Quality and functional characteristics of tofu prepared rapidly from soybeans dried after soaking in water
Abstract
In this study, Soybeans dried after soaking (SDSs) were prepared for rapid tofu production, and quality and functional characteristics of SDS-tofu were analyzed. SDS40, SDS50, and SDS60 were prepared by soaking for 12 hr and then drying at 40, 50, and 60℃, respectively. During the soaking process in tofu production, the water absorption rate of SDSs was higher than that of control soybeans. SDS-tofu was prepared by soaking SDSs for 1 hr. Quality, texture, and sensory characteristics were similar among tofu samples. SDS-tofu had a higher aglycone isoflavone content (AIC) than did control tofu. SDS60-tofu had the highest AIC, indicating high nitric oxide inhibitory effect. Antioxidant components, activities and cell viabilities were similar among control tofu and SDS-tofu. Consequently, this study presents the possibility of enhancing efficiency of tofu production process and functionality of tofu by using SDSs because of reduction of the soaking time and the improvement of aglycone isoflavone content.
Novelty impact statement
Soybeans dried after soaking (SDSs) contained much higher contents of aglycone isoflavones than did general-use soybeans because the former was prepared by drying soybeans at 40–60℃ after they had been completely soaked for a long time, thus allowing endogenous β-glucosidase (optimal temperature, 60℃) to be activated, and in particular, glycitein, which was not detected in general-use soybeans, was detected in the SDSs. The use of SDSs can shorten the moisture reabsorption time because the cross-sectional area for moisture absorption is larger in SDSs than in general-use soybeans, likely as the result of porous structures that form in the soybean as it dries after swelling from being soaked. By manufacturing tofu using SDSs, tofu with enhanced characteristics, can be produced quickly due to improvement of the manufacturing process, namely, the time needed for soaking is shorter, and the resulting tofu has a higher content of aglycone isoflavones from the conversion of isoflavones to aglycone isoflavones.
1 INTRODUCTION
Soybean (Glycine max L. Merrill) has been cultivated throughout East Asia, including Korea and Southeast Asia, for a long time (Mujoo et al., 2003). Soybeans not only are an inexpensive source of abundant protein (protein content of 35%–40% on a dry weight basis) but also have high nutritional value for grain-dependent Asian populations because they contain abundant lysine and sulfur-containing amino acids, which are lacking in grains (Mujoo et al., 2003). Isoflavones, key functional components of soybeans, exert anticancer effects by affecting the metastasis and cell division of cancer cells (Kim et al., 2008) and inhibit the progression of osteoporosis attributable to estrogen deficiency (Choi & Sohn, 1998). Moreover, soybean saponins have a strong antioxidant effect and inhibit colorectal cancer by forming foam that increases the volume of digested food (Park et al., 2009). Along with rice and barley, soybean is the most consumed crop in South Korea. Various processed foods made from soybean are consumed widely in daily life, with tofu and soymilk the most common food types (Kim, 1998).
The soaking of soybeans is a primary and essential process in tofu manufacturing (Deshpande et al., 1984). The soaking process provides moisture that enables isoflavone, a functional component in soybeans, to be hydrolyzed by β-glucosidase to aglycone isoflavone, which is more bioavailable in the body (Góes-Favoni et al., 2010). This process also facilitates heat exchange during chemical reactions, promoting the inactivation of bioactivity inhibitors such as trypsin inhibitors (Morris et al., 1950). Furthermore, the soaking process plays an important role in tofu quality, as the moisture facilitates protein denaturation starch gelatinization (Shih & Chiang, 2017). This soaking process is the most time-consuming aspect of tofu manufacturing (Seo et al., 2010). It is performed at low temperatures to reduce the effects of microorganisms, and soybeans only absorb sufficient moisture to swell after soaking for 12 hr or longer (Kim et al., 2019).
Most studies on the soybean soaking process have focused on improvements in quality and functionality, such as decomposition to produce higher levels of aglycone isoflavones during the soaking period (Falcão et al., 2018) or the effect of soaking soybeans on the quality and flavor of the product (Li et al., 2019), but there is a lack of studies on process improvements, such as shortening the time required for the soaking process.
Webster and Leopold (1977) reported that vacuoles in the beans swell during the soaking process, resulting in an expanded inner space and bean volume. Park et al. (2015) reported that a porous structure forms when moisture escapes from the beans during hot air drying. These studies suggest that we could prepare soybeans dried after soaking (SDSs), in which a porous structure would develop if the soybeans were soaked sufficiently such that they swell up and then dried. Furthermore, the use of SDSs in the manufacturing of tofu would reduce the soaking process time because the porous structure would facilitate rapid moisture absorption from the external environment.
Therefore, in this study, SDSs were prepared by soaking soybeans until they swelled up and drying them, the quality and functional characteristics of the SDSs were analyzed, and the SDSs were used to manufacture tofu to evaluate whether their use improved the manufacturing process by reducing the soaking time. This study also analyzed the quality and functional characteristics of tofu made from SDSs (SDS-tofu) to determine whether the SDSs added value to the tofu.
