Palladium-Catalyzed Alkyl Amination of Olefins via Radical-Polar Crossover at Room Temperature
Dr. Anurag Singh
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorDr. Kuntal Pal
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorDr. Sayan Dutta
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorDr. Arnab Dey
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Dr. Rajesh Kancherla
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDr. Bholanath Maity
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Prof. Dr. Luigi Cavallo
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Magnus Rueping
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDr. Anurag Singh
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorDr. Kuntal Pal
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorDr. Sayan Dutta
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorDr. Arnab Dey
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Dr. Rajesh Kancherla
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDr. Bholanath Maity
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
Search for more papers by this authorCorresponding Author
Prof. Dr. Luigi Cavallo
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Magnus Rueping
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900 Saudi Arabia
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
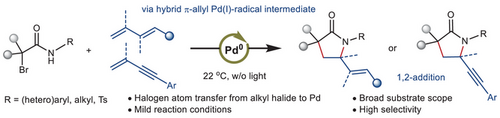
We disclosed a palladium-catalyzed tandem radical Heck/allylic amination of α-bromoamides with 1,3-butadienes and enynes at room temperature to yield γ-lactams. Unlike previous alkyl Heck reactions, this transformation generates hybrid alkyl Pd(I)-radical and a transposed π-allyl Pd(I)-radical under ambient conditions.
Abstract
In contrast to traditional ground-state palladium-catalyzed alkyl Heck reactions, which are thermodynamically unfavorable and endothermic, excited-state palladium catalysis facilitates single-electron mechanisms, with light primarily driving the formation of alkyl radicals from triplet-state Pd(0). Here, we report a novel and mechanistically distinct Pd-catalyzed reaction, where the key hybrid alkyl Pd(I)-radical intermediate is generated by the halogen atom transfer (XAT) from alkyl bromide to the Pd(0) at room temperature, without the need for photoinitiation. This hybrid species engages in the addition to dienes and conjugated enynes, producing a transposed open-shell allyl Pd(I)-radical, which undergoes radical-polar crossover (RPC) to yield the desired products. Density functional theory (DFT) studies offer insights into the reaction mechanism, confirming the involvement of hybrid alkyl/allyl Pd(I) radical species as key intermediates.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202503446-sup-0001-SuppMat.pdf11.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. E. Knappke, A. J. von Wangelin, Chem. Rev. 2011, 40, 4948–4962.
- 2C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot, V. Snieckus, Angew. Chem. Int. Ed. 2012, 51, 5062–5085.
- 3P. Ruiz-Castillo, S. L. Buchwald, Chem. Rev. 2016, 116, 12564–12649.
- 4A. C. Frisch, M. Beller, Angew. Chem. Int. Ed. 2005, 44, 674–688.
- 5N. Kambe, T. Iwasaki, J. Terao, Chem. Soc. Rev. 2011, 40, 4937–4947.
- 6S. Bräse, A. de Meijere, In Metal-Catalyzed Cross-Coupling Reactions (Eds: F. Diederich, P. J. Stang), Wiley-VCH, Weinheim, Ch 3.
- 7Z. Feng, Q. Q. Min, Y. L. Xiao, B. Zhang, X. Zhang, Angew. Chem. Int. Ed. 2014, 53, 1669–1673.
- 8Z. Feng, Q. Q. Min, H. Y. Zhao, J. W. Gu, X. Zhang, Angew. Chem. Int. Ed. 2015, 54, 1270–1274.
- 9Y. Yu, U. K. Tambar, Chem. Sci. 2015, 6, 2777–2781.
- 10H. Y. Zhao, Z. Feng, Z. Luo, X. Zhang, Angew. Chem. Int. Ed. 2016, 55, 10401–10405.
- 11D. Kurandina, P. Chuentragool, V. Gevorgyan, Synthesis 2019, 51, 985–1005.
- 12P. Chuentragool, D. Kurandina, V. Gevorgyan, Angew. Chem. Int. Ed. 2019, 58, 11586–11598.
- 13R. Kancherla, K. Muralirajan, A. Sagadevan, M. Rueping, Trends Chem. 2019, 1, 510–523.
- 14K. P. S. Cheung, S. Sarkar, V. Gevorgyan, Chem. Rev. 2022, 122, 1543–1625.
