Synthesis of Diverse 1,4-(Azaindole)[60]fullerenes via Transition-Metal Free Three-Component Coupling Reaction of Azaindoles, C60, and Bromoalkanes/Triphenylamines†
Xinmin Huang
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Xinmin Huang and Zi-Zheng Liu contributed equally to this manuscript.
Search for more papers by this authorZi-Zheng Liu
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Xinmin Huang and Zi-Zheng Liu contributed equally to this manuscript.
Search for more papers by this authorJia-Qi Cheng
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Search for more papers by this authorCheng-Yu Cao
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Search for more papers by this authorPeng-Cheng Li
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Search for more papers by this authorJun Xuan
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Search for more papers by this authorCorresponding Author
Fei Li
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected]Search for more papers by this authorXinmin Huang
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Xinmin Huang and Zi-Zheng Liu contributed equally to this manuscript.
Search for more papers by this authorZi-Zheng Liu
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Xinmin Huang and Zi-Zheng Liu contributed equally to this manuscript.
Search for more papers by this authorJia-Qi Cheng
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Search for more papers by this authorCheng-Yu Cao
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Search for more papers by this authorPeng-Cheng Li
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Search for more papers by this authorJun Xuan
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Search for more papers by this authorCorresponding Author
Fei Li
Department of Chemistry, Anhui University; Key Laboratory of Structure and Functional Regulation of Hybrid Materials (Anhui University), Ministry of Education; Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials and Key Laboratory of Functional Inorganic Materials of Anhui Province, Hefei, Anhui, 230601 China
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Comprehensive Summary
A transition-metal free three-component coupling reaction of azaindoles, C60, and bromoalkanes/triphenylamines has been developed to provide an efficient access to diverse azaindole functionalized 1,4-C60 adducts. This protocol exhibits low cost, operational simplicity, wide substrate scope, and mild and convenient conditions.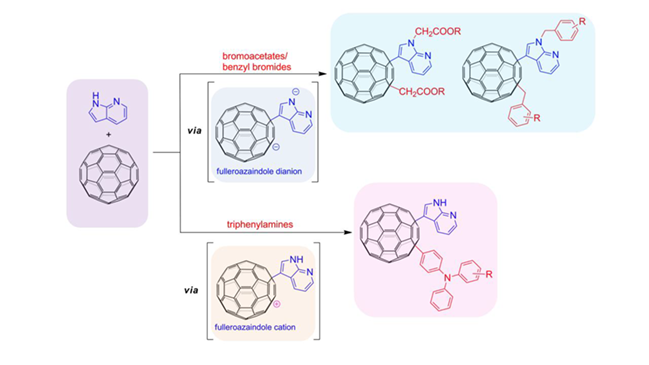
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300292-sup-0001-supinfo.pdfPDF document, 14.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Prokopov, A. A.; L. N. Yakhontov. Azaindole derivatives. Chem. Heterocycl. Compd. 1977, 13, 1224–1227;
10.1007/BF00475952 Google Scholar(b) Fisher, M. H.; Schwartzkopf Jr., G.; Hoff, D. R.; Azaindole anthelmintic agents. J. Med. Chem. 1972, 15, 1168–1171; (c) Smirnov, A. V.; English, D. S.; Rich, R. L.; Lane, J.; Teyton, L.; Schwabacher, A. W.; Luo, S.; Thornburg, R. W.; Petrich, J. W. Photophysics and Biological Applications of 7-Azaindole and Its Analogs. J. Phys. Chem. B 1997, 101, 2758–2769; (d) Zhao, H.-B.; Wang, S. Luminescence and reactivity of 7-azaindole derivatives and complexes. Chem. Soc. Rev. 2010, 39, 3142–3156.
- 2For selected reviews, see: (a) Kannaboina, P.; Mondal, K.; Laha, J. K.; Das, P. Recent advances in the global ring functionalization of 7-azaindoles. Chem. Commun. 2020, 56, 11749–11762; (b) Motati, D. R.; Amaradhi, R.; Ganesh, T. Recent developments in the synthesis of azaindoles from pyridine and pyrrole building blocks. Org. Chem. Front. 2021, 8, 466–513.
- 3For selected reviews, see: (a) Prato, M. [60]Fullerene chemistry for materials science applications. J. Mater. Chem. 1997, 7, 1097–1109; (b) Guldi, D. M.; Illescas, B. M.; Atienza, C. M.; Wielopolski, M.; Martín, N. Fullerene for organic electronics. Chem. Soc. Rev. 2009, 38, 1587–1597; (c) Zieleniewska, A.; Lodermeyer, F.; Roth, A.; Guldi, D. M. Fullerenes – how 25 years of charge transfer chemistry have shaped our understanding of (interfacial) interactions. Chem. Soc. Rev. 2018, 47, 702–714; (d) Megiatto, J. D. Jr.; Guldi, D. M.; Schuster, D. I. Design, synthesis and photoinduced processes in molecular interlocked photosynthetic [60]fullerene systems. Chem. Soc. Rev. 2020, 49, 8–20.
