Enantioconvergent Synthesis of α-Fluoroalkyl Alcohols Enabled by Photocatalytic Radical Brook Rearrangement
Yunhong Niu
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Both authors contributed equally to this work.
Search for more papers by this authorChenyu Jin
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Both authors contributed equally to this work.
Search for more papers by this authorXiaoqian He
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Search for more papers by this authorShenna Deng
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Search for more papers by this authorGang Zhou
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Search for more papers by this authorDr. Shanshan Liu
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao Shen
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
State Key Laboratory of Metabolism and Regulation in Complex Organisms, College of Life Sciences, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Shenzhen Research Institute of Wuhan University, Shenzhen, 518057 China
E-mail: [email protected]
Search for more papers by this authorYunhong Niu
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Both authors contributed equally to this work.
Search for more papers by this authorChenyu Jin
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Both authors contributed equally to this work.
Search for more papers by this authorXiaoqian He
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Search for more papers by this authorShenna Deng
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Search for more papers by this authorGang Zhou
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Search for more papers by this authorDr. Shanshan Liu
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiao Shen
The Institute for Advanced Studies, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
State Key Laboratory of Metabolism and Regulation in Complex Organisms, College of Life Sciences, Wuhan University, 299 Bayi Road, Wuhan, Hubei, 430072 China
Shenzhen Research Institute of Wuhan University, Shenzhen, 518057 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
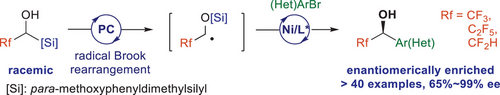
The first application of radical Brook rearrangement in asymmetric cross-coupling reaction has been developed, enabling enantioconvergent synthesis of chiral α-aryl-α-fluoroalkyl alcohols. The reaction proceeded through the combination of photocatalytic generation of masked ketyl radicals and Ni-catalyzed asymmetric cross-coupling with aryl/heteroaryl bromides. The the presence of para-methoxyphenyldimethylsilyl group lowers the oxidation potentials of the racemic alcohol substrates, enabling a radical relay process to form carbon-centered radicals from initial generation of the aryl cation radicals. The reaction features mild conditions, broad substrate scope, and excellent enantioselectivity. The formal synthesis of bioactive Odanacatib and LX-1031 and various down-stream transformations demonstrated the synthetic potential of the reaction.
Abstract
While radical Brook rearrangement has emerged as a powerful strategy in modern organic synthesis, enantioselective cross coupling involving radical Brook rearrangement remains unexplored. Herein, we report a photocatalytic radical Brook rearrangement followed by cross-coupling with aryl/heteroaryl bromides, enabling the enantioconvergent construction of chiral α-fluoroalkyl alcohols. Key to this transformation is a radical relay process involving sequential generation of aryl cation radicals, alkoxy radicals, and carbon-centered radicals through Brook rearrangement. The reaction exhibits exceptional scope (>40 examples), outstanding enantiocontrol (up to 99% ee), and broad functional group tolerance. The synthetic utility is demonstrated through formal syntheses of bioactive Odanacatib and LX-1031, along with diverse downstream derivatizations.
Conflict of Interests
A patent based on this work has been applied, X.S., S.L., and Y.N. may benefit from the royalty payment.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202507789-sup-0001-SupMat.pdf18.5 MB | Supporting Information |
| anie202507789-sup-0002-SupMat.zip100.2 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. G. Brook, Acc. Chem. Res. 1974, 7, 77–84.
- 2P. C. B. Page, S. S. Klair, S. Rosenthal, Chem. Soc. Rev. 1990, 19, 147–195.
- 3I. Fleming, A. Barbero, D. Walter, Chem. Rev. 1997, 97, 2063–2192.
- 4W. H. Moser, Tetrahedron 2001, 57, 2065–2084.
- 5A. B. Smith, III, C. M. Adams, Acc. Chem. Res. 2004, 37, 365–377.
- 6H.-J. Zhang, D. L. Priebbenow, C. Bolm, Chem. Soc. Rev. 2013, 42, 8540–8571.
- 7G. Eppe, D. Didier, I. Marek, Chem. Rev. 2015, 115, 9175–9206.
- 8N. Lee, C.-H. Tan, D. Leow, Asian J. Org. Chem. 2019, 8, 25–31.
- 9Y. Deng, A. B. Smith, III, Acc. Chem. Res. 2020, 53, 988–1000.
- 10M. Agbaria, N. Egbaria, Z. Nairoukh, Synthesis 2024, 56, 2483–2498.
