although a statistically significant effect on fibrosis has not been confirmed. Regarding safety, GLP-1RAs are associated with gastrointestinal and gallbladder-related adverse events, with the latter perhaps related to weight loss. Meta-analyses do not indicate increased risk of acute pancreatitis, pancreatic cancer or other malignancies with GLP-1RAs.
Conclusions
These studies support the use of GLP-1RAs for the improvement of underlying metabolic dysfunction observed in NASH and suggest further long-term phase III trials are warranted.
1 INTRODUCTION
Non-alcoholic steatohepatitis (NASH) is a severe subtype of non-alcoholic fatty liver disease (NAFLD) that is characterised by fat accumulation and inflammation in the absence of secondary causes and significant alcohol consumption.1, 2 In the United States, NAFLD and NASH affect around 30% and 5% of adults, respectively.3 Risk factors for developing NASH include type 2 diabetes (T2D) and obesity, dyslipidaemia, metabolic syndrome and hypertension.3 In a real-world cohort of patients, across the spectrum of NAFLD, 50% had diabetes, 66% had obesity and 19% had cardiovascular (CV) disease (CVD).4 Pathological processes underlying NASH include metabolic substrate overload, hepatocellular cell stress and injury (lipotoxicity), resulting in cell death and activation of inflammation pathways that trigger fibrogenesis (reviewed in Brunt et al5). Histological markers of these processes include triglyceride accumulation (steatosis), ballooning, apoptosis, lobular and portal inflammation, and excess matrix deposition (fibrosis).5 Advancing fibrosis is linked to adverse clinical outcomes, including all-cause and liver-related mortality,6 with ~20% of patients developing cirrhosis and/or hepatocellular carcinoma.7 In addition, there are extra-hepatic complications of NAFLD/NASH that contribute to high mortality rates, including increased risk of CVD, T2D, chronic kidney disease and colorectal cancer (reviewed in Armstrong et al8).
Several genetic variants are associated with the development and progression of NASH (reviewed in Carlsson et al9). There is evidence of a strong interaction between three of these variants (PNPLA3 p.I148M, TM6SF2 p.E167K and GCKR p.P446L) and obesity, which significantly amplifies their effect.
Insulin resistance is a main component of metabolic syndrome10 and an important feature of T2D and obesity, as well as being a key pathogenic driver of NASH.3 Patients with NAFLD and steatosis have severe insulin resistance accompanied by increased hepatic insulin resistance and de novo lipogenesis.11, 12 The overspill of non-esterified fatty acids and release of triglyceride-derived toxic metabolites from adipose tissue via, or through, lipolysis also drives disease progression and extrahepatic complications (reviewed in Armstrong et al8; Cusi et al13).
Currently, there are no approved pharmacological treatments for NASH in Europe and North America, and lifestyle modification remains the cornerstone of management.1, 2 Weight loss induced by a comprehensive 12-month lifestyle programme has been shown to induce important histological changes in patients with NASH.14 However, weight reductions of ≥10% were required to demonstrate resolution of steatohepatitis or improvements in fibrosis and portal inflammation.14 In a real-world cohort of American patients with NAFLD receiving usual care, 32% who were initially overweight or obese were able to achieve ≥5% weight reduction over a median follow-up of 39 months.15 However, such weight loss was not sustainable for all, as 21% of those who lost weight regained it and returned to their baseline weight.
As our understanding of the pathophysiology of NASH has grown, these insights have led to the exploration of new potential therapeutic approaches (reviewed in Vuppalanchi et al16). The association of NASH with metabolic disorders, particularly obesity and T2D, provides a strong rationale for the investigation of the gut-derived incretin hormone, glucagon-like peptide-1 (GLP-1), as a therapeutic target. This review article will discuss the general and hepatic effects of GLP-1 receptor agonists (GLP-1RAs) in T2D and obesity. Evidence from studies of GLP-1RAs in NASH will then be described, along with some practical considerations regarding what hepatologists may need to know about the use of this class in an approved setting (T2D or obesity) where patients may also have liver disease.
2 SEARCH STRATEGY
A narrative literature search was conducted using PubMed on 16 February 2021 to address the three main themes covered in this review: (i) experience of GLP-1RAs in clinical trials to date, (ii) the relationship between T2D and/or obesity, and NASH, and (iii) the mode/mechanism of action of GLP-1RAs in NASH. For each of these themes, a search was performed with keywords restricted to “Title Only” or “Title/Abstract” to refine the breadth and relevance of the publications identified. For the search of data from clinical trials, article type filters were applied to restrict results to clinical trials and meta-analyses, and a manual refinement of search results was performed on a review of the titles and abstracts rather than applying additional restrictions in PubMed that were likely to exclude relevant publications. The complete search strings are provided in Table S1.
Additional references were identified through searching the bibliographies of retrieved articles, and through author suggestions.
3 TARGETING THE INCRETIN PATHWAY
The potential of GLP-1 as a target for the treatment of T2D was initially recognised with the discovery that GLP-1 plays a key role in augmenting insulin secretion in response to nutrient intake.17 GLP-1, released from intestinal enteroendocrine cells during eating, acts via GLP-1 receptors on islet β-cells and δ-cells to control insulin and somatostatin secretion, respectively (reviewed in Drucker18). Somatostatin then inhibits glucagon secretion from islet α-cells via the somatostatin-2 receptor.18 In T2D, the incretin effect, whereby oral glucose enhances insulin secretion more than intravenous glucose, is reduced.17 However, incretin activity can be restored therapeutically with GLP-1RAs and by inhibitors of dipeptidyl peptidase-4 (DPP-4) activity that prevent the degradation of endogenous GLP-1 (reviewed in Nauck19).
