Potential and Challenges of Transcranial Photobiomodulation for the Treatment of Stroke
Funding: This work was supported by research funding from the Research Centre for Chinese Medicine Innovation of the Hong Kong Polytechnic University (Ref No. P0041139) awarded to Professor S Ng and her team and by the PolyU Distinguished Postdoctoral Fellowship Scheme.
ABSTRACT
Photobiomodulation (PBM), also known as low-level laser therapy, employs red or near-infrared light emitted from a laser or light-emitting diode for the treatment of various conditions. Transcranial PBM (tPBM) is a form of PBM that is delivered to the head to improve brain health, as tPBM enhances mitochondrial function, improves antioxidant responses, reduces inflammation, offers protection from apoptosis, improves blood flow, increases cellular energy production, and promotes neurogenesis and neuroplasticity. As such, tPBM holds promise as a treatment for stroke. This review summarizes recent findings on tPBM as a treatment for stroke, presenting evidence from both animal studies and clinical trials that demonstrate its efficacy. Additionally, it discusses the potential and challenges encountered in the translation process. Furthermore, it proposes new technologies and directions for the development of light-delivery methods and emphasizes the need for extensive studies to validate and widen the application of tPBM in future treatments for stroke.
1 Introduction
Photobiomodulation (PBM), formerly known as low-level laser therapy and then low-level light therapy, is a promising modality that involves the irradiation of cells and tissues with photons of specific wavelength ranges. This non-invasive and non-thermal approach stimulates a biological response, particularly at red and near-infrared (NIR) wavelengths (600–700 nm and 760–1200 nm, respectively) and extending to blue and green wavelengths [1-3]. The availability of cost-effective and safe light-emitting diodes (LEDs) rendered the use of expensive lasers unnecessary in many cases, leading to low-level laser therapy being re-named as low-level light therapy (LLLT). Subsequently, to solve the problem of “low level” being ambiguous, LLLT was renamed as PBM [4, 5]. NIR light has gained popularity in PBM due to its tissue penetration properties and overall efficacy being superior to those of other wavelengths of light [6-8]. PBM has been applied in many treatments, such as for wound healing stimulation [9], pain reduction [10], inflammation alleviation in musculoskeletal conditions [11-13], and mitigation of cancer-therapy side effects [14, 15]. Moreover, in recent years, there has been a growing interest in exploring the use of transcranial PBM (tPBM) for treating various neurological diseases, such as stroke [16-18], traumatic brain injury (TBI) [19, 20], Alzheimer's disease (AD) [21, 22], Parkinson's disease (PD) [1, 23], and depression [4].
tPBM is considered to be a safe intervention with no deleterious effects on the structure and function of the brain [24, 25]. However, the inconsistencies in efficacy observed across various studies have raised questions about its optimal application and the underlying mechanisms that govern its effects. Significant challenges, such as the determination of effective dosimetry parameters, and variation in treatment protocols, must be addressed to fully realize its potential for clinical use. In this article, we review recent findings on the potential of tPBM for stroke and summarize lessons learned from previous animal and human studies. We aim to identify potentials and challenges that may be encountered during the translation of tPBM from the laboratory to the clinic for use as a cerebral protective or restorative tool in stroke management. As such, we hope that this review will enhance the understanding and application of tPBM in the treatment of stroke.
2 Roles of tPBM for Stroke Recovery
Stroke is a complex and dynamic process characterized by both acute and reparative phases. In the early acute phase of ischemic stroke, the disruption of oxygen and glucose supply to the brain leads to mitochondrial dysfunction, which is a hallmark of hypoxic-ischemic brain injury [26]. tPBM treatment applied in this phase targets neurons by utilizing wavelengths of light that can be absorbed by chromophores, such as cytochrome C oxidase (CCO), the primary photoreceptor for red-to-NIR light in the mitochondria of neurons. The stimulation of CCO leads to an increase in ATP production, a decrease in mitochondrial nitric oxide (NO) concentrations, and a reduction in reactive oxygen species (ROS) concentrations [27], all of which alleviate oxidative stress and inflammation, thereby helping to mitigate neuronal damage [28]. As stroke progresses to subacute and chronic phases, tPBM treatment enhances hemodynamic and neuroplasticity to provide bioenergetic relief and also enhances various biological reactions. tPBM treatment activates secondary cell-signaling pathways, enhances cerebrovascular oxygenation, increases cerebral blood flow (CBF), exerts anti-inflammatory effects, and promotes the production of endogenous neurotrophic factors, such as nerve growth factor and brain-derived neurotrophic factor (BDNF) [25]. These molecular effects of tPBM treatment contribute to the remodeling of synaptogenesis, neurogenesis, and neuroplasticity, resulting in long-lasting support of stroke recovery. In addition, tPBM has been shown to generate considerable improvements at the behavioral level after stroke, such as improvements in motor skills, memory, mood, and sleep quality [29-31]. In summary, tPBM treatment is able to activate endogenous mechanisms to promote neuroprotection and repair damaged neuronal pathways throughout stroke recovery (Figure 1).
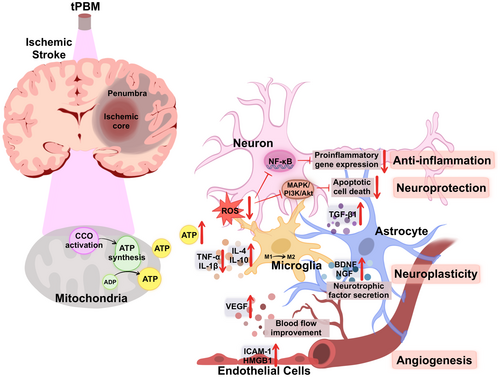
Furthermore, the beneficial effects of tPBM may not solely rely on direct light penetration into brain cells, as it can also have systemic effects. That is, tPBM irradiates the blood, enhancing the activity of circulating blood, resulting in positive effects beyond the irradiated region, a phenomenon known as “remote photobiomodulation” [32, 33]. For example, tPBM treatment of patients with PD led to significant mobility improvements [34], despite it being unlikely that tPBM directly protects vulnerable brain cells located in deep brain regions such as the nigrostriatal pathway. Instead, these benefits may stem from enhanced neural function and communication throughout the brain. Similarly, studies have demonstrated that tPBM had beneficial effects on brain histology even when the head was shielded from direct light [35], highlighting the remote effects of tPBM applied to areas other than the head [36, 37].