- 11F. Yang, J. Wang, Y. Dong, N. Zhang, C. Zhang, Tetrahedron 2024, 168, 134351.
- 12M. D. Paredes, R. Alonso, J. Org. Chem. 2000, 65, 2292–2304.
- 13Y. Zhang, J.-J. Chen, H.-M. Huang, Angew. Chem. Int. Ed. 2022, 61, e202205671; Angew. Chem. 2022, 134, e202205671.
- 14Y. Zhang, G. Zhou, S. Liu, X. Shen, Chem. Soc. Rev. 2025, 54, 1870–1904.
- 15C. Le, T. Q. Chen, T. Liang, P. Zhang, D. W. C. MacMillan, Science 2019, 141, 20031–20036.
- 16G. H. Lovett, S. Chen, X.-S. Xue, K. N. Houk, D. W. C. MacMillan, J. Am. Chem. Soc. 2019, 141, 20031–20036.
- 17Y. Deng, Q. Liu, A. B. Smith, III, J. Am. Chem. Soc. 2017, 139, 9487–9490.
- 18Y. Zhang, Y. Zhang, X. Shen, Chem. Catal. 2021, 1, 423–436.
- 19Y. Zhang, Y. Zhang, Y. Guo, S. Liu, X. Shen, Chem. Catal. 2022, 2, 1380–1393.
- 20Z. Li, Y. Zhang, Y. Zhang, X. He, X. Shen, Angew. Chem. Int. Ed. 2023, 62, e202303218; Angew. Chem. 2023, 135, e202303218.
- 21Y. Zhang, Y. Zhang, C. Ye, X. Qi, L.-Z. Wu, X. Shen, Nat. Commun. 2022, 13, 6111.
- 22T. Qin, C. Xu, G. Zhang, Q. Zhang, Org. Chem. Front. 2023, 10, 1981–1987.
- 23X. Ouyang, B. Shi, Y. Zhao, Z. Zhu, Z. Li, Y. Yang, C. Shu, Chem. Sci. 2024, 15, 11092–11098.
- 24P. Zhou, L. Ding, Y. Liu, H. Song, Q. Wang, Org. Lett. 2024, 26, 7094–7099.
- 25R. Laskar, S. Dutta, J. C. Spies, P. Mukherjee, Á. Rentería-Gómez, R. E. Thielemann, C. G. Daniliuc, O. Gutierrez, F. Glorius, J. Am. Chem. Soc. 2024, 146, 10899–10907.
- 26P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications 2013, Wiley–VCH. Weinheim.
10.1002/9783527651351 Google Scholar
- 27T. Liang, C. N. Neumann, T. Ritter, Angew. Chem. Int. Ed. 2013, 125, 8214–8264; Angew. Chem. 2013, 125, 8372–8423.
- 28J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. Del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432–2506.
- 29C. Ni, M. Hu, J. Hu, Chem. Rev. 2015, 115, 765–825.
- 30E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, N. A. Meanwell, J. Med. Chem. 2015, 58, 8315–8359.
- 31J. B. I. Sap, C. F. Meyer, N. J. W. Straathof, N. Iwumene, C. W. am Ende, A. A. Trabanco, V. Gouverneur, Chem. Soc. Rev. 2021, 50, 8214–8247.
- 32J. Y. Gauthier, N. Chauret, W. Cromlish, S. Desmarais, L. T. Duong, J.-P. Falgueyret, D. B. Kimmel, S. Lamontagne, S. Léger, T. LeRiche, C. S. Li, F. Massé, D. J. McKay, D. A. Nicoll-Griffith, R. M. Oballa, J. T. Palmer, M. D. Percival, D. Riendeau, J. Robichaud, G. A. Rodan, S. B. Rodan, C. Seto, M. Thérien, V.-L. Truong, M. C. Venuti, G. Wesolowski, R. N. Young, R. Zamboni, W. C. Black, Bioorg. Med. Chem. Lett. 2008, 18, 923–928.
- 33M. M. Zhao, H. Zhang, S. Iimura, M. S. Bednarz, R. C. Kanamarlapudi, J. Yan, N.-K. Lim, W. Wu, Org. Process Res. Dev. 2020, 24, 261–273.
- 34H. L. Gelhorn, M. H. Kulke, T. O'Dorisio, Q. M. Yang, J. Jackson, S. Jackson, K. A. Boehm, L. Law, J. Kostelec, P. Auguste, P. Lapuerta, Clin. Ther. 2016, 38, 759–768.