GLP-1RAs have been shown to increase insulin and decrease glucagon secretion in a glucose-dependent manner, resulting in reduced blood glucose levels.19 New prescribers may be concerned that a drug that acts by increasing insulin secretion and suppressing glucagon secretion may cause hypoglycaemia. However, GLP-1RAs have a low intrinsic risk of hypoglycaemia because of the glucose-dependency of their mechanism of action; as glucose levels approach normoglycaemia, the effect of the drug on pancreatic hormone secretion ceases.19
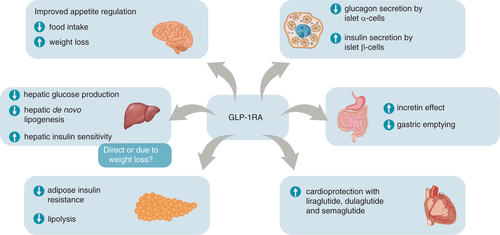
GLP-1RAs also improve multiple pathophysiological defects in T2D beyond glycaemic control (Figure 1)17, 18, 20-25 and suppress appetite leading to weight loss.18, 23 Certain GLP-1RAs also reduce CV outcomes (Figure 1).20-22, 26 Due to their distinct modes of action, DPP-4 inhibitors have different properties from GLP-1RAs, such that DPP-4 inhibitors are associated with more modest glycated haemoglobin (HbA1c) and weight lowering, but a lower incidence of gastrointestinal (GI) adverse events (AEs).19
4 EXPERIENCE WITH GLP-1RAS IN T2D
In T2D, GLP-1RAs are well-established glucose-lowering agents that are generally recommended after first-line metformin therapy, along with other drug classes, including sodium-glucose co-transporter 2 (SGLT2) inhibitors and DPP-4 inhibitors.27 GLP-1RAs are currently available as twice-daily (exenatide), once-daily (liraglutide and lixisenatide) or once-weekly subcutaneous injections (semaglutide, dulaglutide and exenatide extended-release)28-39 (reviewed in Lyseng-Williamson40), and as a once-daily tablet for oral administration (oral semaglutide).41, 42
GLP-1RAs have high glucose-lowering efficacy, but with variations within the drug class.43 In general, the HbA1c-lowering ability of long-acting GLP-1RAs is greater than that of short-acting GLP‑1RAs. In a recent meta-analysis of T2D trials, the mean difference in HbA1c from baseline to week 24 ranged from −0.5% with once-daily lixisenatide to −1.5% with once-weekly subcutaneous semaglutide.43 In a 68-week trial in patients with T2D and obesity, once-weekly subcutaneous semaglutide 2.4 mg reduced HbA1c levels by −1.6% (vs −0.4% with placebo).44 For weight loss in the meta-analysis of T2D trials, the mean difference from baseline to week 24 ranged from −0.8 kg with once-weekly albiglutide (now withdrawn) to −3.4 kg with once-weekly subcutaneous semaglutide.43 Since the meta-analysis of T2D trials was performed, semaglutide in tablet form has been approved for patients with T2D.41, 42 In the PIONEER phase III programme (26- to 78-week trials), once-daily oral semaglutide 14 mg reduced HbA1c over a range of −1.1% to −1.8% and lowered body weight by −3.2 to −4.3 kg (reviewed in Rasmussen45). A meta-analysis comparing oral semaglutide 14 mg with other GLP-1RAs in T2D patients inadequately controlled by one or two oral anti-diabetic drugs46 determined that oral semaglutide was associated with reductions in HbA1c versus most longer- and shorter-acting comparators except for once-weekly semaglutide 1 mg. There were significant reductions in HbA1c with oral semaglutide versus once-weekly dulaglutide 0.75 mg, once-weekly exenatide 2 mg, twice-daily exenatide 5 and 10 μg, once-daily liraglutide 1.2 mg and once-daily lixisenatide 20 μg; reductions versus once-weekly subcutaneous semaglutide 0.5 mg, once-weekly dulaglutide 1.5 mg and once-daily liraglutide 1.8 mg were non-significant. Oral semaglutide 14 mg also reduced body weight significantly more than all other comparators, except once-weekly subcutaneous semaglutide 0.5 and 1 mg.46 Oral semaglutide may provide an option for patients who prefer oral therapy instead of injectable administration.
The effects of GLP-1RAs on liver parameters were also evaluated in trials conducted in patients with T2D (Table S2). Beneficial effects of twice-daily exenatide 10 μg and once-daily liraglutide 1.8 mg on alanine aminotransferase (ALT) were observed.47, 48 A trend towards improvement in hepatic steatosis was also seen with once-daily liraglutide 1.8 mg.48
5 THE EFFECT OF GLP-1RAS ON CVD
CVD is a leading cause of morbidity and mortality for patients with NAFLD (reviewed in Armstrong et al8). The effect of GLP-1RAs on CV events has been thoroughly investigated in multiple large-scale international CV outcomes trials (CVOTs) in patients with T2D who are at high CV risk due to established CVD or multiple CV risk factors (Table 1).20-22, 26, 49-51 In trials with liraglutide, albiglutide, dulaglutide and semaglutide, significant reductions in the rate of major adverse CV events (MACE; death from CV causes, non-fatal myocardial infarction or non-fatal stroke) were noted versus placebo.20-22, 26 Lixisenatide and exenatide extended-release did not statistically significantly reduce MACE versus placebo in their respective trials.49, 50 The CVOT for oral semaglutide was designed and powered to demonstrate CV safety (non-inferiority vs placebo) rather than CV benefit (superiority vs placebo): CV safety was demonstrated and the hazard ratio for MACE was similar to that observed in the subcutaneous semaglutide CVOT.51
| Study | Comparators | Population | Duration | Primary CV finding HR (95% CI) with primary endpoint for GLP-1RA vs placebo | Liver-related findings reported in the main text | General neoplasms findings reported in the main text |
|---|---|---|---|---|---|---|
| ELIXA Pfeffer et al49 | Lixisenatide 20 μg/day vs placebo | 6068 patients with T2D who had had a recent acute coronary event | 2.1 years | Similar rate of MACE plus hospitalisation for unstable angina with lixisenatide and placebo: 1.02 (0.89-1.17); P < 0.001 for non-inferiority | Hepatobiliary SAEs: 1.2% with lixisenatide and 0.9% with placebo Pancreatitis: 0.16% with lixisenatide and 0.26% with placebo Pancreatic cancer: 0.10% with lixisenatide and 0.30% with placebo | Overall neoplasms: 2.4% with lixisenatide and 2.0% with placebo |