3 tPBM Therapy in Animal Models
Preclinical testing of tPBM therapy in animal models has shown promising results in decreasing infarct size, improving motor function, reducing long-term neurological deficits and mortality, and enhancing spatial learning and memory (Table 1) [38-43]. However, there may be variability in the experimental protocols used, and various methodological factors can significantly influence the outcomes of both preclinical and clinical stroke treatment studies.
| Authors | Animal model | Parameters of light | Onset of stroke | Irradiation sites | Duration time | Evaluation tools | Outcomes |
|---|---|---|---|---|---|---|---|
| Leung 2002 [44] | SD rat, 1 h transient MCAO | Laser, 660 nm, average power 8.8 mW, energy density 2.64 J per cm2 /min, pulse frequency 10 kHz | Immediately after stroke | Cerebrum | 1, 5, or 10 min | NOS, TGF-β1 | Low energy laser suppressed NOS activity and up-regulated TGF-β1 expression |
| Lapchak 2004 [45] | Rabbit, RSCEM | Laser, 808 ± 5 nm, 7.5 or 25 mW/cm2, CW | 1, 3, 6, or 24 h after embolization | Posterior to bregma on the midline, both ipsilateral and contralateral sides | 2 or 10 min | Effective stroke dose (P50), clot amount (mg) | Laser treatment improved behavioral performance if initiated within 6 h and the effect was durable |
| DeTaboada 2006 [46] | SD rat, permanent MCAO | Laser, 808 nm, 7.5 mW/cm2, CW | 24 h post-stroke | 3 mm dorsal to the eye and 2 mm anterior to the ear, ipsilateral, contralateral, and bilateral | 2 min at each point | Modified neurological score | LLLT improved neurological deficits at delayed 14, 21, and 28 days post-stroke at different skull locations |
| Oron 2006 [47] | (1) SD rat; (2) Wistar rats, permanent MCAO | Laser, 808 nm, 7.5 mW/cm2, (1) CW; (2) both CW and PW (70 Hz) | 4 and 24 h post-stroke | 3 mm dorsal to the eye and 2 mm anterior to the ear, contralateral | 2 min at each point | Modified neurological severity scores, infarct volume, BrdU, DCX | LLLT issued 24 h after acute stroke induced functional benefit and neurogenesis |
| Lapchak 2007 [48] | Rabbit, RSCEM | Laser, 808 ± 5 nm, power density 7.5 mW/cm, (1) CW; (2) PW, 300 μs pulse at 1 kHz; (3) PW, 2 ms pulse at 100 Hz | 6 or 12 h following embolization | Posterior to bregma on the midline | 2 min | Effective stroke dose (P50), clot amount (mg) | PW mode NILT resulted in significant clinical improvement within 6 h from stroke onset |
| Lapchak 2008 [49] | Rabbits, large clot embolism-induced stroke | Laser, 808 ± 5 nm, power density 10 mW/cm2 | 90 min after embolization | Posterior to bregma on the midline | 2 min | Hemorrhage rate, volume | TLT administration did not affect the tPA-induced increase in hemorrhage incidence. TLT may be administered safely either alone or in combination with tPA |
| Lapchak 2010 [50] | Rabbit, RSCEM | Laser, 808 nm, (1) 7.5 mW/cm2, cortical fluence 0.9 J/cm2, CW; (2) 37.5 mW/cm2, cortical fluence 4.5 J/cm2, PW, 100 Hz; (3) 262.5 mW/cm2, cortical fluence 31.5 J/cm2, PW, 100 Hz | 5 min post-embolization | Posterior to bregma on the midline | 2 min | ATP | NILT increased cortical ATP content, and this was correlated with cortical fluence and the mode of NILT delivery. PW NILT delivered 5 and 35 times more energy than CW |
| Uozumi 2010 [51] | C57BL/6J mice, transient BCCAO | Laser, 808 nm, power densities 0.8, 1.6 and 3.2 W/cm2 | 30 min before BCCAO | Left hemisphere, 2 mm posterior to and 3 mm left of the bregma | 15–45 min | CBF, brain temperature, TUNEL | NIR laser irradiation increased cerebral blood flow and was concerned with NOS activity and NO concentration |
| Yip 2011 [52] | SD rat, 1 h transient MCAO | Laser, 660 nm, average power 8.8 mW, 10 kHz, 2.64 J/cm2, 13.2 J/cm2, and 24.6 J/cm2 | Immediately following MCAO | Cerebrum | 1, 5, or 10 min | Akt, BAD, Bcl-2, caspase 9, caspase 3 | LLI protected the brain by upregulating Akt, pAkt, pBAD, and Bcl-2 expression and downregulating caspase 9 and caspase 3 expression |
| Huisa 2013 [53] | Rabbit, RSCEM | Laser, 808.5 nm, 7.5–20 mW/cm2, 10.8 mW/cm2 for the single therapy and 20 mW/cm2 for the triple therapy | 2, 3, 4 and 5 h post-embolization | Skin overlying the skull | 2 min for each dose | Effective stroke dose (P50), clot amount (mg) | Triple treatment had a greater improvement when compared with single or sham treatment |
| Lapchak 2016 [54] | Rabbit, RSCEM | Laser, 808 ± 5 nm, 7.5 mW/cm2, CW | 1 h post embolization | Posterior to bregma on the midline | 2 min | Effective stroke dose (P50), ICH rate, ATP | Combination of TLT-tPA enhanced ATP production, and produced an additive effect on ATP levels |
| Lee 2016 [55] | C57BL/6J mice, PT | LED, 610 nm, power intensity 1.7 mW/cm2, energy density 2.0 J/cm2 | 2 days before the ischemic event | Right midpoint of the parietal bone and the posterior midline of the seventh cervical vertebra | 20 min, twice a day for 2 days | Infarct volume, COX-2, p65, p38, JNK, ERK, iNOS, TNF-α, IL-1β, IL-6 | LLLT improved functional benefits by suppressing neuroinflammation, such as inhibiting inflammatory mediators and MAPK/NF-κB activation. LLLT also prevented BBB disruption |
| Meyer 2016 [56] | Rabbit, RSCEM | Laser, 808.5 nm, 111 mW, 100 Hz | 2 h post-embolization | Head | 2 min | ES50, the weight of clots (mg) | TLT improved behavior with a triple TLT regimen |