2 MATERIALS AND METHODS
2.1 Materials
The soybeans (Glycine max L. Merrill) used for preparing SDSs were obtained from the Chungju Agricultural Technology Center. Daidzein (16587-10MG), glycitein (43534-10MG), and genistein (92136-10MG) for aglycone isoflavone content analysis; ABTS (A1888-5G), DPPH (D9132-1G), potassium persulfate (216224-100G), Na2CO3 (S7795-500G), NaNO2 (237213-100G), Folin–Ciocalteu phenol reagent (F9252-500Ml), trolox (648471-1G), sodium acetate trihydrate (S7670-1KG), FeCl2.6H2O (F2877-0.5KG), tripyridyltriazine (93285-5G), gallic acid (398225-100G), L-ascorbic acid (A7506-100G), and pyrogallol (254002-10G) for the analysis of antioxidant properties and activities; sulfanilamide (S9251-100G), phosphoric acid (345245-100Ml), sodium nitrite (L4391-1MG), naphthylethylenediamine-HCl (222488-5G), and lipopolysaccharide (LPS; L4391-1MG) for anti-inflammatory assays; and MTT (M5655-100MG) for the MTT assay were obtained from the Sigma-Aldrich Co. Dulbecco's modified Eagle medium (DMEM; LM001-05-500Ml), phosphate-buffered saline (PBS; ML008-01-500Ml), and penicillin–streptomycin (LS202-02-100Ml) were purchased from Welgene (Daegu, Korea). Fetal bovine serum (FBS; US-FBS-500-500Ml) was purchased from A-Frontier (Seoul, Korea).
2.2 Preparation of SDSs
Based on the research of Kim et al. (1988) showing that soybeans need to be soaked at 20℃ or 30℃ for more than 12 hr to swell fully, 600 g of soybeans were placed into 10 times the amount of water and soaked at room temperature for 12 hr. After removing the completely swollen beans from the soaking solution, the beans were dried at 40℃, 50℃, or 60℃ until a constant weight was attained to prepare SDS40, SDS50, and SDS60, respectively. The weight of the prepared SDSs for each group was about 530 g. The SDSs were analyzed for their proximate composition, aglycone isoflavone content, total polyphenol and flavonoid contents, and DPPH and ABTS radical scavenging ability.
2.3 Water content and absorption rate of the SDSs according to soaking time
The water content and absorption rate of the SDSs according to soaking time were analyzed following the method of Lee et al. (1987) with some modifications. General-use soybeans (4 g), SDS40, SDS50, and SDS60 were placed into separate 50-ml conical tubes (SPL Life Sciences Co.), and 40 g of distilled water was added to each tube. The samples were soaked for 10, 20, 30, 60, 90, or 120 min at room temperature, and the soybean weight was measured after the removal of external moisture. Thereafter, the moisture content in each soybean was determined after direct drying at 105℃.
2.4 Preparation of tofu
Tofu was prepared according to the tofu manufacturing method of Lee et al. (2011) and Woo et al. (2009) with modifications to the amount of soybeans used. In 6000 g of water (10 times the weight of the soybeans used), 600 g of control soybeans (general-use soybeans) were soaked for 12 hr, and 530 g of SDSs were soaked for 1 hr. After removing the soaking solution, the soybeans were placed into a tofu-making machine (SD-1500, Daeryuk Food Machine, Co.). According to the manufacturer's method, while adding 5 L of water at a constant rate, the beans were ground wet to produce soymilk while the biji (soy pulp) was filtered out. The separated soymilk was heated at 100℃ for 15 min by steam. After cooling the soymilk to 80℃, 300 ml of 6% (wt/vol) MgCl2 coagulant solution was added to the soymilk, and coagulation was allowed to take place at 80℃ for 30 min. The obtained coagulum was poured into a mold (30 × 24 × 10 cm) and pressed at pressure of 6.3 g/cm2 for 15 min to produce tofu. The tofu was analyzed for its proximate composition, aglycone isoflavone content, total polyphenol and flavonoid contents, DPPH and ABTS radical scavenging abilities, superoxide dismutase (SOD)-like activity, protein mobility based on fluorescence recovery after photobleaching (FRAP), effect on cell viability, anti-inflammatory ability, and sensory characteristics.
2.5 Proximate composition
The proximate composition was determined according to methods of the Association of Official Analytical Chemists (AOAC, 1995) and Seo et al. (2010). The moisture content was determined using the atmospheric-pressure drying method at 105℃. Crude protein content was determined via the Kjeldahl method using a Kjeldahl auto analyzer (Kjeltec™ 2300, Foss) and calculated using a nitrogen-to-protein conversion factor (6.25). The Soxhlet extraction method was used to determine crude fat content. Mineral contents were analyzed using an electric furnace (FX-14, Daihan Scientific) at 525℃. Carbohydrate weight was determined as the fraction of the total weight of the sample after excluding moisture, crude protein, crude fat, and mineral contents.
2.6 Quality characteristics
The tofu yield was calculated based on the ratio between the total weight of the tofu and the weight of the soybeans. The biji dry weight was measured after drying the biji produced during the tofu manufacturing process at 105℃ for 12 hr.
A colorimeter (CR-300, Minolta Co.) calibrated with a standard white plate (L = 92.700, a = 0.313, b = 0.396) was used to determine the color of the tofu. The “L” value indicates the lightness intensity, the “a” value indicates the redness intensity when positive and the greenness intensity when negative, and the “b” value indicates the yellowness intensity when positive and the blueness intensity when negative. The color difference value (ΔE) was calculated based on the measured L, a, and b values.
The texture of the tofu was evaluated according to the method of Kim et al. (2016). Tofu was prepared in cubes of the same shape and size (2 × 2 × 2 cm), and the hardness, chewiness, and gumminess of the tofu were measured using a TA-XT2 texture analyzer (Texture Technologies Co.) with a 20.0-mm-diameter probe. The pre-test and post-test speeds were 3.0 mm/s, the test speed was 1.0 mm/s, and the trigger force and test distance were 10.0 g and 15.0 mm, respectively.
2.7 Aglycone isoflavone content
Sample pretreatment for aglycone isoflavone content analysis was performed according to the method of Kim et al. (2004) with modifications. The sample was dried using a lyophilizer (FD8508, ilShinBioBase Co.) and then pulverized using a grinder (80,335, Elec-Tech Zhuhai Co.). The freeze-dried sample powder was passed through a 50-mesh sieve (Chunggye Sieve Co.) to make the extraction process easier. Sample powder (1 g) and 80% ethanol (20 ml) were added to a 50-ml conical tube, and ultrasonic extraction was performed using an ultrasonic cleaner (Daihan Scientific Co.) at 50°C for 1 hr. The extracted sample was centrifuged at 3500g for 10 min (Union 5kr, Hanil Science Industrial Co.), and the supernatant was filtered with a 0.45-μm syringe filter (Millipore) before being used as the extraction sample for aglycone isoflavone content analysis.