- 35J. Wouters, F. Moureau, G. Evrard, J.-J. Koenig, S. Jegham, P. George, F. Durant, Bioorg. Med. Chem. 1999, 7, 1683–1693.
- 36For reviews see G. K. S. Prakash, A. K. Yudin, Chem. Rev. 1997, 97, 757–786.
- 37R. P. Singh, J. N. M. Shreeve, Tetrahedron 2000, 56, 7613–7632.
- 38G. K. S. Prakash, M. Mandal, J. Fluorine Chem. 2001, 112, 123–131.
- 39J.-A. Ma, D. Cahard, J. Fluorine Chem. 2007, 128, 975–996.
- 40N. Shibata, S. Mizuta, H. Kawai, Tetrahedron: Asymmetry 2008, 19, 2633–2644.
- 41A. D. Dilman, V. V. Levin, Eur. J. Org. Chem. 2011, 2011, 831–841.
- 42For selected examples see G. K. S. Prakash, P. V. Jog, P. T. D. Batamack, G. A. Olah, Science 2012, 338, 1324–1327.
- 43G. K. Surya Prakash, Z. Zhang, F. Wang, S. Munoz, G. A. Olah, J. Org. Chem. 2013, 78, 3300–3305.
- 44J. B. Geri, M. M. W. Wolfe, N. K. Szymczak, Angew. Chem. Int. Ed. 2018, 130, 1381–1385; Angew. Chem. 2018, 130, 1395–1399.
- 45H. Jia, A. P. Häring, F. Berger, L. Zhang, T. Ritter, J. Am. Chem. Soc. 2021, 143, 7623–7628.
- 46Y. Gong, K. Kato, H. Kimoto, Synlett 1999, 1999, 1403–1404.
- 47G.-W. Zhang, L. Wang, J. Nie, J.-A. Ma, Adv. Synth. Catal. 2008, 350, 1457–1463.
- 48T. Yamazaki, T. Terajima, T. Kawasaki-Taskasuka, Tetrahedron 2008, 64, 2419–2424.
- 49J. Zhang, Y.-J. Chen, L. Zhang, Synth. Commun. 2011, 41, 3045–3052.
- 50D. A. Borkin, S. M. Landge, A. Török, Chirality 2011, 23, 612–616.
- 51K. Funabiki, A. Hayakawa, R. Kani, T. Inuzuka, Y. Kubota, Eur. J. Org. Chem. 2019, 2019, 5978–5984.
- 52R. Kani, T. Inuzuka, Y. Kubota, K. Funabiki, Eur. J. Org. Chem. 2020, 2020, 4487–4493.
- 53K. D. Nguyen, B. Y. Park, T. Luong, H. Sato, V. J. Garza, M. J. Krische, Science 2016, 354, aah5133.
- 54J. L. Jeffrey, J. A. Terrett, D. W. C. MacMillan, Science 2015, 349, 1532–1536.
- 55Á. Péter, S. Agasti, O. Knowles, E. Pye, D. J. Procter, Chem. Soc. Rev. 2021, 50, 5349–5365.
- 56Q. Xia, J. Dong, H. Song, Q. Wang, Chem. - Eur. J. 2019, 25, 2949–2961.
- 57S.-R. Guo, P. S. Kumar, M. Yang, Adv. Synth. Catal. 2017, 359, 2–25.
- 58S.-Y. Zhang, F.-M. Zhang, Y.-Q. Tu, Chem. Soc. Rev. 2011, 40, 1937–1949.
- 59H.-M. Huang, P. Bellotti, S. Kim, X. Zhang, F. Glorius, Nat. Synth. 2022, 1, 464–474.
- 60L. Niu, J. Liu, X.-A. Liang, S. Wang, A. Lei, Nat. Commun. 2019, 10, 467–473.
- 61J. Twilton, M. Christensen, D. A. DiRocco, R. T. Ruck, I. W. Davies, D. W. C. MacMillan, Angew. Chem. Int. Ed. 2018, 130, 5369–5373; Angew. Chem. 2018, 130, 5467–5471.
- 62H. Li, J. Tong, Y. Zhu, C. Jiang, P. Liu, P. Sun, Green Chem. 2022, 24, 8406–8411.
- 63H.-M. Huang, P. Bellotti, J. E. Erchinger, T. O. Paulisch, F. Glorius, J. Am. Chem. Soc. 2022, 144, 1899–1909.
