Enantioselective and Z/E-Selective Conjugate Addition of α-Substituted Cyanoacetates to Acetylenic Esters Catalyzed by Bifunctional Ruthenium and Iridium Complexes†
Yasuharu Hasegawa
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo 152-8552 (Japan), Fax: (+81) 3-5734-2637
Search for more papers by this authorProf. Dr. Ilya D. Gridnev
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo 152-8552 (Japan), Fax: (+81) 3-5734-2637
Search for more papers by this authorProf. Dr. Takao Ikariya
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo 152-8552 (Japan), Fax: (+81) 3-5734-2637
Search for more papers by this authorYasuharu Hasegawa
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo 152-8552 (Japan), Fax: (+81) 3-5734-2637
Search for more papers by this authorProf. Dr. Ilya D. Gridnev
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo 152-8552 (Japan), Fax: (+81) 3-5734-2637
Search for more papers by this authorProf. Dr. Takao Ikariya
Department of Applied Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro-ku, Tokyo 152-8552 (Japan), Fax: (+81) 3-5734-2637
Search for more papers by this authorThis work was financially supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (Japan, No. 18065007, 22225004) and by The G-COE Program.
Graphical Abstract
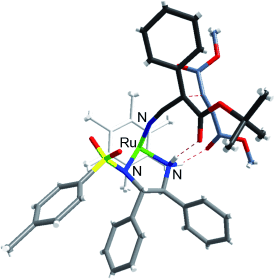
Metal-based catalysts: The title reaction provided the chiral adducts in high yields, excellent enantiomeric excess, and high Z/E selectivity. A combined NMR/DFT study revealed a key intermediate for the stereoselective reaction and a possible reaction mechanism (see the optimized transition-state structure).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201003585_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Christoffers, A. Mann, Angew. Chem. 2001, 113, 4725–4732;
10.1002/1521-3757(20011217)113:24<4725::AID-ANGE4725>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4591–4597;10.1002/1521-3773(20011217)40:24<4591::AID-ANIE4591>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 1bE. J. Corey, A. Guzman-Perez, Angew. Chem. Int. Ed. 1998, 37, 389–401;
- 1cC. J. Douglas, L. E. Overman, Proc. Natl. Acad. Sci. USA 2004, 101, 5363–5367;
- 1dJ. Comelles, M. Moreno-Mañas, A. Vallribera, ARKIVOC 2005, ix, 207–238;
- 1eI. Denissova, L. Barriault, Tetrahedron 2003, 59, 10105–10146;
- 1fM. Shibasaki, M. Kanai, Chem. Rev. 2008, 108, 2853–2873;
- 1gM. Shibasaki, M. Kanai, S. Matsunaga, N. Kumagai, Acc. Chem. Res. 2009, 42, 1117–1127.
- 2
- 2aJ. Åhman, J. P. Wolfe, M. V. Troutman, M. Palucki, S. L. Buchwald, J. Am. Chem. Soc. 1998, 120, 1918–1919;
- 2bT. Hamada, A. Chieffi, J. Åhman, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 1261–1268;
- 2cD. J. Spielvogel, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 3500–3501;
- 2dS. Lee, J. F. Hartwig, J. Org. Chem. 2001, 66, 3402–3415;
- 2eX. Xie, Y. Chen, D. Ma, J. Am. Chem. Soc. 2006, 128, 16050–16051;
- 2fB. M. Trost, R. Radinov, E. M. Grenzer, J. Am. Chem. Soc. 1997, 119, 7879–7880;
- 2gX. Liao, Z. Weng, J. F. Hartwig, J. Am. Chem. Soc. 2008, 130, 195–200.
- 3
- 3aS. Mouri, Z. Chen, H. Mitsunuma, M. Frutachi, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 1255–1256;
- 3bT. Mashiko, N. Kumagai, M. Shibasaki, J. Am. Chem. Soc. 2009, 131, 14990–14999;
- 3cI.-H. Chen, L. Yin, W. Itano, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2009, 131, 11664–11665;
- 3dH. Sasai, E. Emori, T. Arai, M. Shibasaki, Tetrahedron Lett. 1996, 37, 5561–5564.
