Management of macular oedema due to retinal vein occlusion: An evidence-based systematic review and meta-analysis
Elisa E. Cornish and Sophia L. Zagora are equal first authors.
Abstract
Background
Central retinal vein occlusion and branch retinal vein occlusion are common causes of visual loss due to associated macular oedema. The aim of this review was to assess the effectiveness of interventions improving vision and treating macular oedema in central retinal vein occlusion and branch retinal vein occlusion.
Methods
Medical search engines and clinical trial registries were systematically searched. Randomised clinical trials with ≥90 eyes and real-world outcome studies with ≥100 eyes each with ≥6 months follow-up were included.
Results
There were 11 randomised controlled trials evaluating treatments for central retinal vein occlusion which met the inclusion criteria and 10 for branch retinal vein occlusion. There were 10 real world outcome studies of central retinal vein occlusion and 5 real world outcome studies of branch retinal vein occlusion. Meta-analysis was performed on studies that met the defined inclusion criteria. Main outcomes were change in visual acuity at 6-, 12-, 24- and 36 months by treatment.
Conclusions
Intravitreal anti-vascular endothelial derived growth factor is recommended as first line treatment over intravitreal corticosteroid due to its effectiveness and lower rate of ocular adverse events. Best outcomes are achieved when intravitreal treatment is started early. Macular laser may have an adjunctive role in branch retina vein occlusion but not central retinal vein occlusion.
1 INTRODUCTION
Retinal vein occlusion (RVO) is the second most common vision-threatening vascular disorder of the retina after diabetic retinopathy. It can be categorised based on the location of the luminal obstruction of the venous outflow system within the retinal vasculature. In central retinal vein occlusion (CRVO), blockage of the central retinal vein occurs within the optic nerve at the level of, or posterior to, the lamina cribrosa. All retinal veins and venules appear dilated and tortuous often with retinal haemorrhages in all four quadrants of the retina. In hemiretinal vein occlusion, signs are restricted to the superior or inferior half of the retina.1 In branch retinal vein occlusion (BRVO), occlusion tends to occur at an arteriovenous crossing with haemorrhages localised to the area drained by the branch retinal vein.2
CRVO is less common than BRVO with a reported prevalence of 0.1%–0.4%. In eyes with CRVO, visual impairment is most commonly due to the development macular oedema (MO) but can also be caused by macular ischemia, and/or neovascular glaucoma.3 The pathophysiology of CRVO is believed to be due to the atherosclerotic changes occurring in the central retinal artery affecting the central retinal vein following the principles of Virchow's triad for thrombogenesis. The vein and artery share a common adventitial sheath and atherosclerotic changes of the artery can cause compression of the vein at or proximal to the lamina cribrosa of the optic nerve. Patients with CRVO have similar systemic vascular risk factors to patients with cardiovascular disease, including hypertension, age over 55 years, hyperlipidaemia, diabetes, and smoking.4 Younger patients are more likely to have other coexistent systemic disease including hypercoagulability.
The estimated prevalence of BRVO is 0.7%.5 Like CRVO, BRVO can have multiple underlying causes, including age, hypertension, diabetic retinopathy, or hypercoagulability. The pathophysiology of BRVO involves increased hydrostatic pressure within thin-walled veins proximal to a luminal obstruction.3 The superotemporal quadrant in the retina is the most commonly affected in 63%–66% of eyes.6 Similar to eyes with CRVO, the most common cause of vision loss in BRVO is due to the development of MO.3, 7
Hypoxia and vascular endothelial growth factor upregulation occur due to the resistance to outflow in eyes with RVO. The resultant hypermeability leads to exudation and MO. The upregulation of vascular endothelial derived growth factor can also lead to further progression of retinal ischaemia. As MO is the primary cause of visual loss in patients with RVO, various treatments have been trialled aimed at treating MO due to RVO.8 Prior to the advent of intravitreal therapy, there was little evidence of any effective treatment to improve vision in eyes with MO due to CRVO. Whereas macular laser offered modest improvement in eyes with BRVO.9
The purpose of this review was to examine the effectiveness of macular laser, current intravitreal anti-VEGF agents and intravitreal corticosteroids that have been studied in large cohorts in randomised controlled trials (RCTs) and real-world outcome studies (RWOS). We aim to provide a narrative review of evidence of individual studies and meta-analyses to overview the strength of the studies included. This contrasts with previously published meta-analyses.