| Lee 2017 [39] | C57BL/6J mice, PT | LED, 610 nm, 1.7 mW/cm2, 2.0 J/cm2 | 4 h post-ischemia | Right midpoint of the parietal bone and the posterior midline of the seventh cervical vertebra | 20 min, twice a day for 3 days | Infarct volume, neurological score, NF-κB p65, IL-1β, IL-18, JNK, ERK, NLRP3, TUNEL | LED therapy reduced infarct, improved function, and decreased neuroinflammation in the ischemic cortex. It attenuated the NLRP3 inflammasome, suppressed TLR-2/MAPK/NF-κB, and decreased cell death |
| Lee 2017 [38] | C57BL/6J mice, PT | LED, 610 nm, power intensity 1.7 mW/cm2, energy density 2.0 J/cm2 | Immediately, 4 days or 10 days after ischemia | Right midpoint of the parietal bone and the posterior midline of the seventh cervical vertebra | 20 min, once a day for 7 days | Behavior tests, brain atrophy, BrdU, Iba-1, NeuN, DCX, CD31, BNDF | Acute and subacute LED therapy improves long-term functional recovery through neuron/astrocyte proliferation, angiogenesis, and increased BDNF |
| Lee 2017 [57] | C57BL/6J wild-type and eNOS-deficient mice, MCAO | LED, 610 nm, power intensity 1.7 mW/cm2, energy density 2.0 J/cm2 | Before cerebral ischemia | Right midpoint of the parietal bone and the posterior midline of the seventh cervical vertebra | Twice a day for 20 min for 2 days | Brain infarct, edema volume, neurological scales, eNOS, Akt | Pretreatment with LED reduced brain damage by the stimulation of eNOS phosphorylation via the PI3K/Akt pathway |
| Sanderson 2018 [58] | SD, global brain ischemia and reperfusion | LED, 750 nm, 810 nm, 950 nm, power density at the scalp 50 mW/cm2 | At the onset of reperfusion | Intact scalp and skull | 120 min | COX, ROS, mitoSOX, Iba-1, NeuN | NIR therapy preserved neurologic function, and protected neuron loss in CA1 hippocampus post-reperfusion |
| Yang 2018 [59] | Rats, PT | Laser, 808 nm, 25 mW/cm2 at cerebral cortex tissue level, 350 mW/cm2 on the scalp, CW | 1 day following PT | Infarct injury area (1.8 mm anterior to the bregma and 2.5 mm lateral from the midline) | 2 min daily, from day 1 to day 7 | Infarct volume, BrdU, NeuN, MAP2, DCX, Iba-1, CCO, ATP, IL-4, IL-10, IL-6, TNF-α, IL-18, CD32, CD86, iNOS, TGF-β, CD206 | PBM promoted neurogenesis after ischemic stroke. The mechanisms may rely on (1) promotion of proliferation and differentiation of internal neuroprogenitor cells in the peri-infarct zone; (2) improvement of the neuronal microenvironment by altering inflammatory status and promoting mitochondrial function |
| Argibay 2019 [60] | SD rat, 1 h transient MCAO | LED, 830 nm, 10 mW/cm2, 0.28 J/cm2 at brain cortex, CW | 24 h after stroke | Head | (1) 30 min, 1 day/week for 12 weeks; (2) 30 min, 3 days/week for 12 weeks | Infarct volume, sensorimotor test, Fox3, Ki-67, DCX | PBM did not reduce infarct size or improve functional recovery |
| Fonseca 2019 [61] | Wistar rat, hemiplegia, electrode implanted in the internal capsule | LED, 904 nm, 110 mW, 7 J/cm2 | Day 4, 7, 21 post-stroke | Frontal region of the brain | 63 s, daily, for 3, 7, and 21 days | Neurological behavior test, H&E staining | LED irradiation may beneficially affect neurogenesis, reduce edema and density of the cerebral parenchyma, increase muscle resistance and animal motor behavior, especially on treatment days 7 and 21 |
| Salehpour 2019 [62] | BALB/c mice, 20 min transient BCCAO | Laser, 810 nm, irradiance 6.66 W/cm2, 200 mW maximum output power | On the day of ischemia | Midline of the dorsal surface of the head in region between eyes and ears | Once a day for 14 days | Neurological severity score, ROS, ATP, SIRT1, PGC1-α, iNOS, TNF-α, IL-1β | PBM and Coenzyme Q10, alone or combined, had beneficial effects by reducing neuroinflammatory markers like iNOS, TNF-α, and IL-1β |
| Vahabzadeh-Hagh 2019 [63] | C57BL/6J mice, mouse focal cerebral ischemia model | Laser, 808 nm, 37 mW/cm2, 4.4 J/cm2, CW | At day 5, 9, and 13 after stroke onset | Head | 2 min | MAP2, HMGB1, CD38, BDNF, IGF-1, FGF-2, VEGF, CD34 | Repeated NIR irradiation increased HMGB1 in the peri-infarct cortex, leading to higher accumulation of EPCs |
| Strubakos 2020 [64] | Rat, 90 min transient right MCAO | LED, 750 and 950 nm, 200 mW/cm2 | Immediately initiated at reperfusion onset | 1.5 cm from the shaved scalp | 120 or 240 min | Infarct volume | NIR attenuated brain injury and evoked a sustained reduction in infarct volume following ischemic stroke |
| Guo 2021 [65] | Rats, four-vessel occlusion, GCI | Laser, 808 nm, irradiance 20 mW/cm2, fluence at cortical surface 2.4 J/cm2 and at the hippocampus 0.8 J/cm2 | 3 days after GCI | 3 mm posterior to the eye and 2 mm anterior to the ear | 2 min, for 5 days | BrdU, DCX, NeuN, NLRP3, IL-1β, Iba-1, ultrastructure of astrocyte and microglia | PBM had long-term protective effects on astrocytes and promoted hippocampal neurogenesis, contributing to neurological recovery |
| Vogel 2021 [41] | Wistar rat, PT | Laser, 780 nm, 10 mW/cm2, intensity of 0.083 W/cm2, energy density of 10 J/cm2 | 24 h after PT | Injury area (positioned 1 mm posterior and 1 mm lateral to bregma) | 2 min, alternate days, 3 times/week, for 60 days | Ischemia volume, Iba, NeuN, TNF-α, IL-1β, IL-6, IL-10, TGF-β | PBM reduced lesion volume, microglial activation, and neuroinflammation, while increasing astroglial activity in the peri-lesioned region |