- 64F. Chen, X.-H. Xu, L. Chu, F.-L. Qing, Org. Lett. 2022, 24, 9332–9336.
- 65N. Holmberg-Douglas, D. A. Nicewicz, Chem. Rev. 2022, 122, 1925–2016.
- 66D. L. Golden, S.-E. Suh, S. S. Stahl, Nat. Rev. Chem. 2022, 6, 405–427.
- 67W.-C. C. Lee, D.-S. Wang, Y. Zhu, X. P. Zhang, Nat. Chem. 2023, 15, 1569–1580.
- 68Z.-L. Yu, Y.-F. Cheng, J.-R. Liu, W. Yang, D.-T. Xu, Y. Tian, J.-Q. Bian, Z.-L. Li, L.-W. Fan, C. Luan, A. Gao, Q.-S. Gu, X.-Y. Liu, J. Am. Chem. Soc. 2023, 145, 6535–6545.
- 69Y. Gao, B. Zhang, L. Levy, H.-J. Zhang, C. He, P. S. Baran, J. Am. Chem. Soc. 2022, 144, 10992–11002.
- 70G.-Q. Xu, W. D. Wang, P.-F. Xu, J. Am. Chem. Soc. 2024, 146, 1209–1223.
- 71A. C. Colgan, R. S. J. Proctor, D. C. Gibson, P. Chuentragool, A. S. K. Lahdenperä, K. Ermanis, R. J. Phipps, Angew. Chem. Int. Ed. 2022, 61, e202200266; Angew. Chem. 2022, 134, e202200266.
- 72Y.-B. Li, D.-D. Hu, W.-R. Ren, H. Liu, Y.-L. Wang, K. Li, W.-C. Ke, R.-X. Jin, X.-S. Wang, Angew. Chem. Int. Ed. 2025, 64, e202424324; Angew. Chem. 2025, 137, e202424324.
- 73L. Lombardi, A. Cerveri, R. Giovanelli, M. C. Reis, C. Silva López, G. Bertuzzi, M. Bandini, Angew. Chem. Int. Ed. 2022, 61, e202211732; Angew. Chem. 2022, 134, e202211732.
- 74X. Chen, X. Gong, Z. Li, G. Zhou, Z. Zhu, W. Zhang, S. Liu, X. Shen, Nat. Commun. 2020, 11, 2756–2764.
- 75S. Qian, T. M. Lazarus, D. A. Nicewicz, J. Am. Chem. Soc. 2023, 145, 18247–18252.
- 76N. A. Romero, K. A. Margrey, N. E. Tay, D. A. Nicewicz, Science 2015, 349, 1326–1330.
- 77N. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075–10166.
- 78H. G. Yayla, H. Wang, K. T. Tarantino, H. S. Orbe, R. R. Knowles, J. Am. Chem. Soc. 2016, 138, 10794–10797.
- 79E. C. Gentry, R. R. Knowles, Acc. Chem. Res. 2016, 49, 1546–1556.
- 80F. Strieth-Kalthoff, F. Sandfort, M. Kuhnemund, F. R. Schafer, H. Kuchen, F. Glorius, Angew. Chem. Int. Ed. 2022, 61, e202204647; Angew. Chem. 2022, 134, e202204647.
- 81F. Schafer, L. Luckemeier, F. Glorius, Chem. Sci. 2024, 15, 14548–14555.
- 82L. Pitzer, F. Schafers, F. Glorius, Angew. Chem. Int. Ed. 2019, 131, 8572–8576; Angew. Chem. 2019, 131, 8660–8664.
- 83 These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 84For representative Ni-catalyzed cross coupling reactions see X. Dai, N. A. Strotman, G. C. Fu, J. Am. Chem. Soc. 2008, 130, 3302–3303.
- 85Z.-P. Yang, G. C. Fu, J. Am. Chem. Soc. 2020, 142, 5870–5875.
- 86Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle, D. W. C. MacMillan, Science 2014, 345, 437–440.
- 87J. C. Tellis, D. N. Primer, G. A. Molander, Science 2014, 345, 433–436.
- 88X. Shu, L. Huan, Q. Huang, H. Huo, J. Am. Chem. Soc. 2020, 142, 19058–19064.
- 89F.-D. Lu, J. Chen, X. Jiang, J.-R. Chen, L.-Q. Lu, W.-J. Xiao, Chem. Soc. Rev. 2021, 50, 12808–12827.
- 90D. Ameen, T. J. Snape, Med. Chem. Commun. 2013, 4, 893-907.