Synthesis of Functionalized Polycyclic Compounds: Rhodium(I)-Catalyzed Intramolecular Cycloaddition of Yne and Ene Vinylidenecyclopropanes†
Bei-Li Lu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Min Shi
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)Search for more papers by this authorBei-Li Lu
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Min Shi
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032 (China)Search for more papers by this authorWe thank the Shanghai Municipal Committee of Science and Technology (08dj1400100-2), National Basic Research Program of China (973)-2009CB825300, and the National Natural Science Foundation of China for financial support (21072206, 20472096, 20872162, 20672127, 20821002 and 20732008).
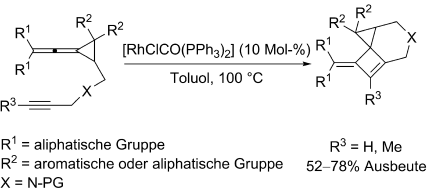
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201105292_sm_miscellaneous_information.pdf1.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aI. Ojima, A. T. Vu, D. Bonafoux, Organometallic Complexes of Rhodium, Thieme, Stuttgart, 2001;
10.1055/sos-SD-001-00399 Google Scholar
- 1bP. van Leeuwen, C. Claver, Rhodium Catalyzed Hydroformylation; Springer, Berlin, 2002;
10.1007/0-306-46947-2 Google Scholar
- 1cP. A. Evans, Modern Rhodium-Catalyzed Organic Reactions, VCH, Weinheim, 2005;
- 1dC. Aubert, L. Fensterbank, P. Garcia, M. Malacria, A. Simonneau, Chem. Rev. 2011, 111, 1954–1993.
- 2For selected reviews, see:
- 2aM. Lautens, W. Klute, W. Tam, Chem. Rev. 1996, 96, 49–92;
- 2bJ. A. Varela, C. Saá, Chem. Rev. 2003, 103, 3787–3802;
- 2cK. Tanaka, Synlett 2007, 1977–1993;
- 2dT. Shibata, K. Tsuchikama, Org. Biomol. Chem. 2008, 6, 1317–1323;
- 2eS. Perreault, T. Rovis, Chem. Soc. Rev. 2009, 38, 3149–3159.
- 3For recent reviews, see:
- 3aC. Slugovc, I. Padilla-Martínez, S. Sirol, E. Carmona, Coord. Chem. Rev. 2001, 213, 129–157;
- 3bV. Ritleng, C. Sirlin, M. Pfeffer, Chem. Rev. 2002, 102, 1731–1770;
- 3cH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861–2904;
- 3dH. M. L. Davies, Ø. Loe, Synthesis 2004, 2595–2608;
- 3eP. M. P. Gois, C. A. M. Afonso, Eur. J. Org. Chem. 2004, 3773–3788;
- 3fH. M. L. Davies, J. Nikolai, Org. Biomol. Chem. 2005, 3, 4176–4187;
- 3gA. G. H. Wee, Curr. Org. Synth. 2006, 3, 499–555;
- 3hY. J. Park, J.-W. Park, C.-H. Jun, Acc. Chem. Res. 2008, 41, 222–234;
- 3iJ. C. Lewis, R. G. Bergman, J. A. Ellman, Acc. Chem. Res. 2008, 41, 1013–1025;
- 3jD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624–655;
- 3kT. Satoh, M. Miura, Chem. Eur. J. 2010, 16, 11212–11222;
- 3lM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704–724;
- 3mC. N. Slattery, A. Ford, A. R. Maguire, Tetrahedron 2010, 66, 6681–6705.
- 4
- 4aJ. C. Namyslo, D. E. Kaufmann, Chem. Rev. 2003, 103, 1485–1537;
- 4bR. J. Griffin, C. E. Arris, C. Bleasdale, F. T. Boyle, A. H. Calvert, N. J. Curtin, C. Dalby, S. Kanugula, N. K. Lembicz, D. R. Newell, A. E. Pegg, B. T. Golding, J. Med. Chem. 2000, 43, 4071–4083;
- 4cN. Butta, A. Martin-Requero, E. Urcelay, R. Parrilla, M. S. Ayuso, J. Pharmacol. 1996, 118, 1797–1805.