| Vogel 2021 [43] | Wistar rats, PT | Laser, 780 nm, 10 mW, 0.083 W/cm2, 10 J/cm2 | 24 h after PT | Injured area (1 mm posterior and 1 mm lateral from bregma) | 2 min, 3 times/week, for 60 days | Electroencephalogram | PBM reduced epileptiform discharges in stroke-induced epilepsy |
| Kim 2022 [66] | C57BL/6 mice, PT and MCAO | LED, 630 nm, 850 nm, 940 nm, 17 mW/ cm2 | Before the induction of ischemia as well as 1 h before the procedure | Sensorimotor cortex of the ipsilesional region | (1) 20 min, twice a day before or after PT for 3 days; (2) immediately after PT, once daily for 7 days | Infarct volume, neurological scores, NeuN, CD31, Iba-1, AIM2, caspase 1, GS- DMD, CD86, CD206, IL-1β, IL-10 | PBM attenuated inflammasome activation, inflammasome-mediated pyroptosis, and modulated microglial polarization in the hippocampus and cortex 7 days post-stroke |
| Feng 2023 [67] | SD rat, PT | Laser, 808 nm, power density 350 mW/cm2 at the scalp level | Initiated 24 h after PT | 1.8 mm anterior to the bregma and 2.5 mm lateral from the midline | 2 min daily, for 7 days | Synaptophysin, IL-1β, Bcl-xL, BAX, Spinophilin, NeuN, MAP2, Caspase 9, C3d, iNOS | PBM inhibited neurotoxic astrocytic polarization, preserved synaptic integrity and protected neurons against stroke injury both in vitro and in vivo |
| Shalaby 2023 [68] | C57BL/6 mice, PT in the olfactory bulb | Laser, 808 nm, 325 mW/cm2, fluence of 40 J/cm2 | From day 2 | The olfactory bulb area | 2 min daily, from day 2 to day 7 | Iba-1, CD31 | PBM improved olfactory recovery after PT by modulating the micro-environment, suppressing inflammatory cytokines and enhancing glial and vascular factors |
| Feng 2024 [69] | SD rats, PT | Laser, 808 nm, 350 mW/cm2, 42 J/cm2 | From day 1 | 1.8 mm anterior to the bregma and 2.5 mm lateral from the midline. | 2 min daily, from day 1 to day 7 | CBF, blood–brain barrier permeability, ZO-1, Claudin 5, testosterone | PBM attenuated cerebrovascular injury and behavioral deficits associated with testosterone/androgen receptor following ischemic stroke |
| Yokomizo 2024 [70] | C57BL/6J, 30 min MCAO | Laser, 808 nm, 1064 nm, 1270 nm, irradiance 50 mW/cm2, CW | Pretreatment | Head | 5 min daily for 4 days before stroke | CBF, infarct volume, eNOS | Laser pretreatment laser improved cerebral blood flow, eNOS phosphorylation, and stroke outcomes |
- Abbreviations: AIM, interferon-inducible protein; BBB, blood–brain barrier; BCCAO, bilateral common carotid artery occlusion; BNDF, brain-derived neurotrophic factor; BrdU, bromodeoxyuridine; CA1, hippocampal cornu ammonis; CBF, cerebral blood flow; CCO, cytochrome c oxidase; CD, cluster of differentiation; COX, cyclooxygenase; CW, continuous wave; DCX, doublecortin; eNOS, endothelial nitric oxide synthase; EPC, endothelial progenitor cell; ERK, extracellular signal-regulated kinase; FGF, fibroblast growth factor; GCI, global cerebral ischemia; HMGB, high-mobility group box 1; Iba, ionized calcium-binding adapter molecule 1; ICH, intracerebral hemorrhage; IGF, insulin like growth factor; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; LED, light-emitting diodes; LLLT, low-level light therapy; MAP, microtubule-associated protein; MAPK, mitogen-activated protein kinase; MCAO, middle cerebral artery occlusion; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NILT, near-infrared light therapy; NIR, near-infrared; NLRP3, NLR family pyrin domain containing 3; NOS, nitric oxide synthase; PGC1-α, peroxisome proliferator-activated receptor gamma coactivator; PT, photo thrombosis; PW, pulse wave; ROS, reactive oxygen species; RSCEM, rabbit small clot embolic stroke model; SIRT, NAD-dependent deacetylase sirtuin; TGF, transforming growth factor; TLR, toll-like receptor; TLT, transcranial laser therapy; TNF, tumor necrosis factor; tPA, tissue-type plasminogen; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; VEGF, vascular endothelial growth factor.
3.1 Therapeutic Window
The timing of tPBM treatment initiation post-stroke has been investigated in animal studies. In the rabbit embolic stroke model (RSCEM), initiation of laser treatment within 3–6 h significantly improved behavioral performance for up to 3 weeks, but initiation at 24 h did not [45]. However, in the rat permanent middle cerebral artery occlusion (MCAO) model, laser treatment at 24 h resulted in neurological function improvement at 3–4 weeks [46, 47]. In addition, LED treatment during the subacute phase (4 days post-stroke onset) significantly improved motor function up to 4 weeks, but a later phase at 10 days post-stroke onset did not [38, 61]. Studies have also examined combining tPBM with tissue-type plasminogen activator (tPA) therapy [49, 54]. The therapeutic window of tPA is 1–1.5 h in the RSCEM, while LLLT had a 4–6 times longer window. Importantly, LLLT did not increase hemorrhagic incidence, volume or mortality rate. In turn, there was a 30% decrease in hemorrhagic incidence when tPA was combined with tPBM [49]. Above evidence suggests that the therapeutic window for tPBM varies by animal model and stroke induction method, but can be extended as a synergistic approach with tPA. It can also exert long-term protective effects and modulate brain responses at different stages of the ischemic cascade.