| LEADER Marso et al20 | Liraglutide 1.8 mg/day vs placebo | 9340 patients with T2D at high CV risk (81% with prior CVD) | 3.8 years | Lower rate of MACE with liraglutide vs placebo: 0.87 (0.78-0.97); P < 0.001 for non-inferiority; P = 0.01 for superiority | Acute gallstone disease: 3.1% with liraglutide and 1.9% with placebo (P < 0.001) Cholelithiasis: 1.5% with liraglutide and 1.1% with placebo (P = 0.09) Acute cholecystitis: 0.8% with liraglutide and 0.4% with placebo (P = 0.046) Acute pancreatitis: 0.4% with liraglutide and 0.5% with placebo (P = 0.44) Chronic pancreatitis: 0% with liraglutide and <0.1% with placebo (P = 0.16) Lipase ≥3 × ULN: 8.3% with liraglutide and 5.3% with placebo Amylase ≥3 × ULN: 1.0% with liraglutide and 0.8% with placebo Pancreatic cancer: 0.3% with liraglutide and 0.1% with placebo (P = 0.06) | Benign neoplasms: 3.6% with liraglutide and 3.1% with placebo (P = 0.18) Malignant neoplasms: 6.3% with liraglutide and 6.0% with placebo (P = 0.46) Medullary thyroid carcinoma: 0% with liraglutide and <0.1% with placebo (P = 0.32) |
| EXSCEL Holman et al50 | Exenatide ER 2 mg/week vs placebo | 14,752 patients with T2D at high CV risk (73% with prior CVD) | 3.2 years | Similar rate of MACE with exenatide and placebo: 0.91 (0.83-1.00); P < 0.001 for non-inferiority | Pancreatitis: 0.4% with exenatide and 0.3% with placebo Pancreatic cancer: 0.2% with exenatide and 0.2% with placebo | Overall neoplasms: 4.8% with exenatide and 4.9% with placebo Medullary thyroid carcinoma: <0.1% with exenatide and <0.1% with placebo |
| HARMONY-OUTCOMESHernandez et al26 | Albiglutide 30-50 mg/week vs placebo | 9463 patients with T2D at high CV risk (71% with prior CAD) | 1.6 years | Lower rate of MACE with albiglutide vs placebo: 0.78 (0.68-0.90); P < 0.0001 for non-inferiority; P = 0.0006 for superiority | Hepatobiliary disorders: 1% with albiglutide and 1% with placebo Pancreatitis: <1% with albiglutide and <1% with placebo Pancreatic cancer: <1% with albiglutide and <1% with placebo ALT ≥3 × ULN: <1% with albiglutide and 1% with placebo ALT ≥5 × ULN: <1% with albiglutide and <1% with placebo Bilirubin ≥2 × ULN: <1% with albiglutide and <1% with placebo | Haematological neoplasm: <1% with albiglutide and <1% with placebo Thyroid cancer: 0% with albiglutide and 0% with placebo |
| REWIND Gerstein et al22 | Dulaglutide 1.5 mg/week vs placebo | 9901 patients with T2D at high CV risk (31% with prior CVD) | 5.4 years | Lower rate of MACE with dulaglutide vs placebo: 0.88 (0.79-0.99); P = 0.026 for superiority | Serious hepatic event: 0.5% with dulaglutide and 0.8% with placebo (P = 0.057) Acute pancreatitis: 0.5% with dulaglutide and 0.3% with placebo (P = 0.11) Pancreatic cancer: 0.4% with dulaglutide and 0.2% with placebo (P = 0.22) | Overall neoplasms: 7.1% with dulaglutide and 7.0% with placebo (P = 0.98) Thyroid cancer: 0.1% with dulaglutide and 0.1% with placebo (P = 0.21) Medullary thyroid carcinoma or C-cell hyperplasia: <0.1% with dulaglutide and 0% with placebo (P = 0.32) |
| SUSTAIN-6 Marso et al21 | Semaglutide 0.5 or 1.0 mg/week vs placebo | 3297 patients with T2D at high CV risk (83% with prior CVD) | 2.1 years | Lower rate of MACE with semaglutide (doses pooled) vs placebo: 0.74 (0.58-0.95); P < 0.001 for non-inferiority; P = 0.02 for superiority | Gallbladder disorders: 3.2%-3.9% with semaglutide 0.5-1.0 mg and 2.8%-4.6% with placebo Cholelithiasis: 2.1%-2.5% with semaglutide 0.5-1.0 mg and 1.5%-2.3% with placebo Acute cholecystitis: 0%-0.5% with semaglutide 0.5-1.0 mg and 0.2%-0.7% with placebo Acute pancreatitis: 0.4%-0.7% with semaglutide 0.5-1.0 mg and 0.4%-1.1% with placebo Pancreatic cancer: 0%-0.1% with semaglutide 0.5-1.0 mg and 0.2% with placebo | Benign neoplasm: 4.8%-6.6% with semaglutide 0.5-1.0 mg and 4.1%-4.4% with placebo Any malignant neoplasm: 3.1%-4.9% with semaglutide 0.5-1.0 mg and 4.2% with placebo |
| PIONEER 6 Husain et al51 | Oral semaglutide 14 mg/day vs placebo | 3183 patients with T2D at high CV risk (85% with prior CVD) | 1.3 years | Similar rate of MACE with oral semaglutide and placebo: 0.79 (0.57-1.11); P < 0.001 for non-inferiority | Acute pancreatitis: 0.1% with oral semaglutide and 0.2% with placebo | Any malignant neoplasm: 2.6% with oral semaglutide and 3.0% with placebo Medullary thyroid carcinoma: 1 patient with oral semaglutide (had pre-existing thyroid nodules) and 0 patients with placebo |
- Abbreviations: ALT, alanine aminotransferase; CAD, coronary artery disease; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; CVOTs, CV outcomes trials; ER, extended-release; GLP-1RA, glucagon-like peptide-1 receptor agonist; HR, hazard ratio; MACE, major adverse cardiovascular event (cardiovascular death, non-fatal myocardial infarction and non-fatal stroke); SAE, serious adverse event; T2D, type 2 diabetes; ULN, upper limit of normal.
Based on the CVOTs, the American Diabetes Association/European Association for the Study of Diabetes guideline recommends that GLP-1RAs with proven CV benefit should be given to patients with T2D and established atherosclerotic CVD, with SGLT2 inhibitors with proven CV benefit as an alternative option.27 GLP-1RAs with proven CV benefit may also be considered in patients with T2D without established CVD, but with indicators of high risk, specifically, those aged ≥55 years with coronary, carotid or lower extremity artery stenosis >50%, left ventricular hypertrophy, estimated glomerular filtration rate <60 ml/minute/1.73 m2 or albuminuria.
