Silver-Catalyzed Isocyanide-Alkyne Cycloaddition: A General and Practical Method to Oligosubstituted Pyrroles†
Jianquan Liu
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
Search for more papers by this authorZhongxue Fang
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
Search for more papers by this authorCorresponding Author
Prof. Qian Zhang
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)Search for more papers by this authorProf. Qun Liu
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
Search for more papers by this authorCorresponding Author
Prof. Xihe Bi
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
State Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China)
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)Search for more papers by this authorJianquan Liu
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
Search for more papers by this authorZhongxue Fang
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
Search for more papers by this authorCorresponding Author
Prof. Qian Zhang
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)Search for more papers by this authorProf. Qun Liu
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
Search for more papers by this authorCorresponding Author
Prof. Xihe Bi
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)
State Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin 300071 (China)
Department of Chemistry, Northeast Normal University, Changchun 130024 (China)Search for more papers by this authorThis work was supported by NSFC (21172029, 21202016, 31101010) and FRFCU (11CXPY005).
Graphical Abstract
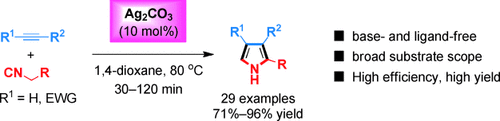
Ag2CO3 is the key: The transition-metal-catalyzed cycloaddition of isocyanides and unactivated terminal alkynes has been realized with Ag2CO3 as a unique and robust catalyst (see scheme). The protocol is highly efficient, allowing a broad range of terminal and internal alkynes to react under base- and ligand-free conditions, generating synthetically useful oligosubstituted pyrroles in high yields.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302024_sm_miscellaneous_information.pdf6.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a general recent review on the reactivity of pyrrole heterocycles, see: B. A. Trofimov, N. A. Nedolya in Comprehensive Heterocyclic Chemistry III, Vol. 3 (Eds.: ), Elsevier, Amsterdam, 2008, p. 45.
- 2For reviews regarding the prevalence and biological importance of pyrrole heterocycles, see:
- 2aC. T. Walsh, S. Garneau-Tsodikova, A. R. Howard-Jones, Nat. Prod. Rep. 2006, 23, 517–531;
- 2bF. Bellina, R. Rossi, Tetrahedron 2006, 62, 7213–7256;
- 2cH. Fan, J. Peng, M. T. Hamann, J.-F. Hu, Chem. Rev. 2008, 108, 264–287.
- 3
- 3aN. T. Patil, Y. Yamamoto, Arkivoc 2007, 10, 121–141;
10.3998/ark.5550190.0008.a11 Google Scholar
- 3bN. T. Patil, Y. Yamamoto, Chem. Rev. 2008, 108, 3395–3442.
- 4 Name Reactions in Heterocyclic Chemistry (Ed.: ), Wiley, Hoboken, 2005.
- 5For examples of intramolecular cyclizations, see:
- 5aB. M. Trost, J.-P. Lumb, J. M. Azzarelli, J. Am. Chem. Soc. 2011, 133, 740–743;
- 5bM. Yamagishi, K. Nishigai, T. Hata, H. Urabe, Org. Lett. 2011, 13, 4873–4875;
- 5cH. M. Peng, J. Zhao, X. Li, Adv. Synth. Catal. 2009, 351, 1371–1377;
- 5dA. Aponick, C.-Y. Li, J. Malinge, E. F. Marques, Org. Lett. 2009, 11, 4624–4627;
- 5eP. W. Davies, N. Martin, Org. Lett. 2009, 11, 2293–2296;
- 5fL. Ackermann, R. Sandmann, L. T. Kaspar, Org. Lett. 2009, 11, 2031–2034;
- 5gJ. T. Kim, A. V. Kel’in, V. Gevorgyan, Angew. Chem. 2003, 115, 102–105; Angew. Chem. Int. Ed. 2003, 42, 98–101;
- 5hA. V. Kel’in, A. W. Sromek, V. Gevorgyan, J. Am. Chem. Soc. 2001, 123, 2074–2075.
- 6For examples of intermolecular cyclizations, see:
- 6aS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585–9587;
- 6bA. Mizuno, H. Kusama, N. Iwasawa, Angew. Chem. 2009, 121, 8468–8470;
10.1002/ange.200904402 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 8318–8320;
- 6cE. Merkul, C. Boersch, W. Frank, T. J. J. Müller, Org. Lett. 2009, 11, 2269–2272;
- 6dY.-F. Wang, K. K. Toh, S. Chiba, K. Narasaka, Org. Lett. 2008, 10, 5019–5022;
- 6eT. J. Harrison, J. A. Kozak, M. Corbella-Pané, G. R. Dake, J. Org. Chem. 2006, 71, 4525–4529.
- 7
- 7aS. Michlik, R. Kempe, Nat. Chem. 2013, 5, 140–144;
- 7bD. Srimani, Y. Ben-David, D. Milstein, Angew. Chem. 2013, 125, 4104–4107;
10.1002/ange.201300574 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 4012–4015.
- 8A. V. Gulevich, A. G. Zhdanko, R. V. A. Orru, V. G. Nenajdenko, Chem. Rev. 2010, 110, 5235–5331.
- 9 Acetylene Chemistry (Eds.: ), Wiley-VCH, Weinheim, 2005.