High-performance liquid chromatography (HPLC; LC-2030C, Shimadzu Corporation) with UV–VIS detection was used for the quantitative analysis of aglycone isoflavones. The column was a YMC-Pack Pro C18 (YMC Korea, Ltd.), and the analysis was performed at a wavelength of 254 nm. For the mobile phase of HPLC, 0.1% acetic acid in acetonitrile was used as solvent A, and 0.1% acetic acid in water was used as solvent B. The flow rate was 0.5 ml/min, and the injection volume was 40 µl. The gradient conditions of the mobile phase were as follows: 0 min, A 15%, B 85%; 50 min, A 30%, B 70%; 60 min, A 30%, B 70%; 75 min, A 15%, B 85%; and 85 min, A 15%, B 85%. The aglycone isoflavone content was determined and quantified via comparison with the standard curves of daidzein, glycitein, and genistein, which are aglycone isoflavones.
2.8 Antioxidant properties and activity
2.8.1 Pretreatment
For sample pretreatment before the antioxidant properties and activity were assayed, each sample was dried using a lyophilizer (FD8508, ilShinBiobase Co.) and then pulverized using a grinder (80335, Elec-Tech Zhuhai Co.). Sample powder (1 g) and 80% ethanol (10 ml) were placed into a 50-ml conical tube and extracted with stirring at 100 rpm in a water bath (WiseStir SMHS6, Daihan Scientific Co.) at 60°C for 2 hr. Thereafter, centrifugation was performed at 3500g for 10 min (Union 5kr, Hanil Science Industrial Co.), and the supernatant was filtered through filter paper (No. 2, Whatman International). The obtained filtrate was concentrated using a rotary vacuum evaporator (Eyela NE-2001), lyophilized, and dissolved in distilled water to prepare a stock solution. Before analysis, the solution was filtered with a 0.45-μm syringe filter (Millipore) and used as the sample.
2.8.2 Total polyphenol and flavonoid contents
The total polyphenol content was measured using the method of Dewanto et al. (2002). The sample extract (100 μl) was combined with Na2CO3 solution (2 ml) and allowed to stand for 3 min. Then, 100 μl of 50% (vol/vol) Folin–Ciocalteu reagent was added, and the solution was incubated in the dark for 30 min. The absorbance at 750 nm was measured using a microplate reader (Synergy HTX, BioTek Instruments, Inc.). The total polyphenol content was expressed as milligrams of gallic acid equivalent (GAE) per 1 g of sample.
The total flavonoid content was determined according to the method of Choi et al. (2003). The sample extract (250 μl) was mixed with 1 ml of distilled water and 75 µl of 5% (wt/vol) NaNO2 and allowed to stand for 5 min. Then, 150 μl of 10% (wt/vol) AlCl3·H2O and 1 M NaOH were added sequentially, and the mixture was left in the dark for 6 and 11 min, respectively. The absorbance at 510 nm was measured using a microplate reader. (+)-Catechin hydrate was used as the standard, and the results were expressed as milligrams of catechin equivalent (CE) per 1 g of sample.
2.8.3 DPPH and ABTS scavenging activity
The DPPH radical scavenging activity was analyzed using the method of Blois (1958). The sample extract (200 μl) was combined with 800 μl of 2 × 10−4 M DPPH solution, and the absorbance at 510 nm was measured using a microplate reader after incubation in the dark for 30 min.
The ABTS radical scavenging activity was determined using the method of Ahn et al. (2012). After mixing 7.4 × 10−3 M ABTS solution with 2.6 × 10−3 M potassium persulfate solution at a ratio of 1:1 [vol/vol] and allowing the mixture to stand in the dark for 24 hr, the mixture was diluted until an absorbance value of 1.4–1.5 at 735 nm was reached. Then, 50 μl of extract was added to 1 ml of the diluted mixed ABTS solution. The absorbance at 735 nm was measured using a microplate reader after the mixture was allowed to stand in the dark for 60 min.
The standard used in the DPPH and ABTS scavenging assays was L-ascorbic acid, and the antioxidant capacity was expressed as milligrams of L-ascorbic acid equivalent (AE) per 1 g of sample.
2.8.4 FRAP and SOD-like activity
FRAP was determined following the method of Benzie and Strain (1999), with modifications. FRAP solution (150 μl) was prepared by mixing 300 mM acetate buffer (pH 3.6) and 10 mM tripyridyltriazine with 40 mM HCl and 20 mM FeCl2.6H2O at a ratio of 10:1:1 (v:v:v). After 50 μl of extract was added to 150 μl of FRAP solution and allowed to react for 4 min, the absorbance at 595 nm was measured using a microplate reader. FRAP was expressed as μmol Trolox equivalent (TE) per 1 g of sample.
SOD-like activity was analyzed according to the method of Marklund and Marklund (1974). First, 3 ml of pH 8.5 tris-HCl buffer solution (0.05 M Tris +10 mM EDTA) and 200 μl of 7.2 mM pyrogallol were added to 200 μl of extract at 30-s intervals, and the mixture was then reacted at 25℃ for 10 min. After the addition of 1 ml of 1 N HCl to the reaction solution, the amount of oxidized pyrogallol in the reaction solution was measured at 420 nm using a microplate reader. The positive control was L-ascorbic acid.