Long-term CVOTs, conducted in thousands of patients with T2D, CVD and other comorbidities, also allow assessment of the general long-term safety of GLP-1RAs.20-22, 26, 49-51 GI AEs (notably nausea, vomiting, constipation, diarrhoea) are a well-known class effect associated with GLP-1RAs, but most cases are transient and mild-to-moderate in severity.52
In the early years following the introduction of GLP-1RAs and DPP-4 inhibitors, concerns were raised from epidemiological reports over the possibility of an increased risk of pancreatitis and pancreatic cancer with these classes.53 However, neither data from individual CVOTs of GLP-1RAs nor their meta-analysis have indicated a significantly elevated risk of acute pancreatitis.54 Furthermore, GLP-1RAs were not associated with a significantly elevated or reduced risk of pancreatic cancer or for the totality of all malignant neoplasms.54
Elevated levels of serum amylase and lipase have been noted with GLP-1RAs,32, 33, 36-39, 41, 42 which is of undetermined/unknown clinical significance. In the LEADER trial, mean levels of serum amylase and lipase were higher in the liraglutide group than in the placebo group.20 In total, 8.3% and 5.3% of liraglutide- and placebo-treated patients, respectively, had lipase ≥3 times above the upper limit of normal at some point during the trial. For amylase, 1.0% and 0.8% of liraglutide- and placebo-treated patients had levels ≥3 times above the upper limit of normal at some point during the trial.20 The clinical significance of elevations in lipase or amylase is unknown in the absence of other signs and symptoms of clinical pancreatitis. Acute gallstone disease was more common with once-daily liraglutide than with placebo (3.1% vs 1.9%),20 which may be related to the rapid weight loss.55
CVOTs also provided the opportunity to investigate the long-term effects of GLP-1RAs on transaminases. Improvements in transaminases were reported in the CVOTs of dulaglutide and once-weekly subcutaneous semaglutide.26, 56 In SUSTAIN-6 with once-weekly subcutaneous semaglutide, elevated baseline ALT was present in 41% of participants.56 In the group with elevated ALT, no significant ALT reduction was noted with once-weekly subcutaneous semaglutide 0.5 mg versus placebo, while a significant 9% reduction was seen with once-weekly subcutaneous semaglutide 1.0 mg (P = 0.0024). Consistent with findings from the LEAD meta-analysis,48 treatment ratios for changes in ALT with semaglutide were not statistically significant after adjustment for weight change.56
6 EXPERIENCE WITH GLP-1RAS IN OBESITY
There is growing recognition that much of the pathophysiology of obesity involves abnormal satiety and feeding signalling in the brain (reviewed in Coulter et al57). When mechanisms responsible for GLP-1-mediated weight reduction were investigated, increased satiety and reduced food intake were observed with subcutaneous and oral semaglutide.23, 24 Animal studies have shown that liraglutide and semaglutide can access the specific areas of the brain involved in appetite regulation.58, 59 In rodents, semaglutide caused weight loss without decreasing energy expenditure through an effect on both homeostatic (appetite, hunger, satiety), as well as hedonic (food choice, control) neural pathways.59
A summary of phase III trials of GLP-1RAs in obesity is presented in Table 2.44, 60-67 In the largest study, the 56-week SCALE Obesity and Prediabetes trial, participants who received once-daily liraglutide 3.0 mg (a higher dose used than that for managing T2D [1.8 mg]), lost a mean of 8.4 kg of body weight compared with 2.8 kg in the placebo group (P < 0.001).62 A total of 63.2% of the participants in the liraglutide group versus 27.1% in the placebo group lost ≥5% of their body weight (P < 0.001), while 33.1% and 10.6%, respectively, lost >10% of their body weight (P < 0.001). On the basis of the SCALE trials, once-daily subcutaneous liraglutide 3.0 mg was approved for chronic weight management as an adjunct to lifestyle intervention in adults with obesity (body mass index [BMI] ≥30 kg/m2), or with overweight (BMI ≥27 kg/m2) who have at least one weight-related comorbid condition (eg, hypertension, T2D or dyslipidaemia).68, 69 The United States Food and Drug Administration has recently approved an updated label for once-daily subcutaneous liraglutide 3.0 mg to include weight management in adolescents aged from 12 years with a body weight ≥60 kg and BMI that corresponds to an adult value of ≥30 kg/m2.68
| Study [country/region] | Comparator(s) | Trial population | Duration | Primary endpoint(s) | Liver-related findings reported in the main text |
|---|---|---|---|---|---|
| Astrup et al60 [Europe] | Liraglutide 1.2, 1.8, 2.4 and 3.0 mg/day vs orlistat 120 mg 3 times daily vs placebo plus lifestyle intervention | 564 patients with BMI 30-40 kg/m2 and no T2D | 20 weeks | Mean weight loss was:
|
No events of pancreatitis were reported |
| SCALE Maintenance Wadden et al61 [North America] | Liraglutide 3.0 mg/day vs placebo plus lifestyle intervention | 422 patients with BMI ≥30 kg/m2 or ≥27 kg/m2 with dyslipidaemia and/or hypertension who lost ≥5% of initial weight during a low-calorie diet run-in | 56 weeks | Additional mean percentage weight loss was:
|
One patient on liraglutide withdrew due to worsening cholelithiasis No cases of acute pancreatitis Lipase and amylase remained within the normal range |
| SCALE Obesity and Prediabetes Pi-Sunyer et al62 [International] | Liraglutide 3.0 mg/day vs placebo plus lifestyle intervention | 3731 patients with BMI ≥30 kg/m2 or ≥27 kg/m2 with dyslipidaemia and/or hypertension | 56 weeks | Mean weight loss was:
|
Cholelithiasis: 0.8% with liraglutide and 0.4% with placebo Acute cholecystitis: 0.5% with liraglutide and 0% with placebo Cholecystitis: 0.2% with liraglutide and 0% with placebo Acute pancreatitis: 0.2% with liraglutide and 0% with placebo Lipase ≥3 × ULN: 2.5% with liraglutide and 1.1% with placebo Amylase ≥3 × ULN: 0.2% with liraglutide and <0.1% with placebo |
| SCALE Diabetes Davies et al63 [International] | Liraglutide 1.8 mg or 3.0 mg/day vs placebo plus lifestyle intervention | 846 patients with BMI ≥27 kg/m2 and T2D | 56 weeks | Mean percentage weight loss was:
|
Gallbladder-related AEs: 1.2% with liraglutide 3.0 mg, 1.9% with liraglutide 1.8 mg and 0.5% with placebo Acute pancreatitis: 0% all groups Lipase ≥3 × ULN: 7.7% with liraglutide 3.0 mg, 9.8% with liraglutide 1.8 mg and 6.3% with placebo Amylase ≥3 × ULN: 0% all groups |
| SCALE Sleep Apnea Blackman et al64 [North America] | Liraglutide 3.0 mg/day vs placebo plus lifestyle intervention | 359 patients with BMI ≥30 kg/m2 and obstructive sleep apnoea | 32 weeks | Apnoea-hypopnoea index was reduced by 12.2 events/hour with liraglutide 3.0 mg vs 6.1 events/hour with placebo (P = 0.0150) Mean percentage weight loss was:
|
Cholelithiasis: 0.6% with liraglutide and 0% with placebo Cholecystitis: 0% with liraglutide and 0.6% with placebo Lipase increased: 5.1% with liraglutide and 2.8% with placebo |
| STEP 1 Wilding et al65 [International] | Semaglutide 2.4 mg/week vs placebo plus lifestyle intervention | 1961 patients with BMI ≥30 kg/m2 or ≥27 kg/m2 with ≥1 weight-related co-existing condition | 68 weeks | Mean percentage weight loss was:
|
Gallbladder-related disorders: 2.6% with semaglutide and 1.2% with placebo Hepatobiliary disorders: 2.5% with semaglutide and 0.8% with placebo Cholelithiasis: 1.8% with semaglutide and 0.6% with placebo Hepatic disorders: 2.4% with semaglutide and 3.1% with placebo Acute pancreatitis: 0.2% with semaglutide and 0% with placebo |
