Immunotherapy and lung cytopathology: Overview and possibilities
Abstract
Immunotherapy has become a promising cancer treatment in the past decade, and IHC is the most commonly used testing method for PDL-1/PD1 evaluation. In general, PD-L1 assays can be performed on both FFPE specimens and cytological samples. However, their use on smears is not yet well-established or validated. Nowadays, digital images and advanced algorithms can aid in interpreting PD-L1 in cytological samples. Understanding the immune environment of non-small cell lung cancer (NSCLC) is critical in developing successful anticancer immunotherapies. The use of a multiplexed immunofluorescence (mIF) assay on cytological samples obtained through minimally invasive methods appears to be a viable option for investigating the immune environment of NSCLC. This review aims to briefly summarize the knowledge of the role of cytopathology in the analysis of PD-L1 by immunocytochemistry (ICC) and future directions of cytopathology in the immunotherapy setting.
Graphical Abstract
Although not formally validated on cytology specimens, cytopathologists are increasingly being asked to assess the expression of PD-L1 in NSCLC to select patients for treatment with PD1/PD-L1 inhibitors. PD-L1 staining and quantitation on cytology samples is feasible, provided that appropriate protocols and validation studies are in place. PD-L1 image analysis algorithms can improve scoring consistency. Digital pathology combined with multiplex immunofluorescence (IF)/IHC assays could be a powerful tool for PD-L1 assessment. Cytological samples can be a reliable source for PD-L1 IHC analysis and have the potential to be used with new approaches, such as digital pathology and multiplex assays.
1 INTRODUCTION
The pathological diagnosis of lung cancer has become a multistep process. Advances in next-generation sequencing (NGS) and other high-throughput genomic profiling platforms have transformed the classification of lung cancer from histopathological descriptions to precise molecular and genetic identities, thereby providing the rationale for therapeutic strategies and the roadmap for the treatment plan. The College of American Pathologists, International Association for the Study of Lung Cancer and Association for Molecular Pathology (CAP, IASLC, AMP) clinical practice guidelines for molecular testing of lung cancer include classifying non-small cell lung cancer (NSCLC) into adenocarcinoma and squamous cell carcinoma by morphology, with the assistance in poorly differentiated tumours, of a limited immunoperoxidase panel of antibodies, as well as saving adequate material for molecular testing.1 Consequently, pathologists face the challenge of performing more tests on smaller specimens.
Patients with NSCLC are often diagnosed at advanced-stage disease and are not candidates for surgical resection of the primary tumour.2 Often, only small biopsies or cytological specimens are available as pathological material for molecular testing. The rapid advances in targeted therapies and cancer immunotherapies in NSCLC have made the optimization and implementation of molecular studies in cytology samples a priority. Several studies have already demonstrated the utility of cytological specimens in molecular testing.1-8
A variety of minimally invasive procedures are available for sampling, depending on the location of the tumour and/or metastasis (Table 1). The most commonly used procedures are computed tomography-guided fine needle aspiration of the lung (CT-FNA; frequently coupled with core needle biopsies), bronchoscopy-guided FNA (BC-FNA) with or without electromagnetic navigation (EMN), endobronchial ultrasound (EBUS)—guided FNAs of lymph nodes, endoscopic ultrasound (EUS)—guided fine needle aspiration and direct aspiration of superficial metastatic lesions. Rapid on-site evaluation (ROSE) is a valuable tool that enhances the performance of all these procedures. ROSE done by a cytopathologist has improved the adequacy of FNA, allowing the assessment of cellular content, tumour cell yields and adequate material for molecular testing.8 The pathologist can help the interventionist to ‘redirect’ the procedure to obtain samples adequate for ancillary studies. ROSE maximizes cytology samples for molecular testing, including important steps for sample acquisition, processing and selection.6, 7
| Approach | Target | Characteristics |
|---|---|---|
| CT-guided | Peripheral lesions | CNB |
| BC | Central lesions | FNA/FNAB |
| EBUS | Central lesions + LN |
Possibility of EMN for more distant lesions Diagnosis an Staging |
| EUS | Central lesions + LN |
Possibility of sampling several distant locations Diagnosis and staging |
| Direct FNA | Superficial lesions | Performed by cytopathologist |
2 PD-L1 IMMUNOHISTOCHEMISTRY (IHC)
Although not formally validated on cytology specimens, cytopathologists are increasingly being asked to assess the expression of PD-L1 in NSCLC to select patients for treatment with PD1/PD-L1 inhibitors. PD-L1 protein expression, as detected by immunohistochemistry (IHC) testing, has been used as a predictive biomarker assay for anti-PD1/PD-L1 therapies.9-12 IHC assays for assessing PD-L1 expression are already approved by the U.S. FDA and the EMA for both first line and second line therapy with immunotherapies. PD-L1 staining and quantitation on cytology samples is feasible, provided that appropriate protocols and validation studies are in place. Inherent challenges associated with the handling of cytology compared with histology specimens, including the use of fixatives, is an important issue. Compared with histology specimens, a broader range of fixatives are used for cytology specimens and, in the case of cells blocks, the preservative solutions used before formalin fixation and paraffin embedding also vary greatly. In case of alcohol-fixed samples, the PD-L1 IHC protocol may require some adjustments. Emerging data from cell blocks and matched histologic specimens from our group and others suggests that cytologic material is as good as histologic material for PD-L1 IHC.13-16 Furthermore, the development of cytologic criteria and interpretation of the PD-L1 IHC on cell blocks has already been proposed. Adjustments of the standard PD-L1 interpretation parameters are required to account for differences in the processing method of the cytologic material.