3.2 Location of Irradiation
Studies have suggested that both focal and whole-brain approaches to tPBM may have advantages for promoting neurological recovery after stroke. Irradiating a stroked hemisphere ipsilaterally, contralaterally, or bilaterally improved neurological outcomes in rats, with effects observed 24 h after stroke induction and persisting for 4 weeks [46]. Notably, evidence suggests that whole-brain treatment may be more effective than focusing solely on the ipsilateral side, as beneficial effects have also been observed with contralateral irradiation [46]. Though the optimal side for irradiation in animal studies remains unclear, targeting the entire brain may generally yield more favorable results. This has prompted subsequent clinical trials to favor whole-brain irradiation [17, 71], while later studies have explored the efficacy of targeting specific brain regions [18, 72].
3.3 Number of Treatments
The numbers and total energy dose of tPBM treatments may optimize the therapeutic response. Double or triple laser treatments with a surface power density of 7.5–20 mW/cm2 within 5 h post-embolization resulted in greater behavioral improvements than a single treatment [53]. Specifically, the triple treatment group showed a remarkable 245% behavioral improvement over controls, exceeding that of neuroprotective agents like tPA [53]. Similarly, triple high-power treatments also provided significant behavioral benefits without causing tissue damage [56].
3.4 Mechanisms of Effects of tPBM on the Brain in Animal Models
3.4.1 Preservation of ATP Production and Protection of Mitochondria
tPBM treatment has been shown to increase ATP concentrations and rescue mitochondrial dysfunction in neurons in ischemic animal stroke models [50, 59], highlighting its potential for stroke treatment. NIR laser irradiation was observed to suppress nitric oxide synthase (NOS) activity and expression, alleviating cerebral concentrations of NO in transient MCAO rat models [44, 51, 52]. Additionally, LED preconditioning stimulates endothelial NOS phosphorylation via the phosphoinositide 3-kinase/Akt pathway, leading to reductions in brain damage in mice after MCAO [57, 70]. Moreover, tPBM treatment targeting mitochondria attenuated ischemia–reperfusion injury by addressing the initial ROS burst, thereby reducing brain injury and infarct volume in the rat MCAO model [64]. tPBM was also observed to protect primary cortical neurons against excitotoxicity induced by glutamate or kainite [67], which are major contributors to neuronal death in ischemic stroke. Furthermore, tPBM has been found to indirectly protect tissues by inhibiting apoptosis. It downregulates pro-apoptotic factors like caspase 9 and caspase 3, and upregulates anti-apoptotic factors like Akt, Bcl-2 [52]. tPBM treatment has also been shown to improve muscle resistance, physical function, and spatial and episodic memory, and increase markers of mitochondrial biogenesis [61, 62].
3.4.2 Increased Neurogenesis
tPBM has been found to promote cortical neurogenesis by stimulating neural progenitor cells and neural stem cells in key neurogenic niches like the subventricular zone (SVZ) and dentate gyrus. Studies have shown that tPBM treatment initiated within 24 h of ischemia can significantly enhance neurogenesis [73]. For example, LED irradiation of the hemisphere contralateral to the stroked region resulted in an increase in newly formed neuronal cells in the ipsilateral SVZ [47]. Similarly, tPBM treatment initiated up to 6 h after an embolic stroke could induce rapid behavioral improvements, along with increased number of nerve-producing cells in the angular gyrus, and elevated BDNF concentrations [48]. Moreover, daily LED irradiation was reported to improve the histological appearances of oligodendrocytes, pyramidal motor neurons, and sensory neurons, reflecting enhanced neurogenesis [61]. In vitro, LED irradiation of rat cortical neurons exposed to ischemic conditions promoted neurite outgrowth and synaptogenesis, mediated by the activation of mitogen-activated protein kinase (MAPK) signaling [74]. However, the positive effects of tPBM treatment on post-stroke neurogenesis may take 2–4 weeks to manifest, as new neurons are required to form and migrate to the damaged site. Studies have shown that tPBM treatment during acute and subacute phases of stroke can induce the proliferation of bromodeoxyuridine (BrdU)-positive cells, as well as BrdU co-labeling with markers of astrocytes, neuronal precursors, mature neurons, and blood vessels [38]. Relatedly, in a photothrombotic stroke model, daily tPBM treatment applied to an infarct area significantly increased BrdU immunostaining of proliferation, neuronal, dendritic spine, and synaptic markers in the peri-infarct zone [59]. Moreover, in a global cerebral ischemia model, tPBM treatment suppressed the activity of reactive astrocytes and maintained astrocyte regeneration as early as 7 days post-reperfusion, and promoted the formation of new neurons at 58 days post-reperfusion [65].
3.4.3 Modulation of Neuroinflammation and Regulation of Cytokine Expression
tPBM treatment has been found to downregulate pro-inflammatory cytokines that can promote neuronal apoptosis, aggravate brain injury, inhibit neurogenesis, and disrupt the integrity of the blood–brain barrier. Conversely, tPBM has been shown to upregulate anti-inflammatory cytokines, indicating its potential to mitigate neuroinflammation [25]. For example, tPBM treatment before photothrombotic cortical ischemia inhibited MAPK activation and NF-κB translocation, prevented leukocyte accumulation, and preserved the integrity of the blood–brain barrier [55]. It also decreased expression of the NLRP3 inflammasome, which is responsible for cleaving pro-inflammatory cytokines IL-1β and IL-18 [65, 75]. tPBM treatment initiated 4 h post-ischemia profoundly reduced neuroinflammatory responses, including neutrophil infiltration and microglial activation, as well as decreases in the concentrations of inducible NOS, TNF-α, and IL-1β in the ischemic cortex [62]. Irradiation of the olfactory bulb with tPBM significantly decreased the expression of pro-inflammatory cytokines, such as IL-1α, IL-1β, and IL-16, while increasing the expression of anti-inflammatory cytokines, such as IL-1 receptor antagonist, IL-4, and IL-10 [68]. In addition, tPBM treatment was found to modulate microglial activation, shifting microglia from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype [41], thereby promoting an environment conducive to regeneration and improved mitochondrial function and structure. Furthermore, tPBM treatment attenuated apoptosis and inflammasome activation in the hippocampus and cortex following ischemic stroke [66, 67], contributing to significant improvements in neurological behavior [74].
