Activation of Dinitrogen-Derived Hafnium Nitrides for Nucleophilic NC Bond Formation with a Terminal Isocyanate†
Scott P. Semproni
Department of Chemistry, Princeton University, Princeton, New Jersey, 08544 (USA)
Search for more papers by this authorCorresponding Author
Prof. Paul J. Chirik
Department of Chemistry, Princeton University, Princeton, New Jersey, 08544 (USA)
Department of Chemistry, Princeton University, Princeton, New Jersey, 08544 (USA)===Search for more papers by this authorScott P. Semproni
Department of Chemistry, Princeton University, Princeton, New Jersey, 08544 (USA)
Search for more papers by this authorCorresponding Author
Prof. Paul J. Chirik
Department of Chemistry, Princeton University, Princeton, New Jersey, 08544 (USA)
Department of Chemistry, Princeton University, Princeton, New Jersey, 08544 (USA)===Search for more papers by this authorWe thank the Director of the Office of Basic Energy Science Chemical Sciences Division, U.S. Department of Energy (DE-FG-02-05ER15659) for financial support. S.P.S. thanks the Natural Sciences and Engineering Research Council of Canada for a predoctoral fellowship.
Graphical Abstract
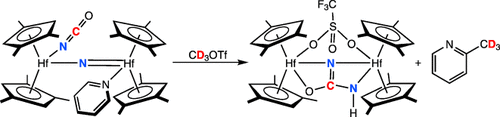
Better by Hf: Anion coordination to a bridging hafnocene nitride complex, prepared from CO-induced N2 cleavage, increases the nucleophilicity of the nitrogen atom, thus promoting additional NC bond formation with a typically inert terminal isocyanate ligand. This cascade sequence allows synthesis of otherwise challenging mono-substituted ureas using N2, CO, and an appropriate electrophile.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201307097_sm_miscellaneous_information.pdf1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. D. Roughley, A. M. Jordan, J. Med. Chem. 2011, 54, 3451.
- 2P. Sobota, Z. Janas, J. Organomet. Chem. 1984, 276, 171.
- 3M. D. Fryzuk, Acc. Chem. Res. 2009, 42, 127.
- 4D. J. Knobloch, E. Lobkovsky, P. J. Chirik, Nat. Chem. 2010, 2, 30.
- 5D. J. Knobloch, E. Lobkovsky, P. J. Chirik, J. Am. Chem. Soc. 2010, 132, 10553.
- 6D. J. Knobloch, S. P. Semproni, E. Lobkovsky, P. J. Chirik, J. Am. Chem. Soc. 2012, 134, 3377.
- 7
- 7aC. E. Laplaza, C. C. Cummins, Science 1995, 268, 861;
- 7bC. E. Laplaza, M. J. A. Johnson, J. C. Peters, A. L. Odom, E. Kim, C. C. Cummins, G. N. George, I. J. Pickering, J. Am. Chem. Soc. 1996, 118, 8623;
- 7cJ. S. Figueroa, N. A. Piro, C. R. Clough, C. C. Cummins, J. Am. Chem. Soc. 2006, 128, 940;
- 7dT. J. Hebden, R. R. Schrock, M. K. Takase, P. Müller, Chem. Commun. 2012, 48, 1851.
- 8aG. K. B. Clentsmith, V. M. E. Bates, P. B. Hitchcock, F. G. N. Cloke, J. Am. Chem. Soc. 1999, 121, 10444;
- 8bY. C. Tsai, M. J. A. Johnson, D. J. Mindiola, C. C. Cummins, W. T. Klooster, T. F. Koetzle, J. Am. Chem. Soc. 1999, 121, 10426;
- 8cA. Caselli, E. Solari, R. Scopelliti, C. Floriani, N. Re, C. Rizzoli, A. Chiese-Villa, J. Am. Chem. Soc. 2000, 122, 3652;
- 8dE. Solari, C. DaSilva, B. Iacono, J. Hesschenbrouck, C. Rizzoli, R. Scopelliti, C. Floriani, Angew. Chem. 2001, 113, 4025;
Angew. Chem. Int. Ed. 2001, 40, 3907;
10.1002/1521-3773(20011015)40:20<3907::AID-ANIE3907>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 8eM. Hirotsu, P. P. Fontaine, A. Epshteyn, P. Y. Zavalij, L. R. Sita, J. Am. Chem. Soc. 2007, 129, 9284;
- 8fA. J. Keane, P. Y. Zavalij, L. R. Sita, J. Am. Chem. Soc. 2013, 135, 9580.
- 9
- 9aH. Henderickx, G. Kwakkenbos, A. Peters, J. van der Spoel, K. de Vries, Chem. Commun. 2003, 2050;
- 9bJ. J. Curley, E. L. Sceats, C. C. Cummins, J. Am. Chem. Soc. 2006, 128, 14036.
- 10G. B. Nikiforov, I. Vidyarante, S. Gambarotta, I. Korobkov, Angew. Chem. 2009, 121, 7551;
10.1002/ange.200903648 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7415.
- 11aF. Akagi, T. Matsuo, H. Kawaguchi, Angew. Chem. 2007, 119, 8934;
10.1002/ange.200703336 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8778;
- 11bF. Akagi, S. Suzuki, Y. Ishida, T. Hatanaka, T. Matsuo, H. Kawaguchi, Eur. J. Inorg. Chem. 2013, 3930.
- 12
- 12aM. M. Rodriguez, E. Bill, W. W. Brennessel, P. L. Holland, Science 2011, 334, 780;
- 12bT. Shima, S. Hu, G. Luo, X. Kang, Y. Luo, Z. Hou, Science 2013, 340, 1549.
- 13
- 13aM. J. A. Johnson, P. M. Lee, A. L. Odom, W. M. Davis, C. C. Cummins, Angew. Chem. 1997, 109, 110;
10.1002/ange.19971090133 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 87;
- 13bJ. C. Peters, A. R. Johnson, A. L. Odom, P. W. Wanandi, W. M. Davis, C. C. Cummins, J. Am. Chem. Soc. 1996, 118, 10175;
- 13cC. J. Carmalt, J. D. Mileham, A. J. P. White, D. J. Williams, New J. Chem. 2000, 24, 929.
- 14S. P. Semproni, P. J. Chirik, J. Am. Chem. Soc. 2013, 135, 11373.
- 15S. P. Semproni, C. Milsmann, P. J. Chirik, Angew. Chem. 2012, 124, 5303;
10.1002/ange.201201361 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5213.
- 16S. P. Semproni, G. W. Margulieux, P. J. Chirik, Organometallics 2012, 31, 6278.
- 17P. Braunstein, D. Nobel, Chem. Rev. 1989, 89, 1927.
- 18L. Maresca, G. Natile, A.-M. Manotti-Lanfredi, A. Tiripicchio, J. Am. Chem. Soc. 1982, 104, 7661.
- 19M. Hvastijová, J. Kohout, J. W. Buchler, R. Boča, J. Kožíšek, L. Jäger, Coord. Chem. Rev. 1998, 175, 17.
- 20Q. Xiao, L. Ling, F. Ye, R. Tan, L. Tian, Y. Zhang, Y. Li, J. Wang, J. Org. Chem. 2013, 78, 3879.
- 21
- 21aW. Chen, K. Li, Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 311;
- 21bE. V. Vinogradova, B. P. Fors, S. L. Buchwald, J. Am. Chem. Soc. 2012, 134, 11132.
- 22CCDC 949474 (2), 949475 (4), and 949476 (6) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.