- 4For selected reviews, see: (a) Thompson, B. C.; Frechet, J. M. J. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77; (b) Li, C.-Z.; Yip, H.-L.; Jen, A. K.-Y. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177; (c) Huang, S.; Zhang, G.; Knutson, N. S.; Fontana, M. T.; Huber, R. C.; Ferreira, A. S.; Tolbert, S. H.; Schwartz, B. J.; Rubin, Y. Beyond PCBM: Methoxylated 1,4-bisbenzyl[60]fullerene adducts for efficient organic solar cells. J. Mater. Chem. A 2016, 4, 416–424; (d) Castro, E.; Murillo, J.; Fernandez-Delgado, O.; Echegoyen, L. Progress in fullerene-based hybrid perovskite solar cells. J. Mater. Chem. C 2018, 6, 2635–2651; (e) Yan, X.-X.; Niu, C.; Yin, Z.-C.; Lu, W.-Q.; Wang, G.-W. Anionic alkene-azide cycloaddition (AAAC) strategy toward electrosynthesis of multifunctionalized [60]fullerene derivatives and further applications, Sci. Bull. 2022, 67, 2406–2410; (f) Lu, W.-Q.; Zhou, D.-B.; Yin, Z.-C.; Liu, Q.-S.; Wang, G.-W. A copper-promoted synthesis of epoxy-bridged [60]fullerene-fused lactones and further derivatization, Chem. Commun. 2021, 57, 7043–7046; (g) Lu, W.-Q.; Yin, Z.-C.; Liu, Q.-S.; Wang, G.-W. Copper-Promoted Cascade Radical Reaction of [60]Fullerene with Arylglyoxals and Further Derivatization. Asian J. Org. Chem. 2022, 11, e202200045.
- 5For selected reviews and examples, see:(a) Nakamura, E.; Isobe, H.; Tomita, N.; Sawamura, M.; Jinno, S.; Okayama, H. Functionalized fullerene as an artificial vector for transfection. Angew. Chem. Int. Ed. 2000, 39, 4254–4257;
10.1002/1521-3773(20001201)39:23<4254::AID-ANIE4254>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar(b) Castro, E.; Garcia, A. H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535; (c) Nierengarten, J.-F. Fullerene hexa-adduct scaffolding for the construction of giant molecules. Chem. Commun. 2017, 53, 11855–11868; (d) Ramos-Soriano, J.; ReinaBeatriz, J. J.; Illescas, M.; Cruz, N. d. l.; Rodríguez-Pérez, L.; Lasala, F.; Rojo, J.; Delgado, R.; Martín, N. Synthesis of highly efficient multivalent disaccharide/[60]fullerene nanoballs for emergent viruses. J. Am. Chem. Soc. 2019, 141, 15403–15412.
- 6For selected reviews, see: (a) Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions, Wiley-VCH, Weinheim, 2005;
10.1002/3527603492 Google Scholar(b) Nakamura, E.; Isobe, H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc. Chem. Res. 2003, 36, 807–815; (c) Giacalone, F.; Martín, N. Fullerene polymers: Synthesis and properties. Chem. Rev. 2006, 106, 5136–5190; (d) Balch, A. L.; Winkler, K. Two-component polymeric materials of fullerenes and the transition metal complexes: A bridge between metal-organic frameworks and conducting polymers. Chem. Rev. 2016, 116, 3812–3882; (e) Zhou, Z.; Han, H.; Chen, Z.; Gao, R.; Liu, Z.; Su, J.; Xin, N.; Yang, X.; Gan, L. Concise synthesis of open-cage fullerenes for oxygen delivery. Angew. Chem. Int. Ed. 2019, 58, 17690–17694; (f) Wang, G.-W. Fullerene Mechanochemistry: Serendipitous Discovery of Dumb-Bell-Shaped C120 and Beyond. Chin. J. Chem. 2021, 39, 1797–1803.
