Asymmetric Ring Opening/Cyclization/Retro-Mannich Reaction of Cyclopropyl Ketones with Aryl 1,2-Diamines for the Synthesis of Benzimidazole Derivatives
Yong Xia
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorDr. Lili Lin
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorFenzhen Chang
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorYuting Liao
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaohua Liu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaoming Feng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, China
Search for more papers by this authorYong Xia
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorDr. Lili Lin
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorFenzhen Chang
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorYuting Liao
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaohua Liu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaoming Feng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, China
Search for more papers by this authorGraphical Abstract
Abstract
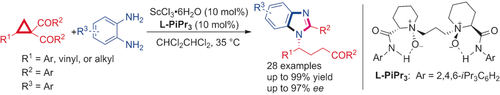
A highly efficient asymmetric ring-opening/cyclization/retro-Mannich reaction of cyclopropyl ketones with aryl 1,2-diamines has been realized using a chiral N,N′-dioxide/ScIII catalyst. Benzimidazoles containing chiral side chains were generated under mild reaction conditions in excellent outcomes (up to 99 % yield and 97 % ee). This method also provides efficient access to chiral benzimidazole-substituted amide and cycloheptene derivatives.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201604735-sup-0001-misc_information.pdf9.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Bansal, O. Silakari, Bioorg. Med. Chem. 2012, 20, 6208;
- 1bG. Yadav, S. Ganguly, Eur. J. Med. Chem. 2015, 97, 419;
- 1cR. S. Keri, A. Hiremathad, S. Budagumpi, B. M. Nagaraja, Chem. Biol. Drug Des. 2015, 86, 19.
- 2
- 2aL. C. R. Carvalho, E. Fernandes, M. M. B. Marques, Chem. Eur. J. 2011, 17, 12544;
- 2bZ. Cheng, Q. Zhang, X. Xu, X. Li, Chin. J. Org. Chem. 2015, 35, 1189, and references therein.
- 3
- 3aZ. Wu, C. Yang, Y. Xiong, Z. Feng, M. Lombardo, A. Verras, R. M. Chabin, et al., Bioorg. Med. Chem. Lett. 2012, 22, 1774;
- 3bY. Qian, K. Conde-Knape, S. D. Erickson, F. Falcioni, P. Gillespie, I. Hakimi, F. Mennona, Y. Ren, H. Salari, S.-S. So, J. W. Tilley, Bioorg. Med. Chem. Lett. 2013, 23, 4216;
- 3cM. Malesevic, D. Gutknecht, E. Prell, C. Klein, M. Schumann, R. A. Nowak, J. C. Simon, C. Schiene-Fischer, A. Saalbach, J. Med. Chem. 2013, 56, 7302.
- 4L. M. Stanley, J. F. Hartwig, J. Am. Chem. Soc. 2009, 131, 8971.
- 5Q. Yang, M. Xie, C. Xia, H. Sun, D. Zhang, K. Huang, Z. Guo, G. Qu, H. Guo, Chem. Commun. 2014, 50, 14809.
- 6Y.-Y. Wang, K. Kanomata, T. Korenaga, M. Terada, Angew. Chem. Int. Ed. 2016, 55, 927; Angew. Chem. 2016, 128, 939.
- 7
- 7aH. U. Reissig, R. Zimmer, Chem. Rev. 2003, 103, 1151;
- 7bT. P. Lebold, M. A. Kerr, Pure Appl. Chem. 2010, 82, 1797;
- 7cD. Zhang, H. Song, Y. Qin, Acc. Chem. Res. 2011, 44, 447;
- 7dM. A. Cavitt, L. H. Phun, S. France, Chem. Soc. Rev. 2014, 43, 804;
- 7eT. F. Schneider, J. Kaschel, D. B. Werz, Angew. Chem. Int. Ed. 2014, 53, 5504; Angew. Chem. 2014, 126, 5608;
- 7fF. de Nanteuil, F. De Simone, R. Frei, F. Benfatti, E. Serrano, J. Waser, Chem. Commun. 2014, 50, 10912;
- 7gH. K. Grover, M. R. Emmett, M. A. Kerr, Org. Biomol. Chem. 2015, 13, 655.