| STEP 2 Davies et al44 [International] | Semaglutide 2.4 mg/week vs placebo plus lifestyle intervention | 1210 patients with BMI ≥27 kg/m2 and T2D | 68 weeks | Mean percentage weight loss was:
|
Gallbladder-related disorders: 0.2% with semaglutide and 0.7% with placebo Hepatobiliary disorders: 0.2% with semaglutide and 0.7% with placebo Cholelithiasis: 0.2% with semaglutide and 0.7% with placebo Hepatic disorders: 2.5% with semaglutide and 3.5% with placebo Acute pancreatitis: 0.2% with semaglutide and 0.2% with placebo |
| STEP 3 Wadden et al66 [USA] | Semaglutide 2.4 mg/week vs placebo plus intensive behavioural therapy with an initial low-calorie diet | 611 patients with BMI ≥30 kg/m2 or ≥27 kg/m2 with ≥1 weight-related co-existing condition | 68 weeks | Mean percentage weight loss was:
|
Gallbladder-related disorders: 4.9% with semaglutide and 1.5% with placebo Cholelithiasis: 3.2% with semaglutide and 1.0% with placebo Hepatic disorders: 2.0% with semaglutide and 2.0% with placebo Acute pancreatitis: 0% with semaglutide and 0% with placebo |
| STEP 4 Rubino et al67 [International] | 20-week run-in period on semaglutide (titrated to 2.4 mg/week) then continued semaglutide 2.4 mg/week vs placebo for 48 weeks plus lifestyle intervention | 902 patients with BMI ≥30 kg/m2 or ≥27kg/m2 with ≥1 weight-related co-existing condition without T2D entered the run-in period | 68 weeks | Mean percentage weight loss from week 20 to week 68 was 7.9% with continued semaglutide 2.4 mg, with a weight gain of 6.9% with placebo (P < 0.001) Over 68 weeks:
|
Gallbladder-related disorders: 2.8% with semaglutide and 3.7% with placebo Hepatic disorders: 2.1% with semaglutide and 1.5% with placebo Acute pancreatitis: 0% with semaglutide and 0% with placebo |
- Abbreviations: AEs, adverse events; BMI, body mass index; GLP-1RA, glucagon-like peptide-1 receptor agonist; T2D, type 2 diabetes; ULN, upper limit of normal.
Until recently, approved anti-obesity drugs required daily (liraglutide) or more frequent administration (eg, orlistat three times daily). Once-weekly subcutaneous semaglutide 2.4 mg was approved for chronic weight management by the United States Food and Drug Administration in June 202170; the once-weekly regimen may improve treatment adherence. The efficacy and safety of once-weekly subcutaneous semaglutide 2.4 mg (a higher dose than for T2D [0.5-1.0 mg]) is being evaluated in the STEP programme.71 As shown in Table 2, the first four STEP trials have been published.44, 65-67 In these trials, semaglutide was initiated at a once-weekly subcutaneous dose of 0.25 mg for the first 4 weeks, with the dose increased every 4 weeks to reach the maintenance dose of 2.4 mg.44, 65-67 In STEP 1 in adults with overweight or obesity, mean change in body weight was −14.9% with semaglutide 2.4 mg versus −2.4% with placebo at week 68 (P < 0.001).65 In STEP 2 in adults with overweight or obesity and T2D, mean change in body weight was −9.6% with semaglutide 2.4 mg versus −3.4% with placebo at week 68 (P < 0.001).44 STEP 3 compared the effects of semaglutide 2.4 mg versus placebo as an adjunct to intensive behavioural therapy with an initial low-calorie diet in adults with overweight or obesity.66 Mean change in body weight was −16.0% with semaglutide 2.4 mg versus −5.7% with placebo at week 68 (P < 0.001).66 STEP 4 compared continued semaglutide 2.4 mg with a switch to placebo in adults with overweight or obesity after a 20-week run-in period on semaglutide.67 With continued semaglutide 2.4 mg treatment, mean body weight change from week 20 to week 68 was −7.9% versus +6.9% with the switch to placebo (P < 0.001). In each STEP trial, significantly more participants in the semaglutide group than in the placebo group achieved weight reductions of ≥5% (68.8%-88.7% vs 28.5%-47.6%) or ≥10% (45.6%-79.0% vs 8.2%-27.0%) at week 68 (all P < 0.001).44, 65-67
As seen in T2D, gallbladder-related disorders, particularly cholelithiasis, were more common in some STEP trials with GLP-1RAs versus placebo. In the STEP trials, gallbladder-related disorders were reported in 0.2%-4.9% and 0.7%-3.7% of participants in the semaglutide and placebo groups, respectively.44, 65-67 In STEP 1, mild acute pancreatitis (according to the Atlanta classification72) was reported in three participants in the semaglutide group (one participant had a history of acute pancreatitis, the other two participants had both gallstones and pancreatitis); all recovered during the trial period.65 In STEP 2, acute pancreatitis was reported in one patient in each of the semaglutide and placebo groups.44 No cases of acute pancreatitis were observed in STEP 3 or 4.66, 67
7 THE POTENTIAL OF GLP-1RAS IN PATIENTS WITH NASH
The benefits of GLP-1RAs on NASH are thought to be due to their effect on insulin resistance, reduction in body weight and a possible direct effect on the liver, thus targeting multiple facets of metabolic syndrome.10 Pre-clinical evidence suggests that GLP-1RAs can reduce de novo lipogenesis, stimulate oxidation of fatty acids and improve multiple elements of insulin signalling pathways.73-76 GLP-1RAs may also reduce hepatic inflammation through mechanisms at least partly independent of body weight reduction.74
The potential role of GLP-1RAs in the treatment of NAFLD and NASH has since been investigated in several phase II trials and investigator sponsored studies, summarised in Table 3.77-85 One of these, the LEAN investigator sponsored study, also provided some mechanistic insight,25, 78 suggesting that there are liver-specific benefits of GLP-1RAs that extend beyond weight loss.25 The LEAN study assessed the efficacy and safety of once-daily liraglutide 1.8 mg compared with placebo after 48 weeks in 52 patients who were overweight and showed clinical evidence of NASH.78 Liraglutide met the primary endpoint and led to resolution of NASH in 39% of patients compared with 9% of patients in the placebo group (relative risk: 4.3; 95% confidence interval [CI]: 1.0-17.7; P = 0.019). Progression of fibrosis was noted in 9% of patients in the liraglutide group versus 36% of patients in the placebo group (relative risk: 0.2; 95% CI: 0.1-1.0; P = 0.04). A substudy determined the effect of liraglutide versus placebo on organ-specific insulin sensitivity, adipose dysfunction and hepatic lipid handling in 14 patients participating in the LEAN study.25 Unlike placebo, liraglutide improved liver enzymes from baseline, particularly aspartate transaminase (AST; 64 vs 37 IU/L; P < 0.05) and ALT (90 vs 36 IU/L; P < 0.05). Liraglutide also increased hepatic insulin sensitivity (−9.36% vs −2.54% suppression of hepatic endogenous glucose production with low-dose insulin; P < 0.05). Liraglutide increased adipose tissue insulin sensitivity, enhancing the ability of insulin to suppress both whole-body lipolysis (−24.9 vs +54.8 pmol/L insulin required for half-maximal suppression of serum non-esterified fatty acids; P < 0.05) and lipolysis within subcutaneous adipose tissue (P < 0.05). Liraglutide decreased hepatic de novo lipogenesis in vivo (−1.26% vs +1.30%; P < 0.05) measured using deuterium labelling, a result that was supported by the finding that the GLP-1 receptor analogue, exendin-4, reduced hepatic steatosis in vitro through a reduction in de novo lipogenesis (24.6% decrease in lipogenesis vs untreated controls; P < 0.01). The authors postulated that the effects of GLP-1RAs on de novo lipogenesis may occur in the absence of weight loss, but the exact mechanisms remain unknown.