- 7For selected representative examples, see: (a) Sawamura, M.; Iikura, H.; Nakamura, E. The first pentahaptofullerene metal complexes. J. Am. Chem. Soc. 1996, 118, 12850–12851; (b) Gan, L.; Huang, S.; Zhang, X.; Zhang, A.; Chen, B.; Chen, H.; Li, X.; Shang, G. Fullerenes as a tertbutylperoxy radical trap, metal catalyzed reaction of tert-butyl hydroperoxide with fullerenes, and formation of the first fullerene mixed peroxides C60(O)(OOtBu)4 and C70(OOtBu)10. J. Am. Chem. Soc. 2002, 124, 13384–13385; (c) Iwashita, A.; Matsuo, Y.; Nakamura, E. AlCl3-mediated mono-, di-, and trihydroarylation of [60]fullerene. Angew. Chem. Int. Ed. 2007, 46, 3513–3516; (d) Nambo, M.; Noyori, R.; Itami, K. Rh-catalyzed arylation and alkenylation of C60 using organoboron compounds. J. Am. Chem. Soc. 2007, 129, 8080–8081; (e) Matsuo, Y.; Iwashita, A.; Abe, Y.; Li, C.-Z.; Matsuo, K.; Hashiguchi, M.; Nakamura, E. Regioselective synthesis of 1,4-di(organo)[60]fullerenes through DMF-assisted monoaddition of silylmethyl grignard reagents and subsequent alkylation reaction. J. Am. Chem. Soc. 2008, 130, 15429–15436; (f) Tzirakis, M. D.; Orfanopoulos, M. Acyl radical reactions in fullerene chemistry: Direct acylation of [60]fullerene through an efficient decatungstat-photomediated approach. J. Am. Chem. Soc. 2009, 131, 4063–4069; (g) Lu, S.; Jin, T.; Bao, M.; Yamamoto, Y. Cobalt-catalyzed hydroalkylation of [60]fullerene with active alkyl bromides: Selective synthesis of monoalkylated fullerenes. J. Am. Chem. Soc. 2011, 133, 12842–12848; (h) Lu, S.; Jin, T.; Bao, M.; Yamamoto, Y. NaOH-catalyzed dimerization of monofunctionalized hydrofullerenes: Transition-metal-free, general, and efficient synthesis of single-bonded [60]fullerene dimers. Org. Lett. 2012, 14, 3466–3469; (i) Lu, S.; Si, W.; Bao, M.; Yamamoto, Y.; Jin, T. Co-catalyzed radical cycloaddition of [60]fullerene with active dibromides: Selective synthesis of carbocycle–fused fullerene monoadducts. Org. Lett. 2013, 15, 4030–4033.
- 8for selected Pd-catalyzed examples, see: (a) Mori, S.; Nambo, M.; Chi, L.-C.; Bouffard, J.; Itami, K. A bench-stable Pd catalyst for the hydroarylation of fullerene with boronic acids. Org. Lett. 2008, 10, 4609–4612; (b) Nambo, M.; Wakamiya, A.; Yamaguchi, S.; Itami, K. Regioselective unsymmetrical tetraallylation of C60 through palladium catalysis. J. Am. Chem. Soc. 2009, 131, 15112–15113; (c) Nambo, M.; Itami, K. Palladium-catalyzed carbon-carbon bond formation and cleavage of organo(hydro)fullerenes. Chem. - Eur. J. 2009, 15, 4760–4764; (d) Zhu, B.; Wang, G.-W. Palladium-catalyzed heteroannulation of [60]fullerene with anilides via C–H bond activation. Org. Lett. 2009, 11, 4334–4337; (e) Nambo, M.; Wakamiya, A.; Itami, K. Palladium- catalyzed tetraallylation of C60 with allyl chloride and allylstannane: mechanism, regioselectivity, and enantioselectivity. Chem. Sci. 2012, 3, 3474–3481; (f) Li, F.; Liu, T.-X.; Wang, G.-W. Synthesis of [60]fullerene–fused sultones via sulfonic acid group–directed C–H bond activation. Org. Lett. 2012, 14, 2176–2179; (g) Hashikawa, Y.; Murata, M.; Wakamiya, A.; Murata, Y. Regioselectivity and structure of arene-fused C60 derivatives. J. Am. Chem. Soc. 2017, 139, 16350–16358; (h) Liu, Q.; Liu, T.-X.; Ma, J.; Zhang, G. Palladium-catalyzed three-component tandem coupling-carboannulation reaction leading to polysubstituted [60]fullerene-fused cyclopentanes. Org. Lett. 2020, 22, 284–289; (i) Su, Y.-T.; Yin, Z.-C.; Wang, G.-W. Palladium-catalyzed three-component 1,4-aminoarylation of [60]fullerene with aryl iodides and N-methoxysulfonamides, and further transformations. Org. Chem. Front. 2022, 9, 2739–2745.