- 5B. Alcaide, P. Almendros, C. Aragoncillo, Chem. Soc. Rev. 2010, 39, 783–816, and references therein.
- 6For recent reviews, see:
- 6aW. Tam, J. Goodreid, N. Cockburn, Curr. Org. Synth. 2009, 6, 219–238. For selected examples, please see:
- 6bT. Shibata, K. Takami, A. Kawachi, Org. Lett. 2006, 8, 1343–1345;
- 6cR. E. Forslund, J. Cain, J. Colyer, M. P. Doyle, Adv. Synth. Catal. 2005, 347, 87–92;
- 6dJ. M. Joo, Y. Yuan, C. Lee, J. Am. Chem. Soc. 2006, 128, 14818–14819;
- 6eH. Kim, C. Lee, J. Am. Chem. Soc. 2005, 127, 10180–10181;
- 6fP. A. Wender, M. P. Croatt, B. Kühn, Organometallics 2009, 28, 5841–5844;
- 6gR. S. Jolly, G. Luedtke, D. Sheehan, T. Livinghouse, J. Am. Chem. Soc. 1990, 112, 4965–4966;
- 6hG. Hilt, A. Paul, J. Treutwein, Org. Lett. 2010, 12, 1536–1539.
- 7B. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921–2944.
- 8For examples about rhodium-catalyzed allylic CH bond activation, see:
- 8aH. M. L. Davies, P. Ren, Q. Jin, Org. Lett. 2001, 3, 3587–3590;
- 8bH. M. L. Davies, R. E. J. Beckwith, J. Org. Chem. 2004, 69, 9241–9247;
- 8cT. Makino, K. Itoh, J. Org. Chem. 2004, 69, 395–405;
- 8dK. M. Brummond, H. Chen, B. Mitasev, A. D. Casarez, Org. Lett. 2004, 6, 2161–2163;
- 8eH. M. L. Davies, Q. Jin, Org. Lett. 2005, 7, 2293–2296;
- 8fH. M. L. Davies, M. G. Coleman, D. L. Ventura, Org. Lett. 2007, 9, 4971–4974;
- 8gD. N. Zalatan, J. D. Bois, J. Am. Chem. Soc. 2008, 130, 9220–9221;
- 8hE. Nadeau, D. L. Ventura, J. A. Brekan, H. M. L. Davies, J. Org. Chem. 2010, 75, 1927–1939;
- 8iQ. Li, Z.-X. Yu, J. Am. Chem. Soc. 2010, 132, 4542–4543;
- 8jS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585–9587;
- 8kW. Li, W. Yuan, M. Shi, E. Hernandez, G. Li, Org. Lett. 2010, 12, 64–67;
- 8lQ. Li, Z.-X. Yu, Angew. Chem. 2011, 123, 2192–2195; Angew. Chem. Int. Ed. 2011, 50, 2144–2147.
- 9For the synthesis of VDCPs, see:
- 9aK. Isagawa, K. Mizuno, H. Sugita, Y. Otsuji, J. Chem. Soc. Perkin Trans. 1 1991, 2283–2285, and references therein;
- 9bA. R. Al Dulayymi, M. S. Baird, J. Chem. Soc. Perkin Trans. 1 1994, 1547–1548.