2.9 Cell viability and anti-inflammatory ability
Cell viability was assessed using the MTT assay (Van Meerloo et al., 2011). Sample pretreatment was performed following the same method used for analyzing antioxidant properties and activity. RAW 264.7 macrophages were cultured in an incubator at 37°C under 5% CO2 on DMEM containing 100 units/ml of penicillin–streptomycin and 10% FBS. RAW 264.7 cells were aliquoted into 96-well plates at 4.0 × 104 cells/well and cultured for 24 hr. The cells were exposed to extracts at different concentrations and cultured for 24 hr. After dissolving 5 mg/ml MTT solution into PBS, it was added to the RAW 264.7 cells, and the mixture was left to react in the dark for 4 hr. Next, centrifugation was performed at 2000g for 10 min, the supernatant was removed, and 150 μl of DMSO was added to each well to dissolve formazan. The absorbance at 540 nm was measured using a microplate reader.
Anti-inflammatory effects were analyzed using the nitric oxide (NO) production inhibition assay as described by Marcocci et al. (1994). NO generated from RAW 264.7 cells in the form of+ 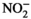 was measured in the cell culture medium using Griess reagent (1% sulfanilamide in 5% phosphoric acid +0.1% naphthylethylenediamine-HCl). RAW 264.7 cells were aliquoted into a 24-well plate at 3 × 105 cells/ml and cultured for 24 hr, and the cultured RAW 264.7 cells were treated with a sample extract and LPS (1 μg/ml). After 24 hr, 100 μl of the supernatant was treated with 100 μl of Griess reagent and reacted at 37°C for 10 min. Then, the absorbance at 540 nm was measured using a microplate reader (BioTek Instruments). NO levels were quantified based on a standard curve of NaNO2.
was measured in the cell culture medium using Griess reagent (1% sulfanilamide in 5% phosphoric acid +0.1% naphthylethylenediamine-HCl). RAW 264.7 cells were aliquoted into a 24-well plate at 3 × 105 cells/ml and cultured for 24 hr, and the cultured RAW 264.7 cells were treated with a sample extract and LPS (1 μg/ml). After 24 hr, 100 μl of the supernatant was treated with 100 μl of Griess reagent and reacted at 37°C for 10 min. Then, the absorbance at 540 nm was measured using a microplate reader (BioTek Instruments). NO levels were quantified based on a standard curve of NaNO2.
2.10 Sensory characteristics
Sensory evaluation of the tofu was conducted with research approval from the Bioethics Committee of Chungbuk National University (institutional review board number: CBNU-202102-HR-0211). The sensory evaluation panel members were selected following the methods of Kim et al. (2012) and Lee et al. (2018), with some modifications. Thirty-two college students (male:female = 19:13) with an ability to discriminate differences among samples were recruited, and the evaluation panel members were pre-educated about the evaluation list and the purpose of the experiment before starting the sensory evaluation. Tofu cubes of the same size (1.0 × 1.0 × 1.0 cm) were provided in white dishes of the same shape and size, which were cleaned beforehand by rinsing with water (20 ± 1℃). Before evaluating the tofu, respondents were asked to rinse their mouth 2 to 3 times to minimize any influence from the previous sample. On the evaluation list, the color, taste, smell (flavor), softness, and overall preference were evaluated using a nine-point scale, with a high score indicating a strong preference.
2.11 Statistical analysis
F-values and p-values were calculated using Statistical Analysis System (SAS) ver. 9.4 (SAS Institute Inc.). For each experiment, average values and standard deviations were calculated by analyzing three or more repetitions, and differences were considered significant at p < .05 using Duncan's multiple range test.
3 RESULTS AND DISCUSSION
3.1 SDS characteristics
3.1.1 Proximate composition
A comparison of the proximate composition of the SDSs is provided in Table 1. The SDSs had a lower moisture content than did the control soybeans, which could be attributed to the drying process used during SDS preparation. The moisture content of SDS40 was higher than the contents of SDS50 and SDS60, suggesting that the lower drying temperature allowed more water to remain bound in the SDS40 samples during the drying process compared to the other SDSs (Trabelsi & Nelson, 2004). Therefore, the residual water content at constant weight was higher for the lower temperatures (Lee & Cho, 2014). Although higher crude protein, crude fat, crude ash, and carbohydrate contents were found in the SDSs than in the control soybeans, the proportions remained constant, despite variation in the moisture content in the dried SDSs. Hence, there were no differences in the proximate composition ratios in the SDSs, except for moisture content. Additionally, there were no differences in the ratios of general components in the SDSs, except for moisture content. When the moisture content was lower, the contents of crude protein, crude fat, ash, and carbohydrate were higher. In consideration of the above, there was almost no loss of nutritional components during the SDS processing. Moreover, the SDSs had high nutrient contents per unit weight. Therefore, SDSs can be used as a semi-processed product for manufacturing high-quality soybean products, given the high nutrient contents per unit weight of the SDSs.
| Control soybean | SDS40 | SDS50 | SDS60 | F-value | |
|---|---|---|---|---|---|
| Moisture content (%) | 12.26 ± 0.13a | 5.24 ± 0.15b | 2.38 ± 0.21c | 2.31 ± 0.10c | 2,878.76*** |
| Crude protein content (%) | 35.88 ± 0.18c | 40.35 ± 0.46b | 41.70 ± 1.11a | 41.55 ± 0.35a | 77.81*** |
| Crude fat content (%) | 12.47 ± 0.10c | 14.19 ± 0.59b | 17.15 ± 0.65a | 16.91 ± 0.20a | 74.45*** |
| Mineral content (%) | 4.53 ± 0.08b | 5.21 ± 0.13a | 5.32 ± 0.17a | 5.40 ± 0.16a | 22.99*** |
| Carbohydrate content (%) | 32.94 ± 0.19b | 34.98 ± 0.24a | 34.84 ± 0.08a | 34.46 ± 0.70a | 17.82*** |
Note
- Means represented by different superscripts in the same row are significantly different at p < .05.
