Easy Access to L-Mannosides and L-Galactosides by Using CH Activation of the Corresponding 6-Deoxysugars†
Tobias Gylling Frihed
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)
Search for more papers by this authorMads Heuckendorff
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)
Search for more papers by this authorCorresponding Author
Dr. Christian Marcus Pedersen
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)Search for more papers by this authorCorresponding Author
Prof. Dr. Mikael Bols
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)Search for more papers by this authorTobias Gylling Frihed
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)
Search for more papers by this authorMads Heuckendorff
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)
Search for more papers by this authorCorresponding Author
Dr. Christian Marcus Pedersen
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)Search for more papers by this authorCorresponding Author
Prof. Dr. Mikael Bols
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen Ø (Denmark)Search for more papers by this authorThis work was supported by the Lundbeck Foundation.
Graphical Abstract
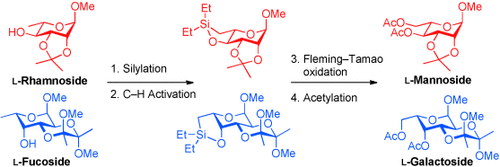
Rare sugars by CH activation: The title compounds have been prepared from the corresponding 6-deoxysugars by an intramolecular CH activation in high yields. Diethyl silane is attached to the 4-OH group followed by formation of a SiC bond; both transformations are catalyzed by iridium in a one-pot fashion. The subsequent Fleming–Tamao oxidation provided the L-sugars.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201206880_sm_miscellaneous_information.pdf4.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Gutiérrez, T. Capson, H. M. Guzmán, E. Quiñoá, R. Riguera, Tetrahedron Lett. 2004, 45, 7833–7836.
- 2
- 2aC.-Y. Tsai, X. Huang, C.-H. Wong, Tetrahedron Lett. 2000, 41, 9499–9503;
- 2bC. Filser, D. Kowalczyk, C. Jones, M. K. Wild, U. Ipe, D. Vestweber, H. Kunz, Angew. Chem. 2007, 119, 2155–2159;
10.1002/ange.200604442 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2108–2111.
- 3
- 3aP.-E. Jansson, B. Lindberg, G. Widmalm, P. A. Sandford, Carbohydr. Res. 1985, 139, 217–223;
- 3bP.-E. Jansson, N. S. Kumar, B. Lindberg, Carbohydr. Res. 1986, 156, 165–172;
- 3cT. A. Chowdhury, B. Lindberg, U. Lindquist, J. Baird, Carbohydr. Res. 1987, 161, 127–132.
- 4Most recent method: Y. Li, Z. Yin, B. Wang, X.-B. Meng, Z.-J. Li, Tetrahedron 2012, 68, 6981–6989.
- 5Recently reviewed in: D. A’Alonzo, A. Guaragna, G. Palumbo, Curr. Org. Chem. 2009, 13, 71–98.
- 6For a recent example see: R. S. Babu, Q. Chen, S. W. Kang, M. Zhou, G. A. O’Doherty, J. Am. Chem. Soc. 2012, 134, 11952–11955, and references therein.
- 7D. Crich, L. Li, J. Org. Chem. 2009, 74, 773–781.
- 8
- 8aJ. F. Hartwig, Acc. Chem. Res. 2012, 45, 864–873;
- 8bE. M. Simmons, J. F. Hartwig, J. Am. Chem. Soc. 2010, 132, 17092–17095;
- 8cT. Newhouse, P. S. Baran, Angew. Chem. 2011, 123, 3422–3435;
10.1002/ange.201006368 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3362–3374.
- 9E. M. Simmons, J. F. Hartwig, Nature 2012, 483, 70–73.
- 10B. G. David, R. J. Nash, A. A. Watson, C. Smith, G. W. J. Fleet, Tetrahedron 1999, 55, 4501–4520.
- 11
- 11aK. Tamao, N. Ishida, T. Tanaka, K. Kumada, Organometallics 1983, 2, 1694–1696;
- 11bI. Fleming, R. Henning, H. Plaut, J. Chem. Soc. Chem. Commun. 1984, 29–31;
- 11cI. Fleming, R. Henning, D. C. Parker, H. E. Plaut, P. E. J. Sanderson, J. Chem. Soc. Perkin Trans. 1 1995, 317–337;
- 11dreview: G. R. Jones, Tetrahedron 1996, 52, 7599–7662.
- 12Z. H. Peng, K. A. Woerpel, Org. Lett. 2002, 4, 2945–2948.
- 13J. H. Smitrovich, K. A. Woerpel, J. Org. Chem. 1996, 61, 6044–6046.
- 14
- 14aJ. C. López, A. M. Gómez, B. Fraser-Reid, J. Chem. Soc. Chem. Commun. 1993, 762–764;
- 14bK. Tamao, T. Yamauchi, Y. Ito, Chem. Lett. 1987, 171–174.
- 15J.-L. Montchamp, F. Tian, M. E. Hart, J. W. Frost, J. Org. Chem. 1996, 61, 3897–3899.
- 16S. V. Ley, D. R. Owen, K. E. Wesson, J. Chem. Soc. Perkin Trans. 1 1997, 2805–2806.
- 17U. Zehavi, N. Sharon, J. Org. Chem. 1972, 37, 2141–2145.