- 8
- 8aR. P. Wurz, A. B. Charette, Org. Lett. 2005, 7, 2313;
- 8bO. Lifchits, A. B. Charette, Org. Lett. 2008, 10, 2809;
- 8cM. R. Emmett, M. A. Kerr, Org. Lett. 2011, 13, 4180;
- 8dY.-Y. Zhou, L.-J. Wang, J. Li, X.-L. Sun, Y. Tang, J. Am. Chem. Soc. 2012, 134, 9066;
- 8eS. S. So, T. J. Auvil, V. J. Garza, A. E. Mattson, Org. Lett. 2012, 14, 444;
- 8fH. K. Grover, M. R. Emmett, M. A. Kerr, Org. Lett. 2013, 15, 4838;
- 8gS. M. Wales, M. M. Walker, J. S. Johnson, Org. Lett. 2013, 15, 2558;
- 8hM. C. Martin, D. V. Patil, S. France, J. Org. Chem. 2014, 79, 3030;
- 8iH. Nambu, M. Fukumoto, W. Hirota, T. Yakura, Org. Lett. 2014, 16, 4012;
- 8jL. K. B. Garve, P. Barkawitz, P. G. Jones, D. B. Werz, Org. Lett. 2014, 16, 5804;
- 8kY. Xia, X. H. Liu, H. F. Zheng, L. L. Lin, X. M. Feng, Angew. Chem. Int. Ed. 2015, 54, 227; Angew. Chem. 2015, 127, 229;
- 8lY. Xia, L. L. Lin, F. Z. Chang, X. Fu, X. H. Liu, X. M. Feng, Angew. Chem. Int. Ed. 2015, 54, 13748; Angew. Chem. 2015, 127, 13952;
- 8mQ.-K. Kang, L. Wang, Q.-J. Liu, J.-F. Li, Y. Tang, J. Am. Chem. Soc. 2015, 137, 14594;
- 8nL.-W. Feng, P. Wang, L.-J. Wang, Y. Tang, Sci. Bull. 2015, 60, 210;
- 8oV. Ortega, A. G. Csákÿ, J. Org. Chem. 2016, 81, 3917;
- 8pR. Karmakar, A. Suneja, V. K. Singh, Org. Lett. 2016, 18, 2636.
- 9
- 9aK. S. Halskov, F. Kniep, V. H. Lauridsen, E. H. Iversen, B. S. Donslund, K. A. Jørgensen, J. Am. Chem. Soc. 2015, 137, 1685;
- 9bE. Sanchez-Diez, D. L. Vesga, E. Reyes, U. Uria, L. Carrillo, J. L. Vicario, Org. Lett. 2016, 18, 1270.
- 10
- 10aS. K. Jackson, A. Karadeolian, A. B. Driega, M. A. Kerr, J. Am. Chem. Soc. 2008, 130, 4196;
- 10bS. Xing, W. Pan, C. Liu, J. Ren, Z. Wang, Angew. Chem. Int. Ed. 2010, 49, 3215; Angew. Chem. 2010, 122, 3283;
- 10cA. T. Parsons, A. G. Smith, A. J. Neel, J. S. Johnson, J. Am. Chem. Soc. 2010, 132, 9688;
- 10dF. de Nanteuil, J. Waser, Angew. Chem. Int. Ed. 2011, 50, 12075; Angew. Chem. 2011, 123, 12281;
- 10eH. Xu, J.-P. Qu, S. Liao, H. Xiong, Y. Tang, Angew. Chem. Int. Ed. 2013, 52, 4004; Angew. Chem. 2013, 125, 4096;
- 10fH. Xiong, H. Xu, S. Liao, Z. Xie, Y. Tang, J. Am. Chem. Soc. 2013, 135, 7851;
- 10gF. de Nanteuil, E. Serrano, D. Perrotta, J. Waser, J. Am. Chem. Soc. 2014, 136, 6239;
- 10hJ. Zhu, Y. Liang, L. Wang, Z.-B. Zheng, K. N. Houk, Y. Tang, J. Am. Chem. Soc. 2014, 136, 6900;
- 10iS. Chakrabarty, I. Chatterjee, B. Wibbeling, C. G. Daniliuc, A. Studer, Angew. Chem. Int. Ed. 2014, 53, 5964; Angew. Chem. 2014, 126, 6074;
- 10jA. R. Rivero, I. Fernández, C. R. de Arellano, M. A. Sierra, J. Org. Chem. 2015, 80, 1207;
- 10kJ. Sabbatani, N. Maulide, Angew. Chem. Int. Ed. 2016, 55, 6780; Angew. Chem. 2016, 128, 6892.