- Abbreviations: SDS40, soybean dried at 40℃ after soaking for 12 hr; SDS50, soybean dried at 50℃ after soaking for 12 hr; SDS60, soybean dried at 60℃ after soaking for 12 hr.
- *** Significant at p < .001 respectively.
3.1.2 Aglycone isoflavone content
In comparison to the control soybeans, the daidzein and genistein contents of the SDSs were significantly increased at higher drying temperatures. In particular, the daidzein and genistein contents of SDS60 were 144.70 μg/g (3.8-fold increase) and 157.87 μg/g (7.2-fold increase), respectively, which were much higher than the contents in the control soybeans. Although glycitein was not detected in the control soybeans, SDS40, SDS50, and SDS60 had glycitein contents of 12.91, 13.83, and 14.37 μg/g, respectively. When soybeans are subjected to long-term hydrothermal treatment, malonyl isoflavone is converted to β-glucoside isoflavone and then further hydrolyzed by the endogenous β-glucosidase present in the cotyledons of soybeans to form aglycone isoflavone (Góes-Favoni et al., 2010). This suggests that the isoflavone glycoside in the raw soybeans was converted into aglycone isoflavone, resulting in increased contents in the SDSs because soybeans, which have a high moisture content, were subjected to prolonged hot air drying after soaking during SDS preparation. Furthermore, aglycone isoflavone contents tended to increase at higher drying temperatures, with the maximum value observed in the SDS60 samples. This suggests that more β-glucoside isoflavone was decomposed by β-glucosidase during the drying process, because the activity of β-glucosidase is optimal at 60°C (Ajisaka et al., 1987). The absorption capacity and bioavailability of aglycone isoflavone are reportedly higher than those of glycoside isoflavone (Izumi et al., 2000). Therefore, SDSs with a higher isoflavone aglycone content would have more functional properties compared to regular soybeans.
3.1.3 Antioxidant components and activity
A comparison of the antioxidant components and activities of the SDSs is presented in Table 2. The total polyphenol and flavonoid contents were similar across all samples, with only minute differences found. The antioxidant components of soybeans are divided into phenolic components and flavonoids. The major phenolic components are ferulic acid, benzoic acid and protocatechuic acid, and their contents are about 95, 57, and 44 μg/g, respectively. Most of the flavonoids are isoflavone, and (+) catechin and quercetin were also studied (Chung et al., 2011). There were no apparent differences in the contents of antioxidant components between the control soybeans and SDSs because, although they can be decomposed or deformed at temperatures exceeding 100℃, such compounds are stable at the drying temperatures used in the SDS drying process (Hwang et al., 2011).
| Control soybean | SDS40 | SDS50 | SDS60 | F-value | |
|---|---|---|---|---|---|
| Daidzein content (μg/g) | 38.00 ± 1.23d | 96.74 ± 0.51c | 116.71 ± 2.55b | 144.70 ± 1.44a | 2,370.68*** |
| Glycitein content (μg/g) | ND | 12.91 ± 0.21c | 13.83 ± 0.07b | 14.37 ± 0.24a | 5329.84*** |
| Genistein content (μg/g) | 21.84 ± 1.23d | 120.36 ± 3.91c | 142.68 ± 2.22b | 157.87 ± 2.32a | 1,658.24*** |
| Total polyphenol content (mg GAE/g) | 4.62 ± 0.37 | 4.59 ± 0.22 | 4.71 ± 0.19 | 4.83 ± 0.13 | 0.57 |
| Total flavonoid content (mg CE/g) | 1.05 ± 0.03 | 1.02 ± 0.04 | 1.09 ± 0.03 | 1.10 ± 0.06 | 2.34 |
| DPPH radical scavenging activity (mg AE/g) | 3.46 ± 0.18 | 3.36 ± 0.17 | 3.47 ± 0.38 | 3.45 ± 0.25 | 0.11 |
| ABTS radical scavenging activity (mg AE/g) | 8.32 ± 0.21 | 8.31 ± 0.39 | 8.42 ± 0.13 | 8.55 ± 0.09 | 0.68 |
Note
- Sample description shown as Table 1. Means represented by different superscripts in the same row are significantly different at p < .05.
- *** Significant at p < .001 respectively.
Furthermore, the DPPH and ABTS radical scavenging activities of all samples were largely similar. The ABTS radical scavenging ability is assessed to analyze the antioxidant activity of insoluble and water-soluble antioxidant components because the compound forms cation radicals in both polar and organic solvents, whereas the DPPH radical scavenging assay is used to measure the antioxidant activity of only insoluble antioxidant components because free radicals only form in organic solvents (Arnao, 2000). Therefore, as polyphenols in soybeans are water-soluble components and flavonoids are insoluble components (Park et al., 2007), the ABTS radical scavenging ability is affected by both the polyphenol and flavonoid contents, whereas the DPPH radical scavenging ability is only affected by the flavonoid content. Thus, it is thought that both the ABTS and the DPPH radical scavenging activities were similar because the contents of antioxidant components in the control soybeans were similar to those in the SDSs. In addition, the ABTS radical scavenging activity might have been higher than the DPPH radical scavenging activity because the antioxidative components of plant foods are composed mainly of water-soluble antioxidants and colorant materials (Lee et al., 2014). Therefore, the absence of any difference in antioxidant properties between the control soybeans and SDSs was likely due to the similar antioxidant components and activities between the two types of soybeans.