- 10
- 10aH. Saikachi, T. Kitagawa, H. Sasaki, Chem. Pharm. Bull. 1979, 27, 2857–2861;
- 10bS. Kamijo, C. Kanazawa, Y. Yamamoto, J. Am. Chem. Soc. 2005, 127, 9260–9266;
- 10cO. V. Larionov, A. de Meijere, Angew. Chem. 2005, 117, 5809–5813;
10.1002/ange.200502140 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5664–5667;
- 10dQ. Cai, F. Zhou, T. Xu, L. Fu, K. Ding, Org. Lett. 2011, 13, 340–343;
- 10eM. Adib, B. Mohammadi, E. Sheikhi, H. R. Bijanzadeh, Chin. Chem. Lett. 2011, 22, 314–317.
- 11Only one report involving the tedious in situ preparation of copper acetylides is known to date, see: A. V. Lygin, O. V. Larionov, V. S. Korotkov, A. de Meijere, Chem. Eur. J. 2009, 15, 227–236.
- 12A. S. Hay, J. Org. Chem. 1962, 27, 3320–3321.
- 13
- 13aC. Kanazawa, S. Kamijo, Y. Yamamoto, J. Am. Chem. Soc. 2006, 128, 10662–10663;
- 13bR. Grigg, M. I. Lansdell, M. Thornton-Pett, Tetrahedron 1999, 55, 2025–2044.
- 14 Silver in Organic Chemistry (Eds.: ), Wiley, Hoboken, 2010.
- 15
- 15aC. He, S. Guo, J. Ke, J. Hao, H. Xu, H. Chen, A. Lei, J. Am. Chem. Soc. 2012, 134, 5766–5769;
- 15bC. He, J. Hao, H. Xu, Y. Mo, H. Liu, J. Han, A. Lei, Chem. Commun. 2012, 48, 11073–11075.
- 16
- 16aT. Hayashi, Y. Uozumi, A. Yamazaki, M. Sawamura, H. Hamashima, Y. Ito, Tetrahedron Lett. 1991, 32, 2799–2802.
- 17
- 17aZ. Wang, X. Bi, P. Liao, X. Liu, D. Dong, Chem. Commun. 2013, 49, 1309–1311;
- 17bZ. Wang, X. Bi, P. Liao, R. Zhang, Y. Liang, D. Dong, Chem. Commun. 2012, 48, 7076–7078;
- 17cT. Xiong, Y. Li, X. Bi, Y. Lv, Q. Zhang, Angew. Chem. 2011, 123, 7278–7281; Angew. Chem. Int. Ed. 2011, 50, 7140–7143;
- 17dY. Wang, X. Bi, D. Li, P. Liao, Y. Wang, J. Yang, Q. Zhang, Q. Liu, Chem. Commun. 2011, 47, 809–811;
- 17eK. Wang, X. Bi, S. Xing, P. Liao, Z. Fang, X. Meng, Q. Zhang, Q. Liu, Y. Ji, Green Chem. 2011, 13, 562–565.
- 18Although the exact role of the counter anion of silver salts remains unclear presently, the alkalinity of Ag2CO3 may account for the observed unique catalytic activity. For related references, see:
- 18aS. Mitsuda, T. Fujiwara, K. Kimigafukuro, D. Monguchi, A. Mori, Tetrahedron 2012, 68, 3585–3590;
- 18bM. S. Shmidt, I. A. Perillo, M. González, M. M. Blanco, Tetrahedron Lett. 2012, 53, 2514–2517.
- 19For the biological importance of 2,3-disubstituted pyrroles, see for example:
- 19aW. W. Wilkerson, W. Galbraith, K. Gans-Brangs, M. Grubb, W. E. Hewes, B. Jaffee, J. P. Kenney, J. Kerr, N. Wong, J. Med. Chem. 1994, 37, 988–998;
- 19bW. W. Wilkerson, R. A. Copeland, M. Covington, J. M. Trzaskos, J. Med. Chem. 1995, 38, 3895–3901.
- 20N. Ono, T. Okujima in Isocyanide Chemistry: Applications in Synthesis and Material Science First Edition (Ed.: ), Wiley-VCH, Weinheim, 2012, p. 385.
10.1002/9783527652532.ch11 Google Scholar
- 21For the synthesis of 3-formylpyrroles, see: A. R. Kelly, M. H. Kerrigan, P. J. Walsh, J. Am. Chem. Soc. 2008, 130, 4097–4104.
- 22J. R. Garabatos-Perera, B. H. Rotstein, A. Thompson, J. Org. Chem. 2007, 72, 7382–7385.
- 23A. Vitérisi, A. Orsini, J.-M. Weibel, P. Pale, Tetrahedron Lett. 2006, 47, 2779–2781.
- 24For a review on carboxylate-assisted transition-metal-catalyzed CH bond functionalizations, see: L. Ackermann, Chem. Rev. 2011, 111, 1315–1345.
- 25E. Barnea, T. Andrea, M. Kapon, J.-C. Berthet, M. Ephritikhine, M. S. Eisen, J. Am. Chem. Soc. 2004, 126, 10860–10861.
- 26S. Ito, K. Wakamatsu, K. Glass, J. D. Simon, Anal. Biochem. 2013, 434, 221–225.
- 27This Communication is published back-to-back with the following study: M. Gao, C. He, H. Chen, R. Bai, B. Cheng, A. Lei, Angew. Chem. 7096–7099; Angew. Chem. Int. Ed. 6958–6961.