| Study [country/region] | Comparators | Trial population | Duration | Key hepatic-related efficacy findings | Hepatic-related safety findings |
|---|---|---|---|---|---|
| Shao et al77 [China] | Exenatide 10 μg twice daily vs insulin aspart plus insulin glargine | 60 patients with T2D, obesity and NAFLD with elevated liver enzymes | 12 weeks | Levels of ALT, AST and GGT in the exenatide group were significantly lower than in the intensive insulin group (P < 0.001) Reversal rate of fatty liver (assessed by ultrasound) was significantly higher in the exenatide group (93.3%) than the intensive insulin group (66.7%) (P < 0.01) | None reported |
| LEAN Armstrong et al78 [UK] | Liraglutide 1.8 mg/day vs placebo | 52 patients with NASH | 48 weeks | Primary endpoint Proportion of patients with resolution of NASH with no worsening of fibrosis: 39% with liraglutide 1.8 mg and 9% with placebo (P = 0.019) Secondary endpoint Progression of fibrosis: 9% with liraglutide 1.8 mg and 36% with placebo (P = 0.04) | No cases of pancreatitis, hepatitis or liver failure |
| Feng et al79 [China] | Liraglutide 1.8 mg/day vs gliclazide 120 mg/daily vs metformin 1000 mg twice daily | 87 patients with T2D and NAFLD | 24 weeks | Intrahepatic fat content decreased from 36.7% to 13.1% with liraglutide, from 33.0% to 19.6% with gliclazide, and from 35.1% to 18.4% with metformin (P < 0.01 for liraglutide vs gliclazide) Liver function enzymes significantly improved with liraglutide and metformin | None reported |
| Light-On Yan et al80 [China] | Liraglutide 1.8 mg/day vs sitagliptin 100 mg/day vs insulin glargine 0.2 IU/kg/day plus metformin | 75 patients with T2D and NAFLD | 26 weeks | Liraglutide and sitagliptin, but not insulin glargine, reduced intrahepatic lipid content (primary endpoint), visceral adipose tissue and body weight | None reported |
| Khoo et al81 [Singapore] | Liraglutide 3.0 mg/day vs diet and exercise programme | 30 patients with obesity and NAFLD | 26 weeks | Both liraglutide and exercise reduced body weight, hepatic steatosis and hepatocellular apoptosis, but liraglutide’s benefits were not sustained after discontinuation, in contrast with exercise | No cases of pancreatitis |
| Guo et al82 [China] | Liraglutide 1.8 mg/day vs insulin glargine vs placebo plus metformin | 96 patients with T2D, obesity and NAFLD | 26 weeks | Intrahepatic lipid content decreased significantly from baseline with liraglutide, but not insulin glargine Subcutaneous adipose tissue and visceral adipose tissue decreased significantly in the liraglutide group and in the insulin glargine group (P < 0.05), but subcutaneous adipose tissue changes were greater with liraglutide than insulin glargine AST and ALT decreased significantly from baseline with liraglutide | None reported |
| Liu et al83 [China] | Exenatide 10 μg twice daily vs insulin glargine 0.1-0.3 IU/kg | 76 patients with T2D and NAFLD | 24 weeks | Liver fat content, visceral adipose tissue, subcutaneous adipose tissue and Fibrosis-4 index were significantly reduced with exenatide (all P < 0.05), while only liver fat content (P < 0.05) was reduced with insulin glargine Greater reductions in ALT, AST and GGT with exenatide vs insulin glargine (P < 0.05) | None reported |
| Kuchay et al84 [North India] | Dulaglutide 1.5 mg/week vs usual care | 64 patients with T2D and NAFLD | 24 weeks | Dulaglutide significantly reduced liver fat content and improved GGT levels (P < 0.05) Non-significant reductions in pancreatic fat content, liver stiffness, AST and ALT with dulaglutide | None reported |
| Newsome et al85 [Patients were from multiple countries] | Semaglutide 0.1, 0.2 or 0.4 mg/day vs placebo | 320 patients with NASH | 72 weeks | Primary endpoint Proportion of patients with resolution of NASH with no worsening of fibrosis: 40% with semaglutide 0.1 mg, 36% with semaglutide 0.2 mg, 59% with semaglutide 0.4 mg and 17% with placebo (P < 0.001 for semaglutide 0.4 mg vs placebo) Secondary endpoint Proportion of patients with an improvement of ≥1 fibrosis stage and no worsening of NASH: 49% with semaglutide 0.1 mg, 32% with semaglutide 0.2 mg, 43% with semaglutide 0.4 mg, 33% with placebo (P = 0.48 for semaglutide 0.4 mg vs placebo) | Similar incidence of hepatic AEs Gallbladder-related disorders: 6% with semaglutide 0.1 mg, 5% with semaglutide 0.2 mg, 7% with semaglutide 0.4 mg and 2% with placebo Acute pancreatitis: no cases |
- Abbreviations: AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma glutamyl transpeptidase; GLP-1RA, glucagon-like peptide-1 receptor agonist; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; T2D, type 2 diabetes.