3.2 Tofu characteristics
3.2.1 Water content change in the SDSs according to soaking time
To assess whether the soaking time could be shortened to improve the tofu manufacturing process, we monitored the moisture content of the SDSs as a function of soaking time (Figure 1). Compared to the moisture content of the control soybeans, the moisture contents of the SDS40, SDS50, and SDS60 samples increased with increasing soaking time. Therefore, the control soybeans reached an equilibrium moisture condition at 12 hr (Kim et al., 2019), whereas the SDSs reached an equilibrium condition after only 1 hr of soaking. The water reabsorption time of the SDSs in the soaking process may be shorter because of the increase in surface area attributable to porous structures that formed as the soybeans were swelling during the soaking process from the hydration of proteins and the development of vacuoles (Webster & Leopold, 1977). Thereafter, the pores that had developed could not be shrunk due to the hardness of soybeans (Zhang et al., 2008). In addition, the moisture content increased the fastest in SDS60 samples. This is thought to have occurred because the drying rate of SDS60 was higher than those of the other SDSs with a higher drying temperature; thus, the swollen soybeans with porous structures were less affected by shrinkage during the drying process (Kerdpiboon et al., 2007). Therefore, the use of SDSs can shorten the time required for soaking in the tofu manufacturing process because they absorb moisture much faster than regular soybeans.
3.2.2 Proximate composition and quality characteristics
A comparison of the proximate compositions and quality characteristics of the tofu is presented in Table 3. The control tofu and tofu made from SDS40 (SDS40-tofu), SDS50 (SDS50-tofu), and SDS60 (SDS60-tofu) had similar moisture, crude protein, crude fat, crude ash, and carbohydrate contents (all p > .05). Therefore, although using SDSs instead of regular soybeans reduced the soaking time during the tofu manufacturing process from 12 to 1 hr, the nutritional composition of the tofu remained largely unaffected, suggesting that the soluble components could be eluted effectively despite the shortened soaking time. Regarding chromaticity, the L value for the tofu made from SDSs (SDS-tofu) was significantly lower than the value for the control tofu (p < .05), whereas the a and b values were similar. According to the research that the chromaticity changes due to the Maillard reaction by the reducing sugar and amino compounds of the soybean as the soybean is heated (Woo et al., 2018; You & Choi, 2016; Zhang et al., 2018), The reason for the different L values of control and SDS-tofu was probably attributable to the Maillard reaction during drying of SDSs. But when comparing the color difference values (ΔE) of the tofu groups according to the US National Bureau of Standards protocol (Woods & Shouse, 1972), trace differences in chromaticity values (trace 0.0–0.5, slight 0.5–1.5, noticeable 1.5–3.0, appreciable 3.0–6.0, much 6.0–12.0, and very much >12.0) were found across all SDS-tofu groups. Regarding the texture of the tofu, there was no significant difference in hardness, chewiness, or gumminess (all p > .05) among all tofu groups. The tofu yield and amount of tofu residue produced during the manufacturing process were also similar. Therefore, the production of tofu using SDSs, even with the shortened soaking time, does not affect the quality of the final product, suggesting that this ingredient could be used to reduce the manufacturing time.
| Control tofu | SDS-tofu40 | SDS-tofu50 | SDS-tofu60 | F-value | |
|---|---|---|---|---|---|
| Moisture content (%) | 81.82 ± 0.51 | 82.17 ± 0.05 | 82.20 ± 0.09 | 82.01 ± 0.16 | 1.23 |
| Crude protein content (%) | 6.98 ± 0.15 | 6.89 ± 0.23 | 6.81 ± 0.18 | 6.83 ± 0.08 | 1.23 |
| Crude fat content (%) | 5.85 ± 0.12 | 5.86 ± 0.03 | 5.85 ± 0.03 | 5.84 ± 0.05 | 0.59 |
| Mineral content (%) | 0.79 ± 0.01 | 0.76 ± 0.01 | 0.76 ± 0.04 | 0.76 ± 0.02 | 0.02 |
| Carbohydrate content (%) | 4.56 ± 0.61 | 4.31 ± 0.22 | 4.39 ± 0.25 | 4.56 ± 0.11 | 0.38 |
| Chromatility | |||||
| L | 86.77 ± 12.25a | 86.03 ± 0.33ab | 86.16 ± 0.16ab | 85.22 ± 0.60b | 0.58* |
| a | −2.13 ± 0.08 | −2.04 ± 0.19 | −2.03 ± 0.12 | −2.14 ± 0.20 | 2.35 |
| b | 13.17 ± 0.47 | 12.88 ± 0.26 | 13.22 ± 0.40 | 13.35 ± 0.40 | 0.80 |
| ΔE | — | 0.22 ± 0.02a | 0.16 ± 0.02b | 0.17 ± 0.01b | 12.89** |
| Hardness (g) | 892.49 ± 17.76 | 874.92 ± 52.55 | 878.74 ± 64.29 | 848.81 ± 30.23 | 0.49 |
| Chewiness | 333.43 ± 19.31 | 357.64 ± 18.37 | 341.13 ± 14.56 | 357.46 ± 18.67 | 1.39 |
| Gumminess | 315.98 ± 14.46 | 347.18 ± 32.14 | 326.78 ± 35.12 | 318.42 ± 24.19 | 1.84 |
| Yield (%) | 250.50 ± 1.46 | 246.53 ± 3.39 | 249.95 ± 1.67 | 250.53 ± 4.44 | 1.22 |
| Biji weight (dry basis, g) | 127.74 ± 6.93 | 127.23 ± 2.00 | 123.25 ± 5.81 | 125.15 ± 4.19 | 0.49 |
Note
- Means represented by different superscripts in the same row are significantly different at p < .05.
- Abbreviations: SDS-tofu40, tofu prepared with SDS40; SDS-tofu50, tofu prepared with SDS50; SDS-tofu60, tofu prepared with SDS60.
- *, **Significant at p < .05, p < .01 respectively.