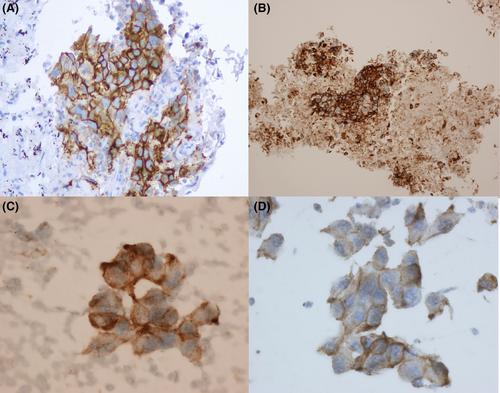
On direct smears, the PD-L1 IHC can be performed and evaluated in the same way as with histologic material, using the corresponding clinically validated commercial assay. As with the histologic material, PD-L1 positive staining in lung cancer cytology specimens is defined as complete circumferential or partial linear cell membrane staining of tumour cells at any intensity. Only cytoplasmic staining in tumour cells is not considered as positive for scoring purposes. Evaluating at least 100 viable tumour cells is recommended. Because entire cells are present on direct smears, tumour cells often display a folded cell membrane showing a thick and strong membranous positivity.12 Cytoplasmic staining is often present, light and diffuse due to the 3-dimensionality of the cell (Figure 1A–D). Heterogeneity within and across the monolayered areas of tumour cells is also observed frequently. The PD-L1 IHC 22C3 pharmDx assay often displays a perinuclear dot-like staining with variable intensity. Quantification of PD-L1-positive immune cells will likely be complicated.17
Scoring PD-L1 IHC is often a challenging task for pathologists. The main problem of PD-L1 scoring is that may not always be reproducible, as a number of factors can generate challenges for pathologists, such as the temporal and spatial heterogeneity of PD-L1 expression in tumours, the pre-analytical variables that can affect staining quality (e.g. fixation time, antigen stability), the presence of endogenous material (e.g. anthracotic pigment), the non-specific staining (e.g. necrotic tissue), among others. These challenges can potentially lead to significant inter-observer variability.17
3 ALGORITHM-BASED ASSESSMENT OF PD-L1 EXPRESSION
New computational tools, such as machine learning and deep learning, can be used by pathologists to assess digital slide images confidently and objectively, with the promise of improved and unbiased diagnostics. Recently, Roche® has introduced the uPath PD-L1 (SP263) image analysis tool, a CE-IVD-approved automated digital pathology algorithm for PD-L1 scoring in NSCLC. The algorithm is integrated within the Roche uPath enterprise software and has been validated on the VENTANA PD-L1 (SP263) assay. It detects PD-L1 positive and negative tumour cells within a pathologist-annotated viable tumour region (Figures 2 and 3). The PD-L1 image analysis algorithm can improve scoring consistency and provide more accurate treatment decisions for patients with NSCLC.