A recently published phase II trial on semaglutide appeared to show an improvement on results reported for liraglutide.85 Newsome et al85 compared once-daily (rather than once weekly) subcutaneous semaglutide at doses of 0.1, 0.2 or 0.4 mg and placebo in 320 patients with biopsy-confirmed NASH and liver fibrosis (stage F1, F2 or F3), but not cirrhosis.85 The percentage of patients in whom NASH resolution was achieved with no worsening of fibrosis, the primary endpoint, was 40% with semaglutide 0.1 mg, 36% with semaglutide 0.2 mg, 59% with semaglutide 0.4 mg and 17% with placebo after 72 weeks (P < 0.001 for semaglutide 0.4 mg vs placebo). There was no significant difference in the confirmatory secondary endpoint of percentage of patients with improvement in liver fibrosis stage without worsening of NASH (43% with semaglutide 0.4 mg and 33% with placebo; P = 0.48). Worsening of fibrosis occurred in 5% of patients in the semaglutide 0.4-mg group compared with 19% of patients in the placebo group. Other beneficial effects included mean change in body weight of −12.5% with semaglutide 0.4 mg versus −0.6% with placebo, and reductions in ALT, AST, enhanced liver fibrosis score, liver stiffness (assessed by transient elastography) and exploratory biomarker levels (eg, caspase-cleaved cytokeratin-18 fragments M30 and M65, markers of hepatocyte apoptosis). The authors postulated that, given the lack of hepatic GLP-1 receptor expression, the potential mechanism of action of GLP-1RAs in NASH may relate to indirect beneficial effects on weight and insulin resistance, as well as reductions in metabolic dysfunction, lipotoxic effects and inflammation.85
Regarding safety and tolerability, the incidence of nausea, constipation and vomiting was higher in the semaglutide 0.4 mg group than in the placebo group (nausea, 42% vs 11%; constipation, 22% vs 12%; and vomiting, 15% vs 2%, respectively).85 Furthermore, GI AEs were the most common reasons for discontinuation in the once-daily semaglutide group (4% of patients), with no GI-related discontinuations in the placebo group. Severe hypoglycaemic episodes were rare over the 72-week trial, occurring in two or fewer patients per group. Gallbladder-related AEs were more common with semaglutide than with placebo, and increases in amylase and lipase levels were greater with semaglutide than with placebo. Malignant neoplasms were reported in 1% patients who received semaglutide and in no patients who received placebo. Overall neoplasms (benign, malignant or unspecified, including cysts and polyps) were reported in 15% of the patients in the semaglutide groups and in 8% in the placebo group; however, there was no pattern of occurrence in specific organs. It should be noted that in the meta-analysis of long-term CVOTs, GLP-1RAs were not associated with an increased risk of any malignant neoplasms.54
Results from a phase II trial evaluating the safety and efficacy of semaglutide monotherapy and combination regimens involving once-weekly subcutaneous semaglutide 2.4 mg with once-daily farnesoid X receptor agonist cilofexor, and/or acetyl-CoA carboxylase inhibitor firsocostat in 108 patients with non-cirrhotic NASH have been published in abstract form.86 Semaglutide 2.4 mg alone reduced ALT, hepatic steatosis by magnetic resonance imaging (MRI)-derived proton density fat fraction and liver stiffness by transient elastography over 24 weeks; however, reductions were greater when semaglutide 2.4 mg was combined with cilofexor (30 or 100 mg), firsocostat 20 mg or both cilofexor 30 mg and firsocostat 20 mg. Median change from baseline in hepatic steatosis as measured by MRI-derived proton density fat fraction was −47% with semaglutide once-weekly alone, −66% with semaglutide plus firsocostat and −68% with semaglutide plus cilofexor and firsocostat. Least square mean reduction in liver stiffness by transient elastography was −2.5 kPa with semaglutide alone, −3.8 kPa with semaglutide plus firsocostat and −3.5 kPa with semaglutide plus cilofexor and firsocostat.
Additional data on the impact of semaglutide in NAFLD using MRI-based metrics to assess disease was recently published by Flint et al.87 A total of 67 patients were randomised to subcutaneous semaglutide 0.4 mg once daily or placebo for 72 weeks. The primary endpoint was change from baseline to week 48 in liver stiffness assessed by magnetic resonance elastography (MRE) with secondary outcomes of liver steatosis assessed by MRI proton density fat fraction, liver enzymes, body weight and HbA1c. At 48 weeks, MRE-measured liver stiffness was not significantly different between semaglutide and placebo. Overall reductions in liver steatosis were significantly greater with semaglutide and more patients in the semaglutide arm achieved at least a 30% reduction in liver fat versus placebo. Improvements in other outcomes were also observed, including decreases in liver enzymes, body weight and HbA1c.
Data from phase II studies of other GLP-1RAs in the treatment of NAFLD and NASH are limited. In a 12-week study of exenatide 10 μg twice daily compared with insulin aspart plus insulin glargine, in 60 patients with T2D, obesity and NAFLD with elevated liver enzymes, levels of ALT, AST and gamma glutamyl transpeptidase (GGT) were significantly lower in the exenatide group than in the intensive insulin group (P < 0.001) and the reversal rate of fatty liver was significantly higher (93.3% vs 66.7%, respectively; P < 0.01).77 In a study of 76 patients with T2D and NAFLD who received 24 weeks of exenatide 10 μg twice daily or insulin glargine 0.1-0.3 IU/kg, liver fat content, visceral adipose tissue, subcutaneous adipose tissue and Fibrosis-4 index were significantly reduced with exenatide (all P < 0.05), while only liver fat content (P < 0.05) was reduced with insulin glargine. Greater reductions in ALT, AST and GGT were also seen with exenatide (P < 0.05).83 In a 24-week study of dulaglutide 1.5 mg/week versus usual care in 64 patients with T2D and NAFLD, dulaglutide significantly reduced liver fat content and improved GGT levels (P < 0.05). Reductions in pancreatic fat content, liver stiffness, ALT and AST were not significant with dulaglutide.84
A meta-analysis of 11 placebo- or active-controlled phase II randomised controlled trials (RCTs) of liraglutide (n = 6), semaglutide (n = 1), exenatide (n = 3) or dulaglutide (n = 1) for the treatment of NAFLD or NASH detected by liver biopsy (n = 2) or imaging techniques (n = 9) in 935 adults with overweight or obesity (72.4% with T2D) was recently conducted.10 GLP-1RA treatment for a median of 26 weeks was associated with significant reductions in the absolute percentage of liver fat content (pooled weighted mean difference: −3.92%, 95% CI –6.27 to –1.56) and serum liver enzyme levels, together with greater histological resolution of NASH without worsening of liver fibrosis (pooled random-effects odds ratio 4.06, 95% CI 2.52-6.55; for liraglutide and semaglutide only) compared with placebo or reference therapy. Treatment with GLP-1RAs was also associated with significant reductions in body weight versus placebo or reference therapy (pooled weighted mean difference: −4.06 kg, 95% CI −5.44 to 2.68).
Reduction in body weight is likely a key mechanism through which GLP-1RAs achieve their effects on NASH.10 Therefore, patients with overweight or obesity may potentially demonstrate a greater benefit from GLP-1RA therapy.