3.2.3 Aglycone isoflavone content
When comparing the aglycone isoflavone content among the tofu groups, the contents of SDS-tofu samples were significantly higher than that of the control tofu (p < .05), with SDS60-tofu having the highest content (Table 4). This result indicates that the aglycone isoflavone content is higher in the SDSs than in the control soybeans. In addition, although the soaking time for SDSs in the tofu manufacturing process was shorter than that use for the control soybeans (12 vs. 1 hr), the SDS-tofu still had high contents of aglycone isoflavones. This was probably attributable to the large amounts of isoflavones distributed in the cotyledons of soybeans (Kim et al., 2007) and the more efficient penetration by water into the cotyledons and elution of isoflavones due to the porous structure of the SDSs. In particular, glycitein, which is an aglycone, was detected in the SDS-tofu but not in the control tofu, and the content of glycitein was similar across the different types of SDS-tofu. Given the report of Ajisaka et al. (1987) indicating that β-glucosidase, which converts glycosides into non-glycosides, is inactivated at a temperature of 100℃ (optimal temperature of 60℃), it is thought that glycitin, a glycoside, was not converted to glycitein in the control tofu because it was manufactured by heating soybeans that were soaked for 12 hr, whereas SDS-tofu was prepared from SDSs containing glycitein that had already been converted from glycitin. Therefore, because the contents of aglycone isoflavones, which have high in vivo utility, were higher in the SDS-tofu made with a shorter soaking process than in the control tofu, if SDSs are used, functional components can be easily eluted and tofu with useful functional properties can be manufactured.
| Control tofu | SDS-tofu40 | SDS-tofu50 | SDS-tofu60 | F-value | |
|---|---|---|---|---|---|
| Daidzein content (μg/g) | 16.60 ± 0.07d | 56.54 ± 2.69c | 66.35 ± 3.11b | 88.79 ± 4.34a | 305.19*** |
| Glycitein content (μg/g) | ND | 8.15 ± 0.81c | 8.82 ± 0.87b | 9.12 ± 0.35a | 147.27*** |
| Genistein content (μg/g) | 22.21 ± 0.08d | 62.90 ± 3.31c | 76.48 ± 4.06b | 99.82 ± 5.19a | 233.84*** |
| Total polyphenol content (mg GAE/g) | 1.03 ± 0.09 | 0.99 ± 0.04 | 1.03 ± 0.03 | 1.00 ± 0.04 | 0.42 |
| Total flavonoid content (mg CE/g) | 0.24 ± 0.01 | 0.20 ± 0.04 | 0.22 ± 0.04 | 0.23 ± 0.06 | 0.51 |
| DPPH radical scavenging activity (mg AE/g) | 0.29 ± 0.02 | 0.27 ± 0.04 | 0.29 ± 0.03 | 0.30 ± 0.02 | 0.58 |
| ABTS radical scavenging activity (mg AE/g) | 1.06 ± 0.03 | 1.02 ± 0.02 | 1.05 ± 0.03 | 1.08 ± 0.04 | 1.97 |
- Note Sample description shown as Table 3. Means represented by different superscripts in the same row are significantly different at p < .05.
- *** Significant at p < .001 respectively.
3.2.4 Antioxidant components and activity
The total polyphenol and total flavonoid contents did not differ significantly between the control tofu and SDS-tofu (both p > .05) (Table 4). Thus, the antioxidant components of the control soybeans and SDSs were similar, indicating that the ratios of soybean antioxidant components eluted during the tofu manufacturing process were also similar. Therefore, even when SDSs are prepared with a shorter soaking time (1 vs. 12 hr), sufficient antioxidant component elution is possible.
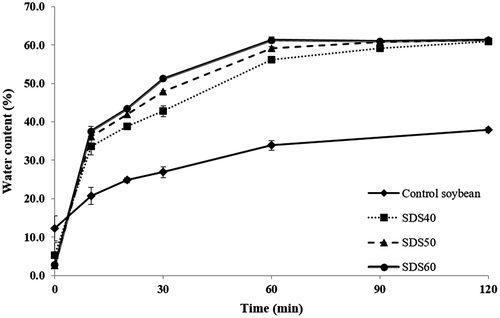
In addition, the control tofu and SDS-tofu exhibited similar DPPH and ABTS radical scavenging activities. Given the above, because the contents of antioxidant components are proportional to the antioxidant activity (Hwang et al., 2011), the activity level should follow a trend similar to those of the antioxidant components. There was no significant difference in FRAP measurements between the control tofu and SDS60-tofu (Figure 2). Similarly, there were no significant differences in SOD-like activity between the control tofu and SDS60-tofu extracts at all concentrations (0.5, 1, or 5 mg/ml) (Figure 2). From the comparison and analysis of the antioxidant activity using diverse methods, the SDS-tofu and control tofu exhibited similar antioxidant activities. Therefore, this result supports the possibility of manufacturing tofu rapidly using SDSs because the efficient elution of antioxidant components can be achieved despite the shorter soaking time, and the antioxidant activity is maintained in the product.
3.2.5 Comparison of cell viability via the MTT assay and anti-inflammatory activity via the NO assay
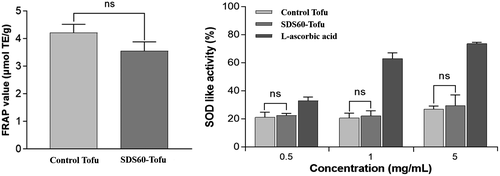
None of the control tofu or SDS60-tofu extract concentrations assessed here (0.1, 0.5, or 1 mg/ml) caused observable cytotoxic effects on RAW 264.7 cells (Figure 3) based on a survival rate of 80% or more. Therefore, we concluded that no cytotoxic substances were generated during the processing of SDSs or their derivatives, confirming the safety of SDSs.
