Transient ‘NeoBRAF wild type’ state in a patient with BRAFV600E mutant metastatic colorectal cancer
Graphical Abstract
We report the case of a patient with a BRAFV600E mutant mCRC, with evidence of acquired ‘NeoBRAF wild-type’ (wt) state. The patient, longitudinally assessed by liquid biopsy, obtained a remarkable clinical outcome with a multimodal approach including surgery, systemic treatment and targeted therapy.
In patients with newly diagnosed RAS and BRAFV600E mutant mCRC, longitudinal assessment with liquid biopsy is not routinely used in clinical practice. We report the case of a patient with a BRAFV600E mutant mCRC, with evidence of acquired ‘neoBRAF wild-type’ (wt) state. The patient obtained a remarkable clinical outcome and has been longitudinally assessed by liquid biopsy.
1 INTRODUCTION
Upfront RAS and BRAF tissue testing are required to guide first-line treatment for metastatic colorectal cancer (mCRC). The use of liquid biopsy, which involves targeted molecular profiling using plasma-derived circulating tumour DNA (ctDNA), is being increasingly explored in patients with mCRC. Circulating tumour DNA has been adopted in the management of mCRC to exclude RAS and BRAF mutations when an adequate tissue sample is not available and to identify patients with maintained RAS and BRAF wild-type (wt) status who may benefit from anti-epidermal growth factor receptor (EGFR) rechallenge treatment.1, 2
The presence of RAS and BRAF mutations is an established mechanism of resistance to anti-EGFR therapy. Therefore, in patients with newly diagnosed RAS and BRAFV600E mutant mCRC, longitudinal assessment with liquid biopsy is not routinely used in clinical practice, as a targeted approach with anti-EGFR is not possible.
Recent evidence suggests that in patients with mCRC, RAS mutant clones may be cleared in a phenomenon known as ‘neoRAS wt’, which is characterized by RAS wt disease in plasma. Studies have shown that this phenomenon may occur during treatment and during disease progression and is associated with improved outcomes.3 Limited data is available for patients with the BRAFV600E mutant disease. Herein, we report the case of a patient with BRAFV600E mutant mCRC, longitudinally assessed by liquid biopsy, with evidence of acquired ‘neoBRAF wt’ state and a remarkable clinical outcome.
2 CLINICAL CASE
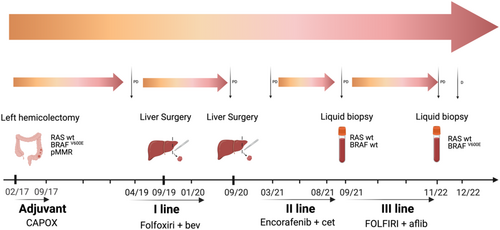
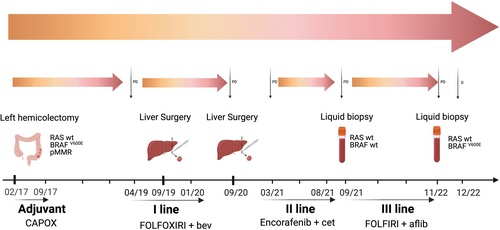
In February 2017, a 58-year-old woman with left-sided CRC underwent left hemicolectomy with the diagnosis of poorly differentiated adenocarcinoma pT3 pN2a (4/21) pV0 pR0. In the absence of distant metastases, CAPOX adjuvant chemotherapy was administered for 6 months from April to September 2017.
In March 2019, during regular follow-up, CEA increased and a CT scan showed a new liver lesion, at the VII–VI segment, confirmed by liver MRI. Molecular testing was performed on the primary tumour, by the Oncomine™ Solid tumour DNA kit using Ion Torrent S5 XL system (ThermoFisher Scientific), covering 22 genes with a limit of detection of 2%–5%. BRAFV600E was the only mutation identified by the analysis.
Upon multidisciplinary evaluation, perioperative chemotherapy was recommended. Based on the available results from a non-preplanned analysis of the TRIBE2 study, chemotherapy with FOLFOXIRI plus bevacizumab was initiated, resulting in a partial response.4
Robotic resection of the VIII liver segment was performed in September 2019. The patient completed perioperative treatment in January 2020. A liver relapse was detected in September 2020. After multidisciplinary assessment, in October 2020, the patient underwent robotic liver resection, with histological evidence of poorly differentiated adenocarcinoma.
In February 2021, a CT scan revealed multiple metastases in the lung, peritoneum and liver. Based on the BEACON trial findings, cetuximab plus encorafenib was started within an expanded access program in March 2021.5
The patient experienced clinical benefit, with an early reduction in symptom burden and improved performance status.
The first CT scan showed stable disease based on RECIST 1.1, with evidence of lung response in May 2021. In August 2021, after 12 cycles, the patient's clinical condition worsened, CT scan revealed liver progression.