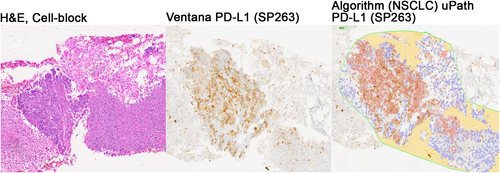
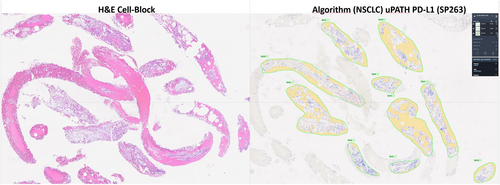
It has been shown that image analysis algorithms are likely to identify a higher number of PD-L1-positive patients as compared with manual scoring.18 This is likely a result of multiple factors. Image analysis algorithms exhaustively analyse and classify every cell on the tissue image, thereby providing a highly precise measure of the true PD-L1 positivity on tumour cells. Although the algorithm is trained to accurately classify cells, some level of misclassification is expected. Misclassifications could be due to factors such as the presence of clustered, membranous PD-L1-positive tumour cells overlapping with PD-L1-negative tumour cells or misclassification of PD-L1-positive immune cells as PD-L1-positive tumour cells.18 New computational tools have been shown to be precise, reproducible, scalable and exhaustive approaches to quantifying PD-L1 expression in routine practice.
4 MULTIPLEX IMMUNOLABELLING ASSAYS IN CYTOLOGICAL SAMPLES
The study of the immune environment of tumours can give valuable insights into their complex and heterogeneous composition. Specifically, understanding the immune environment of NSCLC is important for designing effective anticancer immunotherapies.19 Digital pathology combined with multiplex immunofluorescence (IF)/IHC assays could be a powerful tool for PD-L1 assessment. The use of multiplex immunolabelling protocols allows for the simultaneous visualization and quantification of several cellular markers in a single tissue section. Several high-throughput image analysis workflows have been already proposed. They permit the assessment of PD-L1 expression in different cellular compartments, such as the tumour compartment, defined by the positivity of the epithelial cell marker cytokeratin, and the stromal compartment, defined by the negativity of the cytokeratin marker. In addition, spatial architecture and arrangement of tumour-infiltrating immune cells, studied by the expression of the macrophage marker CD68 and the T-cell marker CD8, can be depicted with the potential to increase the level of confidence in the identification of specific immune cell subtypes co-expressing PD-L1. Therefore, multiplex assays may enable cytopathologists to refine PD-L1 scores in lung cancer cytology. However, cytological samples pose practical problems due to a lack of tissue architecture. This issue can be addressed by having a sequential H&E slide to guide the annotation of regions of interest for image analysis.
Up to 70% of patients with NSCLC are diagnosed at advanced stages and tissue biopsies are often limited or cannot be taken.16 With the increasing diversification of ancillary applications applied to cytological samples, the development of multiplex assays is critical.20 We have previously shown that multiplex immunofluorescence assays might be used on cytological samples taken with minimally invasive methods to explore the immune environment of NSCLC. The staining pattern of every individual marker within the multiplex antibody panel is similar to the one described for lung tissue using conventional immunohistochemistry. More importantly, the multiplex immunolabelling workflow and image analysis could be fully performed on cytological samples, enabling future routine analysis of tissue and cancer morphology and cell phenotypes (Figure 4).
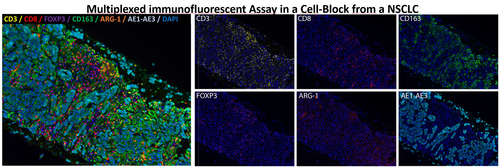
Whole-tissue images from multiplex immunofluorescence assays can be analysed. During image analysis workflow, the corresponding H&E digital section can aid in the selection of better cases or areas where tumour architecture is preserved. This is relevant in cytological samples obtained by transoesophageal ultrasound-guided fine needle aspiration (EUS-FNA).
Further validation studies using large series of paired cytological samples and surgical resection specimens are needed to establish the real value of multiplex assays on samples taken with minimally invasive procedures.
5 CONCLUSION
This review shows that cytological samples can be a reliable source for PD-L1 IHC analysis and have the potential to be used with new approaches, such as digital pathology and multiplex assays. Nonetheless, there are challenges, which still need to be addressed. In particular, the standardization of PD-L1 IHC staining in cytological samples could be reliably implemented across laboratories.
ACKNOWLEDGEMENTS
We thank Nerea Gomez, Bsc; Vanesa Ocon Cruz, Bsc; and Jaione García Martínez, Bsc, for the expert technical help.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
De-identified study data may be made available at publication upon request to the corresponding author. Data sharing will only be available for academic research, instead of commercial use or other objectives. A data use agreement and institutional review board approval will be required as appropriate.