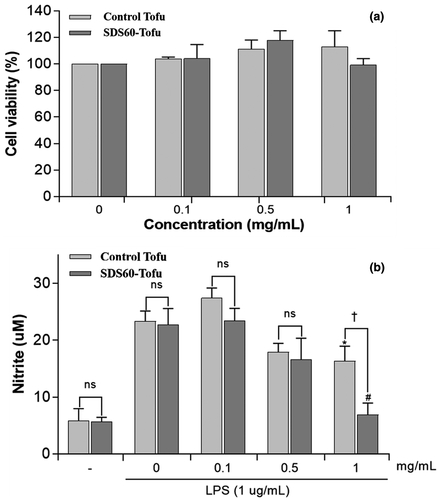
Next, we assessed the NO inhibitory effect of different concentrations of control tofu and SDS60-tofu extracts (0.1, 0.5, or 1 mg/ml) in RAW 264.7 cells (Figure 3). Both the control tofu and SDS60-tofu 1 mg/ml extracts significantly inhibited LPS-induced NO production (both p < .05). Moreover, this NO inhibitory activity was ~40% higher with SDS60-tofu than in with control tofu. In general, when cells are treated with LPS, expression of the iNOS enzyme is induced through the activation of NF-κB, and NO is excessively produced. NO, an intermediate product of an immune response regulated by enzymes, raises the levels of expression of inflammatory mediators such as cytokines more than necessary, causing inflammation leading to pain and fever (Hu et al., 2008). Isoflavones, the representative functional components of tofu and soybean, suppress NO production by lowering the expression of iNOS, which regulates NO, and thereby have an anti-inflammatory effect (Yu et al., 2019). As aglycone isoflavones exhibit stronger anti-inflammatory effects than do glycoside isoflavones (Takano-Ishikawa et al., 2006), the NO production inhibition ability of SDS60-tofu, with its high content of aglycone isoflavones, is expected to be higher than that of control tofu.
3.2.6 Sensory characteristics
The sensory characteristics of the control tofu and SDS-tofu, i.e., color, smell (flavor), taste, and softness, as well as the overall preferences of the panel members are shown in Table 5. The control tofu scored higher for overall sensory characteristics compared to the SDS-tofu. In addition, with an increase in the drying temperature used for preparing the SDSs, the preference for the corresponding SDS-tofu type also increased, but the differences were not significant (p > .05). This was probably attributable to the fact that personal preferences for the sensory characteristics of tofu likely varied widely among the panelists. Factors that control the sensory characteristics of tofu include the quality of the soybeans (Han et al., 2006), the tofu manufacturing process (Kim et al., 2001), and the types of coagulants added (Lee & Hwang, 1997). This study presents the possibility of rapid tofu manufacturing using SDSs because the SDS-tofu was manufactured using the same process, except the soaking time, the same variety of soybeans, and the same coagulant. Thus, the sensory characteristics of the SDS-tofu were not affected despite the shortened soaking time (1 hr instead of 12 hr).
| (n = 32) | |||||
|---|---|---|---|---|---|
| Control tofu | SDS-tofu40 | SDS-tofu50 | SDS-tofu60 | F-value | |
| Color | 7.16 ± 1.46 | 6.19 ± 2.43 | 6.53 ± 1.54 | 7.00 ± 1.59 | 0.18 |
| Flavor | 6.72 ± 1.61 | 5.13 ± 2.04 | 5.72 ± 1.25 | 6.09 ± 1.40 | 0.52 |
| Taste | 6.34 ± 2.07 | 4.84 ± 2.36 | 5.38 ± 1.52 | 5.81 ± 1.64 | 0.33 |
| Softness | 5.69 ± 1.93 | 5.06 ± 2.38 | 5.34 ± 1.99 | 5.50 ± 2.08 | 0.05 |
| Overall preference | 6.22 ± 1.83 | 4.59 ± 2.00 | 5.28 ± 1.63 | 5.47 ± 1.78 | 0.41 |
- Note Sample description shown as Table 3.
4 CONCLUSIONS
In this study, SDSs were prepared, and the quality and functional characteristics of the SDSs were compared with those of regular soybeans. SDS is a semi-processed soybean that had a porous structure which formed in soaking and drying process. Although the SDSs had a higher water absorption rate and aglycone isoflavone content, their general quality and functional characteristics were similar to those of regular soybeans, indicating that soybean characteristics can improved by drying them after soaking. When SDS used as a food raw material, tofu is prepared rapidly by shortening the soaking time of soybean, and it can also be applied to other soybean processing products. In addition, tofu was manufactured using the SDSs, and the quality and functional characteristics of the SDS-tofu were compared with those of tofu made with regular soybeans. According to the results, the soaking time in the tofu manufacturing process can be shortened from 12 to 1 hr by using SDSs, with the quality and functional characteristics of the SDS-tofu remaining similar to those of the control tofu. Because the SDS-tofu was made using SDSs, it also had a higher aglycone isoflavone content. Therefore, this study provides information useful for enhancing tofu products and improving soybean processing. Namely, the use of SDSs can shorten the time required for soaking soybeans, thus enabling a rapid tofu manufacturing process and improving the functional properties of soybean products, as the aglycone isoflavone content is increased in both the prepared soybeans and SDS-tofu.
ACKNOWLEDGEMENTS
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01528501)” Rural Development Administration, Republic of Korea.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
AUTHOR CONTRIBUTIONS
In-Beom Han: Formal analysis; Investigation; Writing – original draft; Writing – review & editing. Seung-Hyeon Cha: Data curation; Formal analysis. Woo-Hyeon Park: Formal analysis. Sang-Beom Park: Formal analysis. Se-Lim Bak: Formal analysis. Eun Woo Jeong: Formal analysis. Seyoung Jung: Formal analysis. Tan Kyung Woo: Formal analysis. Hyeon Gyu Lee: Conceptualization; Methodology; Writing – original draft. Tae Kyung Hyun: Conceptualization; Methodology; Writing – original draft; Writing – review & editing. KEUM-IL JANG: Conceptualization; Funding acquisition; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