3.4.4 Enhancement of CBF and Repair of the Neurovascular Unit
tPBM treatment has shown a potential to enhance local CBF by 30% in a mouse model [51], likely due to increases in NO and reductions in hippocampal apoptosis. tPBM treatment of the olfactory bulb not only accelerated the recovery of impaired olfaction by enhancing the expression of glial and vascular factors, such as GFAP, Iba-1, and CD31 [68] but also increased the concentration of soluble intercellular adhesion molecule-1, which is associated with cerebral microbleeds and hemorrhagic transformation in stroke. Studies also indicate that tPBM treatment can modulate intercellular signaling within the neurovascular unit. It triggers reactive astrocytes to release high-mobility group box 1, which may enhance the accumulation of endothelial progenitor cells during stroke recovery [63, 76]. Increasing CBF through tPBM treatment could ensure that ischemic or damaged areas of the brain are provided with oxygen and nutrients, thus removing toxins byproducts and inflammatory mediators, reducing neuroinflammation and creating a favorable environment for neuronal repair and regeneration. Additionally, tPBM engages the neurovascular coupling mechanism, supporting long-term synaptic plasticity and neuroplasticity.
4 tPBM Therapy in Human Clinical Trials
4.1 tPBM Treatments for Acute Ischemic Stroke: NeuroThera Effectiveness and Safety Trials (NESTs)
The first human trial of transcranial laser therapy (TLT) was the NeuroThera Effectiveness and Safety Trial-1 (NEST-1), a prospective, double-blind, sham-controlled trial that enrolled 120 stroke participants [17]. NEST-1 delivered 808 nm wavelength light for 2 min to each of 20 predetermined locations on the scalp within 24 h of stroke symptom onset and found that the TLT group demonstrated significantly greater improvements in the primary outcome measure of complete recovery on the National Institutes of Health Stroke Scale (NIHSS) at day 90, compared with the sham-treatment group. Importantly, the NEST-1 also demonstrated the safety of TLT, as there were no significant between-group differences in mortality rates or numbers of serious adverse events. The following NEST-2 recruited 660 stroke participants with more severe strokes [71], but did not demonstrate statistically significant improvements in the primary outcome of the modified Rankin Scale (mRS) score, though post hoc analyses suggested a meaningful benefit in the subgroup with moderate to moderately severe strokes. A final pooled analysis of 778 participants revealed that the TLT group had a significantly higher success rate the on 90-day mRS score [77]. Subgroup analyses revealed that moderate strokes and treatment 12 h after stroke onset predicted better response, suggesting delayed treatment may be more effective than early treatment. Subsequently, the large NEST-3 was terminated early after a futility analysis of 566 participants showed no clinical difference in the primary 90-day mRS score between the TLT group and sham-treatment group [78]. The concurrent trial evaluating the safety of tPA plus TLT was also halted prematurely [79]. Additionally, infarct volume analyses had mixed results, with the NEST-2 study showing no reduction in overall infarct volume or cortical infarct volume from tPBM within 24 h [80], but other research finding that tPBM within 72 h with frequent subsequent treatments reduced infarct volume and improved NIHSS scores [81], highlighting the complexity of stroke treatment.
In summary, the positive outcomes in NEST-1 and the signal toward efficacy in NEST-2 provided evidence of the potential benefits of tPBM treatment for acute ischemic stroke (Table 2). However, the negative results from the NEST-3 are not entirely surprising, given the inherent challenges in acute stroke treatment. Further research and refinement of trial designs are needed to fully understand the potential benefits and limitations of tPBM for the treatment of stroke.
| Authors | Study design | Participants, age, number | Parameters of light | Onset of stroke | Irradiation sites | Duration time | Evaluation tools | Outcomes | SAEs |
|---|---|---|---|---|---|---|---|---|---|
| Lampl 2007 [17], NEST-1 | A prospective, intention-to-treat, multicenter, international, double-blind, trial | 120 ischemic stroke patients (79 TLT vs. 41 sham), age 40–85 years, NIHSS score 7–22 | Laser, 808 nm, 1 J/cm2 of energy (NeuroThera Laser System, PhotoThera Inc.) | Within 24 h from stroke onset | 20 predetermined locations, over the entire surface of the cortex regardless of stroke location | 2 min at each site | NIHSS, mRS, Barthel Index, and Glasgow Outcome Scale at 90 days | NILT initiated within 24 h of ischemic stroke onset showed initial safety and effectiveness, as measured by improved NIHSS and mRS scores | Mortality rates and SAEs did not differ between groups |
| Zivin 2009 [71], NEST-2 | A double-blind, randomized study | 660 ischemic stroke patients (331 TLT vs. 327 sham), age 40–90 years, NIHSS score 7–22, without tPA and hemorrhagic infarct | 808 nm, (NeuroThera Laser System, PhotoThera Inc.) | Within 24 h from stroke onset | 20 predetermined locations | 2 min at each site | mRS at 90 days | TLT within 24 h was safe but did not meet formal statistical significance for efficacy. However, predefined analyses showed a favorable trend, consistent with NEST-1 | Mortality rates and SAEs did not differ between groups |
| Huisa 2013 [77] | A pooled analysis | A total of 778 patients from NEST-1 and NEST-2 | — | — | — | — |
mRS 0–2 at 90 days |
TLT had a significantly higher 90-day mRS success rate compared to sham. Moderate strokes were identified as a predictor of better treatment response | — |
| Kasner 2013 [80] | — | 640 subjects from NEST-2, had scans on day 5 (576 CT, 64 MRI) | — | — | — | — | Infarct volumes by CT or MRI | TLT was not associated with a reduction in overall or cortical infarct volume as measured on CT in the subacute phase | — |