- 9for selected Cu-mediated examples, see: (a) Nakamura, E.; Tahara, K.; Matsuo, Y.; Sawamura, M. Synthesis, structure, and aromaticity of a hoop-shaped cyclic benzenoid [10]cyclophenacene. J. Am. Chem. Soc. 2003, 125, 2834–2835; (b) Filippone, S.; Maroto, E. E.; Martín- Domenech, Á.; Suarez, M.; Martín, N. An efficient approach to chiral fullerene derivatives by catalytic enantioselective 1,3-dipolar cycloadditions. Nat. Chem. 2009, 1, 578–582; (c) Xiao, Z.; Matsuo, Y.; Nakamura, E. Copper-catalyzed formal [4+2] annulation between alkyne and fullerene bromide. J. Am. Chem. Soc. 2010, 132, 12234–12236; (d) Nambo, M.; Segawa, Y.; Itami, K. Aziridinofullerene: A versatile platform for functionalized fullerenes. J. Am. Chem. Soc. 2011, 133, 2402–2405; (e) Lu, S.; Jin, T.; Kwon, E.; Bao, M.; Yamamoto, Y. Highly efficient Cu(OAc)2-catalyzed dimerization of monofunctionalized hydrofullerenes leading to single-bonded [60]fullerene dimers. Angew. Chem. Int. Ed. 2012, 51, 802–806; (f) Liu, Z.; Yin, Z.-C.; Lu, W.-Q.; Niu, C.; Chen, M.; Yang, S.; Wang, G.-W. Cu(I)-catalyzed synthesis of [60] fullerene-fused lactams and further electrochemical functionalization. Org. Lett. 2021, 23, 4051–4056.
- 10(a) Li, F.; Elhussin, I. E. H.; Li, S.; Zhou, H.; Wu, J.; Tian, Y. KOtBu-Mediated coupling of indoles and [60]fullerene: transitionmetal-free and general synthesis of 1,2-(3-indole)(hydro)[60]fullerenes. J. Org. Chem. 2015, 80, 10605–10610; (b) Li, F.; Wang, L.; Wang, J.; Peng, D.; Zhao, Y.; Li, S.; Zhou, H.; Wu, J.; Tian, X.; Tian, Y. KOtBu-mediated, three-component coupling reaction of indoles, [60]fullerene, and haloalkanes: One-pot, transition-metal–free synthesis of various 1,4-(3-indole)(organo)[60]fullerenes. Org. Lett. 2017, 19, 1192–1195; (c) Chen, X.-R.; Li, Y.-M.; Li, X.; Xuan, J.; Zhou, H.-P.; Tian, Y.-P.; Li, F. An “umpolung relay” strategy: one-pot, twice polarity inversion cascade synthesis of diversified[60]fulleroindoles. Org. Lett. 2021, 23, 1302–1308; (d) Chen, X.-R.; Zhang, Q.-W.; Tao, G.-G.; Xuan, J.; Zhou, H.-P.; Tian, Y.-P.; Li, F. One-pot, three-component regioselective coupling reaction of triphenylamine/carbazole derivatives with [60]fullerene and indoles via an “umpolung relay” strategy. Org. Chem. Front. 2021, 8, 5994–5999.
- 11(a) Li, F.; Xun, J.; Zhang, S.; Liu, B.; Yang, J.; Liu, K.; Liu, D.; Zhang, Q.; Zhou, H.; Wu, J.; Tian, Y. KOtBu-promoted C4 selective coupling reaction of phenols and [60]fullerene: one-pot synthesis of 4-[60]fullerephenols under transition–metal–free conditions. J. Org. Chem. 2018, 83, 5431–5437; (b) Li, F.; Shang, Y.; Niu, C.; Li, C.; Huang, X.; Xu, G.; Xuan, J.; Zhou, H.; Yang, S. Potassium salt promoted regioselective three–component coupling synthesis of 1,4–asymmetrical [60]fullerene bisadducts with superior electron transport properties. Chem. Commun. 2020, 56, 9513–9516; (c) Chen, X.-R.; Zhang, J.-X.; Zhu, S.-K.; Li, Y.-W.; Yang, R.; Xuan, J., Li, F. Transition-Metal-Free Domino Reaction of [60]Fullerene, Indole, and DMSO/HCl: One-Pot Access to Diverse N-Substituted [60]Fulleroindole Derivatives. J. Org. Chem. 2022, 87, 7945–7954; (d) Zhang, J.-X.; Liu, M.-W.; Wang, W.-Y.; Jia, R.-L.; Yan, M.-Q.; Xuan, J.; Li, F. KOtBu–Promoted, Three-Component Domino Reaction of Arenes(indoles/phenols), C60, and (Per/poly)fluoroarenes: Achieving Direct C−C Cross–Coupling of Fullerene with (Per/poly)fluoroarenes. J. Org. Chem. 2023, 88, 116–131; (e) Zhang, D.-K.; Ma, W.-B.; Wei, S.-Y.; Chen, D.-Y.; Hu, X.; Xuan, J.; Li, F. Synthesis of diverse unsymmetric 1,4-adducts via a three–component coupling reaction of malonate derivatives, [60]fullerene and electrophiles/nucleophiles. Org. Chem. Front. 2023, 10, 1626–1632.
15 November 2023
Pages 2975-2980