- 11
- 11aI. S. Young, M. A. Kerr, Angew. Chem. Int. Ed. 2003, 42, 3023; Angew. Chem. 2003, 115, 3131;
- 11bM. P. Sibi, Z. Ma, C. P. Jasperse, J. Am. Chem. Soc. 2005, 127, 5764;
- 11cY.-B. Kang, X.-L. Sun, Y. Tang, Angew. Chem. Int. Ed. 2007, 46, 3918; Angew. Chem. 2007, 119, 3992;
- 11dW. J. Humenny, P. Kyriacou, K. Sapeta, A. Karadeolian, M. A. Kerr, Angew. Chem. Int. Ed. 2012, 51, 11088; Angew. Chem. 2012, 124, 11250;
- 11eY.-Y. Zhou, J. Li, L. Ling, S.-H. Liao, X.-L. Sun, Y.-X. Li, L.-J. Wang, Y. Tang, Angew. Chem. Int. Ed. 2013, 52, 1452; Angew. Chem. 2013, 125, 1492;
- 11fH.-H. Zhang, Y.-C. Luo, H.-P. Wang, W. Chen, P.-F. Xu, Org. Lett. 2014, 16, 4896;
- 11gR. Talukdar, D. P. Tiwari, A. Saha, M. K. Ghorai, Org. Lett. 2014, 16, 3954;
- 11hQ.-Q. Cheng, Y. Qian, P. Y. Zavalij, M. P. Doyle, Org. Lett. 2015, 17, 3568;
- 11iQ.-J. Liu, W.-G. Yan, L. Wang, X. P. Zhang, Y. Tang, Org. Lett. 2015, 17, 4014;
- 11jH. Liu, C. Yuan, Y. Wu, Y. Xiao, H. Guo, Org. Lett. 2015, 17, 4220;
- 11kC. M. Braun, E. A. Congdon, K. A. Nolin, J. Org. Chem. 2015, 80, 1979;
- 11lL. K. B. Garve, M. Petzold, P. G. Jones, D. B. Werz, Org. Lett. 2016, 18, 564.
- 12
- 12aO. A. Ivanova, E. M. Budynina, Y. K. Grishin, I. V. Trushkov, P. V. Verteletskii, Angew. Chem. Int. Ed. 2008, 47, 1107; Angew. Chem. 2008, 120, 1123;
- 12bH. Xu, J.-L. Hu, L. Wang, S. Liao, Y. Tang, J. Am. Chem. Soc. 2015, 137, 8006.
- 13R. Tejero, A. Ponce, J. Adrio, J. C. Carretero, Chem. Commun. 2013, 49, 10406.
- 14
- 14aX. H. Liu, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2011, 44, 574;
- 14bK. Shen, X. H. Liu, L. L. Lin, X. M. Feng, Chem. Sci. 2012, 3, 327;
- 14cX. H. Liu, L. L. Lin, X. M. Feng, Org. Chem. Front. 2014, 1, 298;
- 14dM. S. Xie, X. X. Wu, G. Wang, L. L. Lin, X. M. Feng, Acta Chim. Sin. 2014, 72, 856;
- 14eX. Xiao, L. L. Lin, X. J. Lian, X. H. Liu, X. M. Feng, Org. Chem. Front. 2016, 3, 809.
- 15
- 15aY.-S. Lee, Y.-H. Cho, S. Lee, J.-K. Bin, J. Yang, G. Chae, C.-H. Cheon, Tetrahedron 2015, 71, 532;
- 15bK. M. H. Nguyen, M. Largeron, Eur. J. Org. Chem. 2016, 1025.
- 16
- 16aJ. D. White, D. C. Ihle, Org. Lett. 2006, 8, 1081;
- 16bS. Roy, M. P. Davydova, R. Pal, K. Gilmore, G. A. Tolstikov, S. F. Vasilevsky, I. V. Alabugin, J. Org. Chem. 2011, 76, 7482;
- 16cM. S. Mayo, X. Yu, X. Zhou, X. Feng, Y. Yamamoto, M. Bao, Org. Lett. 2014, 16, 764;
- 16dC.-X. Zhuo, Y. Zhou, Q. Cheng, L. Huang, S.-L. You, Angew. Chem. Int. Ed. 2015, 54, 14146; Angew. Chem. 2015, 127, 14352;
- 16eL. Huang, L.-X. Dai, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5793.
- 17CCDC 1414840 (3 ea) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 18For more details, see the Supporting Information.