8 PRACTICAL CONSIDERATIONS AND LIMITATIONS
Although GLP-1RAs are not currently approved for the treatment of NASH, hepatologists should be aware of some practical considerations regarding the use of GLP-1RAs in patients being treated with T2D and/or obesity. As mentioned, GI AEs are commonly seen with GLP-1RAs, but most cases are mild-to-moderate in severity and occur in the initial dose-escalation period. GI effects appear to be dose related and escalating from a low dose may improve tolerability. Patients experiencing GI AEs could be advised to eat smaller meals and stop when they feel full, since reducing food intake may lessen nausea and vomiting.88 Monitoring renal function is recommended in patients with renal impairment who also experience severe GI AEs or dehydration, due to reports of acute kidney injury in this group of patients.32-39, 41, 42
Hypoglycaemia may be a cause for concern for patients with T2D; however, the general risk of hypoglycaemia with GLP-1RAs is minimal,89 as supported by published data. The risk of hypoglycaemia may be increased when GLP-1RAs are used in combination with sulphonylureas or insulin.28-39, 41, 42 Reducing the dose of the sulphonylurea or insulin should therefore be considered when GLP-1RAs are used with these drug classes.28-39, 41, 42
As reports of pancreatitis in patients treated with GLP-1RAs are generally infrequent, prescribing information recommends that the GLP-1RA is discontinued as soon as pancreatitis is suspected.28-39, 41, 42 If pancreatitis is subsequently confirmed, the recommendation is for the GLP-1RA not to be started.
GLP-1RAs cause a delay in gastric emptying, and thus have the potential to reduce the rate of absorption of concomitantly administered oral medications.28-39, 41, 42 Caution should be exercised when oral medications are concomitantly administered with GLP-1RAs, and it is advised that drug levels of oral medications with a narrow therapeutic index should be adequately monitored.28-39, 41, 42 There have, for example, been post-marketing reports of increased international normalised ratio when exenatide is given concomitantly with warfarin, sometimes associated with bleeding, and it is recommended that the international normalised ratio should be monitored more frequently after initiation or alteration of exenatide doses.28, 29, 34, 35 In general, however, GLP-1RAs did not affect the absorption of tested, orally administered medications to a clinically relevant degree in clinical pharmacology studies.28-39, 41, 42
GLP-1RAs have several limitations, notably frequent GI side effects which can limit their tolerability,52, 85 together with possible risks of gallbladder-related events, pancreatitis and malignant neoplasms.20, 53, 85 While no prospective phase III trials of GLP-1RAs for the treatment of NASH have been conducted, many patients with NASH would have been eligible to participate in the trials of T2D or obesity. The phase II trial of semaglutide in the treatment of NASH did not include patients with cirrhosis85 and thus, the efficacy and safety in this population is unknown. However, phase II studies are planned, or ongoing, to assess semaglutide monotherapy and combination regimens with cilofexor and/or firsocostat in patients with compensated cirrhosis due to NASH.90, 91
9 THE POTENTIAL OF OTHER GLUCOSE-LOWERING THERAPIES IN PATIENTS WITH NASH
Other glucose-lowering therapies have also been investigated in the treatment of NAFLD and NASH, although data are limited. A small, open-label study of metformin was conducted in 26 patients with biopsy-proven NASH and overweight or obesity; 22 patients had fibrosis and one patient had cirrhosis on pre-treatment liver biopsy.92 After 48 weeks of treatment, improvements in liver histology (defined as a 3-point improvement in NASH activity index with improvements in ≥2 components of the score and no worsening of fibrosis or increase in Mallory bodies) and serum ALT levels were seen in 8/26 patients (31%), with these effects thought to be mediated largely through weight loss. Changes in liver fibrosis scores were minimal.92 Two meta-analyses (nine RCTs of 4-12 months duration that included 417 patients with NAFLD93 and four RCTs of 6-12 months duration that included 115 patients94 with NASH) concluded that metformin therapy did not improve liver histology in this patient population. Metformin is therefore not recommended by the American Association for the Study of Liver Disease for the treatment of NASH.1
Pioglitazone has demonstrated improvements in liver histology in patients with biopsy-proven NASH, with and without T2D,95-98 and is recommended by the American Association for the Study of Liver Disease for the treatment of these patients.1 Pioglitazone was also associated with a significant improvement in fibrosis versus placebo in a meta-analysis of three RCTs in 117 patients with NASH.99 Pioglitazone may be associated with side effects such as moderate weight gain, peripheral oedema and risk of distal bone fractures, particularly in postmenopausal women.10
There are limited data on sodium-glucose cotransporter (SGLT) inhibitors in NASH. In a pre-clinical rodent model, the intestinal SGLT1 inhibitor, SGL5213, demonstrated a protective effect on the NAFLD pathogenesis, which may be related to its action in inhibiting glucose absorption and increasing glucose content in the GI tract.100 SGLT2 inhibitors (including ipragliflozin and luseogliflozin) have been shown to improve NASH in rodent models,101, 102 and ipragliflozin is associated with improvements in liver function tests and fatty liver index in patients with NAFLD and T2D.103-105 Phase III and IV studies are ongoing to assess the efficacy and safety of the SGLT2 inhibitors empagliflozin and dapagliflozin106-108; however, no data have yet been published.
10 CONCLUSIONS
Due to their multiple physiological effects, GLP-1RAs play an important role in the diabetes treatment armamentarium and their benefits have been found to extend to the management of obesity. Given the shared pathogenic drivers, GLP-1RAs have been tested in patients with NASH without cirrhosis and appear to have favourable effects. Phase III trials of GLP-1RAs in NASH are now needed to answer further questions regarding their effects on fibrosis and NASH resolution, and to confirm safety. A phase III trial in approximately 1200 patients with non-cirrhotic NASH is ongoing, to investigate the efficacy and safety of once-weekly semaglutide versus placebo over 240 weeks (NCT04822181).109
ACKNOWLEDGEMENT
Declaration of personal interests: A. Sidney Barritt 4th conducts NASH-related clinical trials with support from Intercept, Genfit, Allergan, Celgene, Galmed, Pfizer, Bristol Myers Squibb, Viking and Gilead. He has provided consulting for Intercept and Target RWE in the past 12 months. Emma Marshman received funding from Novo Nordisk Inc. for medical writing support for this manuscript. Mazen Noureddin has served on the advisory boards for 89BIO, Gilead, Intercept, Pfizer, Novo Nordisk, Blade, EchoSens, Fractyl, Terns, Perspectum, Siemens and Roche Diagnostic. He has received research support from Allergan, Bristol Myers Squibb, Gilead, Galmed, Galectin, Genfit, Conatus, Enanta, Madrigal, Novartis, Shire, Viking and Zydus. He is a minor shareholder and has stocks in Anaetos, Rivus Pharma and Viking.
AUTHORSHIP
Guarantor of the article: A. Sidney Barritt 4th.
Author contributions: Emma Marshman drafted the manuscript under the direction of A. Sidney Barritt 4th and Mazen Noureddin. A. Sidney Barritt 4th and Mazen Noureddin edited and reviewed the manuscript. All authors have approved the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