- 15S. Sarkar, K. P. S. Cheung, V. Gevorgyan, Angew. Chem. Int. Ed. 2024, 63, e202311972.
- 16P.-C. Cui, G.-W. Wang, Org. Lett. 2024, 26, 427–432.
- 17G.-Z. Wang, R. Shang, W.-M. Cheng, Y. Fu, J. Am. Chem. Soc. 2017, 139, 18307–18312.
- 18P. Chuentragool, M. Parasram, Y. Shi, V. Gevorgyan, J. Am. Chem. Soc. 2018, 140, 2465–2468.
- 19R. Kancherla, K. Muralirajan, B. Maity, C. Zhu, P. E. Krach, L. Cavallo, M. Rueping, Angew. Chem. Int. Ed. 2019, 58, 3412–3416.
- 20W.-J. Zhou, G.-M. Cao, G. Shen, X.-Y. Zhu, Y.-Y. Gui, J.-H. Ye, L. Sun, L.-L. Liao, J. Li, D.-G. Yu, Angew. Chem. Int. Ed. 2017, 56, 15683–15687.
- 21Y.-C. Luo, F.-F. Tong, Y. Zhang, C.-Y. He, X. Zhang, J. Am. Chem. Soc. 2021, 143, 13971–13979.
- 22Y. Liu, C. Zhou, M. Jiang, B. A. Arndtsen, J. Am. Chem. Soc. 2022, 144, 9413–9420.
- 23S. Sarkar, S. Ghosh, D. Kurandina, Y. Noffel, V. Gevorgyan, J. Am. Chem. Soc. 2023, 145, 12224–12232.
- 24H. Fang, C. Empel, I. Atodiresei, R. M. Koenigs, ACS Catal. 2023, 13, 6445–6451.
- 25M. Parasram, P. Chuentragool, D. Sarkar, V. Gevorgyan, J. Am. Chem. Soc. 2016, 138, 6340–6343.
- 26M. Parasram, P. Chuentragool, Y. Wang, Y. Shi, V. Gevorgyan, J. Am. Chem. Soc. 2017, 139, 14857–14860.
- 27W.-M. Cheng, R. Shang, Y. Fu, Nat. Commun. 2018, 9, 5215.
- 28K. Yamada, K. P. S. Cheung, V. Gevorgyan, J. Am. Chem. Soc. 2024, 146, 18218–18223.
- 29K. Mukherjee, K. P. S. Cheung, V. Gevorgyan, Angew. Chem. Int. Ed. 2025, 64, e202413646.
- 30K. Pak Shing Cheung, J. Fang, K. Mukherjee, A. Mihranyan, V. Gevorgyan, Science 2022, 378, 1207–1213.
- 31H.-M. Huang, M. Koy, E. Serrano, P. M. Pflüger, J. L. Schwarz, F. J. N. C. Glorius, Nat. Catal. 2020, 3, 393–400.
- 32K. P. S. Cheung, D. Kurandina, T. Yata, V. Gevorgyan, J. Am. Chem. Soc. 2020, 142, 9932–9937.
- 33H.-M. Huang, P. Bellotti, P. M. Pflüger, J. L. Schwarz, B. Heidrich, F. Glorius, J. Am. Chem. Soc. 2020, 142, 10173–10183.
- 34Y. Cai, G. Gaurav, T. Ritter, Angew. Chem. Int. Ed. 2024, 63, e202311250.
- 35X.-Y. Ruan, D.-X. Wu, W.-A. Li, Z. Lin, M. Sayed, Z.-Y. Han, L.-Z. Gong, J. Am. Chem. Soc. 2024, 146, 12053–12062.
- 36X. Zhan, Z. Nie, N. Li, A. Zhou, H. Lv, M. Liang, K. Wu, G. J. Cheng, Q. Yin, Angew. Chem. Int. Ed. 2024, 63, e202404388.
- 37Y. Liang, T. Bian, K. Yadav, Q. Zhou, L. Zhou, R. Sun, Z. Zhang, ACS Cent. Sci. 2024, 10, 1191–1200.
- 38H. Yu, Q. Zhang, W. Zi, Angew. Chem. Int. Ed. 2022, 61, e202208411.
- 39X. Wu, L.-Z. Gong, Synthesis 2019, 51, 122–134.