- 4Chiral Pd:
- 4aY. Hamashima, M. Sodeoka, Chem. Rec. 2004, 4, 231–242;
- 4bN. Umebayashi, Y. Hamashima, D. Hashizume, M. Sodeoka, Angew. Chem. 2008, 120, 4264–4267;
10.1002/ange.200705344 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4196–4199;
- 4cchiral lanthanide: J. Comelles, À. Pericas, M. Moreno-Mañas, A. Vallribera, G. Drudis-Solé, A. Lledos, T. Parella, A. Roglans, García-Granda, S. L. Roces-Fernández, J. Org. Chem. 2007, 72, 2077–2087;
- 4dchiral Cu: M. Marigo, K. Juhl, K. A. Jørgensen, Angew. Chem. 2003, 115, 1405–1407;
10.1002/ange.200390322 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1367–1369;
- 4eS. Ma, N. Jiao, Z. Zheng, Z. Ma, Z. Lu, L. Ye, Y. Deng, G. Chen, Org. Lett. 2004, 6, 2193–2196;
- 4fS. Saaby, M. Bella, K. A. Jørgensen, J. Am. Chem. Soc. 2004, 126, 8120–8121;
- 4gP. M. Pihko, A. Pohjakallio, Synlett 2004, 2115–2118;
- 4hE. Fillion, A. Wilsily, J. Am. Chem. Soc. 2006, 128, 2774–2774;
- 4iC. Hawner, K. Y. Li, V. Cirriez, A. Alexakis, Angew. Chem. 2008, 120, 8334–8337;
10.1002/ange.200803436 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8211–8214;
- 4jL. Yin, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2009, 131, 9610–9611.
- 5
- 5aK.-J. Haack, S. Hashiguchi, A. Fujii, T. Ikariya, R. Noyori, Angew. Chem. 1997, 109, 297–300;
10.1002/ange.19971090333 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 285–288;
- 5bR. Noyori, S. Hashiguchi, Acc. Chem. Res. 1997, 30, 97–102;
- 5cT. Ikariya, A. J. Blacker, Acc. Chem. Res. 2007, 40, 1300–1308.
- 6
- 6aM. Watanabe, A. Ikagawa, H. Wang, K. Murata, T. Ikariya, J. Am. Chem. Soc. 2004, 126, 11148–11149;
- 6bM. Watanabe, K. Murata, T. Ikariya, J. Am. Chem. Soc. 2003, 125, 7508–7509;
- 6cT. Ikariya, K. Murata, R. Noyori, Org. Biomol. Chem. 2006, 4, 393–406;
- 6dT. Ikariya, I. D. Gridnev, Chem. Rec. 2009, 9, 106–123.
- 7
- 7aK. Murata, T. Ikariya, R. Noyori, J. Org. Chem. 1999, 64, 2186–2187;
- 7bK. Mashima, T. Abe, K. Tani, Chem. Lett. 1998, 27, 1199–1200.
- 8Y. Hasegawa, M. Watanabe, I. D. Gridnev, T. Ikariya, J. Am. Chem. Soc. 2008, 130, 2158–2159.
- 9X. Wang, M. Kitamura, K. Maruoka, J. Am. Chem. Soc. 2007, 129, 1038–1039.
- 10The Ru amido complexes 1 a and 1 b usually exist in an equilibrium with the corresponding hydroxy amino complexes because the presence of water cannot be completely excluded from the synthetic procedure.
- 11M. T. Nguyen, G. Raspoet, L. G. Vanquickenborne, J. Phys. Org. Chem. 2000, 13, 46–56.
- 12H. F. Bettinger, P. R. Schreiner, P. V. R. Schleyer, H. F. Schaefer III, J. Phys. Chem. 1996, 100, 16147–16154.