| Hacke 2014 [78], NEST-3 | A double-blind, randomized, sham-controlled, parallel group, multicenter trial | 630 acute ischemic stroke patients (316 TLT vs. 314 sham), age 40–80 years, NIHSS score 7–17, without tPA and hemorrhagic infarct |
808 nm (NeuroThera Laser System, PhotoThera Inc.) |
Within 24 h from stroke onset |
20 predetermined sites |
2 min at each site |
mRS 0–2 at 90 days |
TLT showed no efficacy for the treatment of acute ischemic stroke. When combined with previous study data, there was no net benefit from TLT |
SAEs did not differ between groups |
| Hemmen 2014 [79] | StELLAR, a small exploratory study | 12 subjects (7 received tPA only, 5 tPA+ TLT), age 40–80, NIHSS score 7–17 | 808 nm (NeuroThera Laser System, PhotoThera Inc.) | Within 24 h from stroke onset |
20 predetermined sites |
2 min at each site |
Rate of intracranial hemorrhages at 36 h and mRS 0–1 at 90 days | TLT was well tolerated in combination with IV tPA. Good 90-day outcome in 3 versus 2 patients (42.9 vs. 40%, NS) | No ICH, SAEs were not different between groups |
| Naeser 2020 [82] | Case series report | Six people with aphasia, age 46–49 years | LED, 633 nm and 870 nm, 22.48 cm2, 22.2 mW/cm2. 500 mW (Model 1100 LED cluster head, MedX Health, Toronto) | 2–18 years after single left hemisphere stroke | (1) Bilateral and midline placements including both L and R SMAs at vertex; (2) Only LH ipsilesional LED placements; (3) only LH, plus one midline cortical node of DMN (mPFC) using 13 J/cm2; (4) only LH, plus two midline cortical nodes of DMN (mPFC and precuneus) using 26 J/cm2 | 3 times/week, for 6 weeks |
Language testing, rs-fcMRI |
NIR photons can affect surface brain cortex areas subjacent to where LEDs are applied on the scalp. Improved naming ability was present with optimal ipsilesional plus two midline DMN node placement | The pulsed rates were complete safe, even for patients with a history of seizures, and were on antiseizure medications. No seizures occurred |
| Estrada-Rojas 2023 [18] | Case report | A 38-year-old female, with a stroke on the left side of the brain | (1) An LED cluster (630 nm, 660 nm, and 850 nm), power density of 200 mW/cm2, energy density of 12 J/cm2 per min; (2) an LED helmet (810 nm, power, 15 W; fluence, 28.8 J/cm2; and irradiance, 24 mW/cm2) | 5 months poststroke |
(1) Left side of the scalp, primarily along the Sylvian fissure language areas, at eight target areas; (2) an LED tPBM helmet applied to the scalp |
45 min sessions, 2 times a week, for a total of 30 sessions in 5 months | Language testing | A combination of speech-language therapy plus tPBM within a treatment session where tPBM is first applied to the left hemisphere language areas only, and then to the whole head during simultaneous speech-language therapy was highly beneficial | — |
| Paolillo 2023 [83] | A randomized and placebo-controlled study | Hemiplegia after stroke (more than 12 months from onset), age 35–75 years | Laser, 660 nm, 808 nm, 980 nm, average power of 720 mW/min, 43.2 J energy | More than 12 months from onset, 5 min after NMES | 15 regions for all head | 1 min per region, once a week, for 12 weeks | Grip strength, TUG, modified Ashworth Scale, VAS, MMSE, QoL | Laser and NMES treatments improved cognitive function, pain relief, manual dexterity, social–emotional health, and quality of life |
No adverse effects were observed |
- Abbreviations: CT, computed tomography; DMN, the default mode network; LH, left hemisphere; MMSE, the mini-mental state examination; mPFC, medial prefrontal cortex; MRI, magnetic resonance imaging; mRS, modified Rankin scale; NEST, NeuroThera effectiveness and safety trials; NIHSS, National Institutes of Health Stroke Scale; NMES, neuromuscular and muscular electrical stimulation; QoL, quality of life; rs-fcMRI, resting-state functional-connectivity MRI; SAEs, serious adverse events; SMA, the supplementary motor area; TUG, timed up and go test; VAS, visual analog scale.
4.2 tPBM Treatment of Chronic Ischemic Stroke
A case study involving post-stroke patients with chronic left hemisphere infarction and aphasia applied LED clusters three times per week for 6 weeks [82]. This tPBM treatment increased functional connectivity within neural networks and positively affected cortical surface areas, thereby enhancing neuromodulation. In addition, simultaneous irradiation of the ipsilateral language cortex plus two midline default mode network nodes (the medial prefrontal cortex and praecuneus) emerged as the most effective protocol for improving naming ability and enhancing impaired functional connectivity. Similarly, a combination of tPBM to left hemisphere language areas followed by whole-head tPBM concurrent with speech-language therapy led to significant improvements in speech rate, utterance length, and grammatical complexity compared to speech-language therapy alone [18]. These findings suggest that a targeted tPBM approach, such as by targeting left-hemisphere language network areas, to priming affected neural networks may enhance the benefits of tPBM combined with rehabilitation. Studies have also found that tPBM combined with other therapies can enhance stroke rehabilitation outcomes. A trial using tPBM on 15 regions across the entire head combined with neuromuscular electrical stimulation once a week for 12 weeks improved manual dexterity and motor function in chronic stroke participants [83]. Single sessions of tPBM to the left primary motor cortex increased finger tapping in healthy participants, and high-dose tPBM was shown to instantly upregulate corticomotor excitability [72]. These findings indicate that tPBM has potential as a noninvasive neuromodulation therapy to alleviate aphasia and enhance motor recovery in chronic stroke rehabilitation.
Regarding the safety of tPBM, clinical trials have found no significant differences in adverse events like skin burns, and mortality rates, between tPBM treatment and control groups. Animal studies also show no major adverse events are associated with tPBM therapy. However, the potential cumulative thermal effects of prolonged or multiple tPBM sessions have not been well-established due to limitations in previous trial designs.