- 10
- 10aM. L. Poutsma, P. A. Ibarbia, J. Am. Chem. Soc. 1971, 93, 440–450;
- 10bW. Smadja, Chem. Rev. 1983, 83, 263–320;
- 10cH. Sugita, K. Mizuno, T. Saito, K. Isagawa, Y. Otsuji, Tetrahedron Lett. 1992, 33, 2539–2542;
- 10dK. Mizuno, H. Sugita, T. Kamada, Y. Otsuji, Chem. Lett. 1994, 449–452, and references therein;
- 10eL. K. Sydnes, Chem. Rev. 2003, 103, 1133–1150;
- 10fK. Mizuno, H. Sugita, K. Isagawa, M. Goto, Y. Otsuji, Tetrahedron Lett. 1993, 34, 5737–5738;
- 10gK. Mizuno, H. Sugita, T. Hirai, H. Maeda, Chem. Lett. 2000, 1144–1145;
- 10hK. Mizuno, H. Maeda, H. Sugita, S. Nishioka, T. Hirai, A. Sugimoto, Org. Lett. 2001, 3, 581–584;
- 10iH. Maeda, K. Mizuno, J. Synth. Org. Chem. Jpn. 2004, 62, 1014–1025;
- 10jM. Shi, L.-X. Shao, J.-M. Lu, Y. Wei, K. Mizuno, H. Maeda, Chem. Rev. 2010, 110, 5883–5913;
- 10kG.-C. Xu, L.-P. Liu, J.-M. Lu, M. Shi, J. Am. Chem. Soc. 2005, 127, 14552–14553;
- 10lJ.-M. Lu, M. Shi, Org. Lett. 2007, 9, 1805–1808;
- 10mJ.-M. Lu, Z.-B. Zhu, M. Shi, Chem. Eur. J. 2009, 15, 963–971;
- 10nL.-F. Yao, M. Shi, Chem. Eur. J. 2009, 15, 3875–3881;
- 10oW. Li, W. Yuan, M. Shi, E. Hernandez, G.-G. Li, Org. Lett. 2010, 12, 920–923;
- 10pL. Wu, M. Shi, Chem. Eur. J. 2010, 16, 1149–1152;
- 10qJ.-M. Lu, M. Shi, Org. Lett. 2006, 8, 5317–5320;
- 10rJ.-M. Lu, M. Shi, Org. Lett. 2008, 10, 1943–1946;
- 10sL. Wu, M. Shi, J. Org. Chem. 2010, 75, 2296–2301;
- 10tL. Wu, M. Shi, Eur. J. Org. Chem. 2011, 1099–1105;
- 10uW. Yuan, M. Shi, Tetrahedron 2010, 66, 7104–7111;
- 10vB.-L. Lu, Y. Wei, M. Shi, Chem. Eur. J. 2010, 16, 10975–10979;
- 10wB.-L. Lu, M. Shi, Eur. J. Org. Chem. 2011, 243–248.
- 11CCDC 791963 (3 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12CCDC 816150 (6 b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13
- 13aM. E. Prater, L. E. Pence, R. Clérac, G. M. Finniss, C. Campana, P. Auban-Senzier, D. Jérome, E. Canadell, K. R. Dunbar, J. Am. Chem. Soc. 1999, 121, 8005–8016;
- 13bF. L. Taw, P. S. White, R. G. Bergman, M. Brookhart, J. Am. Chem. Soc. 2002, 124, 4192–4193.
- 14
- 14aZ.-X. Yu, P. H.-Y. Cheong, P. Liu, C. Y. Legault, P. A. Wender, K. N. Houk, J. Am. Chem. Soc. 2008, 130, 2378–2379;
- 14bS. Mao, D. Probst, S. Werner, J. Chen, X. Xie, K. M. Brummond, J. Comb. Chem. 2008, 10, 235–246.
- 15aR. D. Schuetz, F. W. Millard, J. Org. Chem. 1959, 24, 297–300;
- 15bK.-T. Yip, J.-H. Li, O.-Y. Lee, D. Yang, Org. Lett. 2005, 7, 5717–5719.
- 16CCDC-830623 (7 b) CCDC 6.. contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17CCDC-828941 (7 c) CCDC 6.. contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.