Therefore, a third-line systemic treatment with FOLFIRI plus aflibercept was initiated in September 2021. In addition, to better understand the mechanisms of resistance to targeted treatment, a liquid biopsy was performed. As previously reported, plasma fraction was obtained and stored within 2 h from the blood drawing, cfDNA was extracted according to the manufacturer's instructions.6 The NGS analysis was performed using the Oncomine™ Precision Assay on Genexus™ Integrated Sequencer (ThermoFisher Scientific), allowing the detection of somatic mutations, copy number variations and fusions genes for 50 cancer-related genes with a limit of detection of 0.1%. The analysis reported no alterations, showing a wt status, including BRAF.
A clinical benefit was observed early in the course of FOLFIRI plus aflibercept, with pain control and tolerable side effects. Radiological assessment by CT scan was performed every 3 months, with stable disease and response to the lung lesions. In November 2022, after 14 months, the patient reported radiological and clinical progression. To inform the subsequent line and to assess whether treatment with anti-EGFR agents was appropriate, a liquid biopsy was ordered. Following the same procedures, the analysis identified a BRAFV600E mutation. Therefore, standard fourth-line therapy with regorafenib or trifluridine-tipiracil was indicated. To date, persistent COVID-19 delayed the initiation of systemic treatment. A decline in performance status upon readmission prevented the patient from receiving any active treatment. Due to clinical progression, the patient died in December 2022. A timeline overview of the patient's management is summarized in Figure 1.
3 DISCUSSION
The BRAFV600E mutation is found in around 8%–12% of patients with mCRC. It is a known prognostic biomarker associated with poor prognosis and a predictive factor of limited response to anti-EGFR treatment.7
Recently, the introduction of a targeted chemo-free regimen with an anti-BRAF agent, encorafenib, plus an anti-EGFR monoclonal antibody, cetuximab, has led to a significant increase in median survival.5 However, the occurrence of acquired resistance precludes further benefits and limited data is available regarding subsequent therapies.
Recent data demonstrated satisfying concordance in the detection of BRAFV600E mutation between tissue and plasma. Indeed, the presence of liver metastases and unresected primitive tumours have been significantly associated with ctDNA detection.8 These results suggest that ctDNA testing is an appropriate method to assess BRAF status.
Limited data is currently available on ctDNA longitudinal assessment in patients with BRAFV600E mutant disease and on the occurrence of a ‘neoBRAF wt’ state.
Similar to RAS mutant disease, the presence of a ‘neoBRAF wt’ state might be associated with pre-analytical issues, heterogeneous test sensitivity level, inadequate sampling, low tumour DNA levels and specific sites of metastasis, such as lymph nodes, lung or peritoneum.
Further information may be obtained from an additional tissue biopsy. However, in patients with chemo-refractory CRC, without offering an innovative targeted therapy, a less invasive approach with liquid biopsy represents an appropriate method to obtain molecular information outside of clinical trials.
In the present clinical case, a decrease in ctDNA levels in response to anti-BRAF therapy might be associated with the decrease in the number of BRAFV600E mutant clones and the occurrence of a ‘neoBRAF wt’ state. This phenomenon might explain the favourable outcome of the patient, who reported with a multimodal approach an overall survival of 71 months from the initial diagnosis and a progression-free survival in the third line of 14 months.
A recent study reported that BRAF mutant allele fraction (MAF) is a prognostic biomarker and surrogate for tumour load in patients treated with anti-BRAF targeted therapy.9 These observations are consistent with previous findings in RAS mutant cohorts. Recent studies have investigated in patients with RAS mutant mCRC the phenomenon of ‘neoRAS wt’ state and have suggested an association between the clearance of RAS mutations in ctDNA and improved clinical outcomes. The ‘neoRAS wt’ state has led to the hypothesis that patients with RAS mutant mCRC might benefit from a longitudinal ctDNA assessment to inform a strategy including anti-EGFR treatment.3, 10
These findings warrant further investigation also in the BRAFV600E population, emphasizing the development of individualized strategies in the context of a precision medicine approach.
Therefore, despite not currently being recommended in guidelines, in patients with BRAFV600E mutant mCRC, adequate sampling and permissive burden of disease, ctDNA longitudinal assessment might provide further insight into tumour heterogeneity and disease complexity.
AUTHOR CONTRIBUTIONS
CC and AA contributed to the drafting of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content and approved the final version for submission.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
CC reports personal honoraria for advisory roles for Bayer and Sandoz; ADS reports BMS relative employee and personal honoraria for educational activities from Amgen; AA reports personal honoraria for advisory board roles and educational activities from AstraZeneca, Amgen, MSD, Servier; AR and SF have no relevant financial or non-financial interests to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.