5 Translational Gaps and Possible Explanations
The conclusions from the NESTs and other studies using different protocols are inconsistent, and the efficiency of tPBM to enhance the recovery of speech-language function in chronic stroke has only been reported in a few case studies. Several reasons have been proposed for the lack of reproducible efficacy in the NESTs. First, there are challenges in translating animal models into human trials. For example, the TLT used in the NESTs was developed without optimization in multiple species or rigorous efficacy testing across translational animal models [84]. The heterogeneity of human stroke populations is also higher than that of animal models, which can contribute to variability in treatment outcomes. Additionally, for light to effectively reach brain tissue, it must penetrate at least 8 mm to account for the thickness of the human skull and dura [85]. Although studies indicate that wavelengths such as 808 nm can penetrate much deeper, reaching depths of 40–50 mm under optimal conditions [86]. Despite this capability, the complex tissue interfaces in humans also cause significant scattering, limiting photonic energy delivery to affected cortical and subcortical structures.
Second, the optimal dosimetry parameters to traverse the skull and penetrate brain tissue were not determined. For instance, the NEST-1 specified a cortical surface fluence of 1 J/cm2, but the NEST-2 and NEST-3 did not provide details on the power density used. It is possible that the power density was insufficient to adequately penetrate the human skull and activate the biological mechanisms necessary for neuroprotection and recovery. In efforts to solve this problem, higher doses have been tested, showing that fluences of up to 120 J/cm2, which was 25–100 times greater than that used in the NESTs, could significantly increase cerebral metabolism and blood oxygen supply [87], and cognitive function improvements in neurological conditions [88]. NIR LEDs irradiating the scalp for 20 min in patients with TBI, delivering an incident light fluence of approximately 43 J/cm2, also demonstrating feasibility and safety [20, 89]. Thus, the low-power-density continuous-wave (CW) mode regimen used in the NESTs may not be optimal for treating acute ischemic stroke. It has been suggested that tissue penetration can be maximized and tissue heating can be minimized by using pulsed-wave (PW) mode rather than CW mode. PW light, with quench periods following an active pulse, allows higher power to reach deep tissues without excessive heating of surface tissues when compared with CW light at the same energy density [90, 91]. As a result, PW light is preferred for delivering high power densities to target tissues deeper than 5 cm, particularly in transcranial therapy requiring deep tissue penetration without concomitant thermal damage [92]. Researchers found PW mode increased cortical ATP concentrations by 157%–221%, versus only 41% with CW mode, showing PW is optimal for mitochondrial stimulation and potentially preserving tissue in ischemic penumbrae [50].
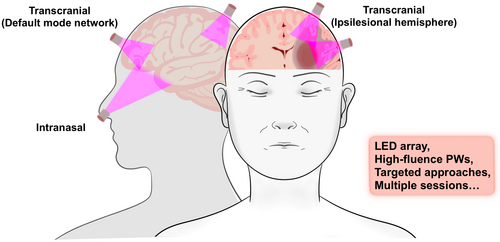
Third, the protocol used for stroke treatment is an important consideration. Animal study showed that a single tPBM treatment can promote behavioral recovery for 21 days [46], and the NESTs demonstrated the effectiveness of a single dose by measuring clinical efficacy 90 days later [17]. However, a single treatment may be insufficient for some brain areas, thereby necessitating repeated treatments. Studies have shown that delivering large total doses in a single session may yield less favorable outcomes than the same doses over multiple sessions [93]. Furthermore, a focused tPBM approach may be more effective than the broad application used in the NESTs, where only a few of the 20 skull sites were likely adjacent to penumbral tissue, and a 2-min application might have been insufficient. Findings suggest that a more targeted tPBM approach to only the ipsilateral side and default mode network nodes with multiple tPBM sessions [18, 82] could be more effective than the NESTs approach (Figure 2).
6 Conclusion and Future Perspective
Currently, research on tPBM therapy remains in its early stages, but the solid mechanistic theory and its potential to be a non-invasive, cost-effective, and safe long-term treatment option for neurological and psychological conditions, make it highly appealing. There is emerging enthusiasm for tPBM treatments to positively influence neuronal activity and achieve favorable outcomes in patients with neurological conditions like stroke, TBI, Parkinson's, Alzheimer's, and depression. Proof-of-concept studies have shown tPBM can improve motor and non-motor symptoms, such as enhancing cognitive scores, verbal memory, and sleep quality, and alleviating post-traumatic stress in TBI patients [42]. Preliminary research also indicates tPBM may improve sleep and reduce anxiety in patients with generalized anxiety disorders [94]. However, these results should be interpreted cautiously due to small sample sizes and lack of control groups. More rigorous double-blind studies are needed to confirm the effectiveness of tPBM for neurological diseases.
The continual development of tPBM equipment is crucial. Initially, tPBM treatment involved irradiating the forehead with laser light due to its good penetration abilities [83, 95]. More recently, the introduction of inexpensive LED arrays has facilitated the creation of light-emitting helmets [96]. Additionally, a NIR LED noseclip has also been developed to deliver photons directly to the olfactory bulbs and enable therapeutic doses of light to penetrate the brain [97-99]. It is suggested that the intranasal approach seems to be more effective in transmitting light to deep brain structures, such as the limbic system and prefrontal lobe, and claimed to improve the symptoms of AD, PD, and depression [100, 101]. Another expected advancement is the use of organic LEDs, which emit light when exposed to an electric current [25, 102], further expanding the potential applications by targeting specific brain structures and enabling various stimulation modes.
Recent research has demonstrated that tPBM with NIR-II (1000–1700 nm) laser light at a wavelength of 1064 nm significantly enhances CBF in mouse models of ischemic stroke, leading to reduced infarct volumes and enhanced neurological function [70]. Additionally, NIR-II laser light at a wavelength of 1267 nm has also been shown to facilitate rapid recovery in newborn rats following intraventricular hemorrhage due to improvements in lymphatic drainage and clearing functions [103]. These findings exemplify the potential of tPBM in ameliorating stroke injury and indicate that there remains considerable opportunity for further research to optimize treatment protocols and explore its applications across various neurological conditions.
In conclusion, tPBM holds great promise as a therapeutic tool for stroke and other neurological conditions. However, a rigorous re-evaluation of tPBM is required, with an emphasis on enhancing device technology, optimizing treatment parameters and protocols, and conducting thorough testing in both animal models and human clinical studies.
Author Contributions
S.L. and S.S.M.N. designed and wrote the manuscript. T.W.L.W. and S.S.M.N. revised the manuscript. S.S.M.N. gave constructive advice and participated in proofreading this paper. All authors contributed to the article and approved the submitted version.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