- 40Z. Zhang, V. Gevorgyan, Angew. Chem. Int. Ed. 2023, 62, e202311848.
- 41G. Manolikakes, P. Knochel, Angew. Chem. Int. Ed. 2009, 48, 205–209.
- 42M. R. Kwiatkowski, E. J. Alexanian, Acc. Chem. Res. 2019, 52, 1134–1144.
- 43M. Besora, F. Maseras, Dalton Trans. 2019, 48, 16242–16248.
- 44J. Rio, H. Liang, M.-E. L. Perrin, L. A. Perego, L. Grimaud, P.-A. Payard, ACS Catal. 2023, 13, 11399–11421.
- 45M. Besora, C. Gourlaouen, B. Yates, F. Maseras, Dalton Trans. 2011, 40, 11089–11094.
- 46M. E. Greaves, E. L. J. Humphrey, D. J. Nelson, Catal. Sci. Technol. 2021, 11, 2980–2996.
- 47K. Muralirajan, R. Kancherla, A. Gimnkhan, M. Rueping, Org. Lett. 2021, 23, 6905–6910.
- 48R. Kancherla, K. Muralirajan, M. Rueping, Chem. Sci. 2022, 13, 8583–8589.
- 49K. Muralirajan, R. Kancherla, B. Maity, S. Karuthedath, F. Laquai, L. Cavallo, M. J. N. C. Rueping, Nat. Commun. 2023, 14, 6622.
- 50V. K. Velisoju, E. V. Ramos-Fernández, R. Kancherla, R. Ahmad, K. Pal, H. Mohamed, J. L. Cerrillo, M. J. Meijerink, L. Cavallo, M. Rueping, P. Castaño, Angew. Chem. Int. Ed. 2024, 63, e202409490.
- 51R. Kancherla, K. Muralirajan, S. Dutta, K. Pal, B. Li, B. Maity, L. Cavallo, M. Rueping, Angew. Chem. Int. Ed. 2024, 63, e202314508.
- 52S. M. Treacy, D. R. Vaz, S. Noman, C. Tard, T. Rovis, Chem. Sci. 2023, 14, 1569–1574.
- 53W. Huang, W. Chen, G. Wang, J. Li, X. Cheng, G. Li, ACS Catal. 2016, 6, 7471–7474.
- 54M. Zhang, W. Li, Y. Duan, P. Xu, S. Zhang, C. Zhu, Org. Lett. 2016, 18, 3266–3269.
- 55Y. Yamane, K. Yoshinaga, M. Sumimoto, T. Nishikata, ACS Catal. 2019, 9, 1757–1762.
- 56Y. Yamane, K. Miyazaki, T. Nishikata, ACS Catal. 2016, 6, 7418–7425.
- 57J.-H. Fan, J. Yang, R.-J. Song, J.-H. Li, Org. Lett. 2015, 17, 836–839.
- 58C. Liu, S. Tang, D. Liu, J. Yuan, L. Zheng, L. Meng, A. Lei, Angew. Chem. Int. Ed. 2012, 51, 3638–3641.
- 59Y. Lv, W. Pu, Q. Wang, Q. Chen, J. Niu, Q. Zhang, Adv. Synth. Catal. 2017, 359, 3114–3119.
- 60Z.-L. Liu, Z.-P. Ye, Y.-X. Chen, Y. Zheng, Z.-Z. Xie, J.-P. Guan, J.-A. Xiao, K. Chen, H.-Y. Xiang, H. Yang, Org. Lett. 2022, 24, 924–928.
- 61M. Ratushnyy, N. Kvasovs, S. Sarkar, V. Gevorgyan, Angew. Chem. Int. Ed. 2020, 59, 10316–10320.
- 62L.-W. Ye, C. Shu, F. Gagosz, Org. Biomol. Chem. 2014, 12, 1833–1845.
- 63J. Caruano, G. G. Muccioli, R. Robiette, Org. Biomol. Chem. 2016, 14, 10134–10156.
- 64S. Liu, Z. Zhuang, J. X. Qiao, K.-S. Yeung, S. Su, E. C. Cherney, Z. Ruan, W. R. Ewing, M. A. Poss, J.-Q. Yu, J. Am. Chem. Soc. 2021, 143, 21657–21666.