2 METHODS
This systematic review is reported in accordance with the preferred reporting items for systematic reviews (http://www.prisma-statement.org; accessed 20 May 2022).
2.1 Types of interventions
Randomised controlled studies for the treatment of MO due to CRVO and BRVO comparing any known therapies (including laser treatment, intravitreal therapies such as anti-VEGF agents or corticosteroid and no/sham treatment) were included in this review. We included all treatment algorithms (fixed, as needed [PRN] and treat and extend [T&E]), as the goal was to assess efficacy of treatments irrespective of their treatment algorithm. We also included RWOS with visual outcomes whether anatomical outcomes were reported or not.
2.2 Outcome measures
Outcome measures included the mean change in best corrected visual acuity (BCVA) from baseline to end of study, and the mean change in MO usually measured as a change in central macular thickness (CMT) on optical coherence tomography (OCT). Where available, ocular adverse events were also reported.
2.3 Study selection process and data extraction and management
Articles were retrieved using a keyword search of medical internet search engines—Cochrane, PubMed, MEDLINE Ovid; the ISRCTN registry; ClinicalTrials.gov; and the WHO ICTRP. The date of the last search was August 2022. Two investigators (E. C., S. Z.) independently identified all RCTs which evaluated the management of MO in CRVO or BRVO with at least 90 patients and RWOS of CRVO or BRVO with more than 100 patients, and with a follow up period of at least 6 months. RCTs that investigated only the dose and duration of treatment without a comparator were excluded. Discrepancies between reviewers were resolved by discussion and referral with a third party (S. F. B.).
Keywords search used was—BRVO/CRVO and macular (o)edema
2.4 Meta-analysis
Two reviewers (S. F. B., K. S.), working independently and in replica, evaluated the risk of bias using the Cochrane Risk of Bias Tool9 for RCTS, and the Risk of Bias in Non-randomised Studies-of interventions (ROBINS-I) tool.10 The risk of bias for RCT studies was considered according to the subsequent fields; selection bias, reporting bias, and other bias. Then the articles were classified into ‘low risk’, ‘high risk’, or ‘unclear risk’ of bias for each domain. For cohort studies, the risk of bias was evaluated through its selection, comparability, and result.
Study findings were summarised with regards to trends in BCVA improvement over time. Meta-analyses of visual outcomes were performed using ETDRS letters. Studies that reported visual acuity in logMAR or decimal values had those values approximated to ETDRS letter scores11 with an approximate SD.12 Studies that presented outcome data at various time intervals were included in the meta-analysis using pairwise associations of treatments evaluated at each of the different time points. The summary measure of primary outcome was the difference in mean change of VA from baseline. Subgroup analyses were performed at 6, 12, 24 and 36 months, as results at these time points were reported most commonly in studies. A post-hoc analysis was performed in order to compare the outcomes of various therapies used to treat MO due to RVO. Treatment effect estimates were assessed for all patients as well as subcategories based on VA.
Comprehensive Meta-Analysis version 4.0 was employed to generate the meta-analysis and to evaluate the data from included studies. The standardised mean difference (SMD) between the two groups was calculated using an inverse variance statistical approach with 95% confidence interval (CI) as the effect measure. To characterise any heterogeneity among studies, random effects analysis was used. The chi-square test and the 12 value were used to evaluate the statistical heterogeneity between studies. p Values below 0.10 in chi-square analyses were regarded as indicators of statistically significant heterogeneity. This was quantified using I2 statistics. I2 > 30% was deemed to indicate moderate heterogeneity, I2 > 50% substantial heterogeneity and I2 > 75%. considerable heterogeneity. Studies with substantial or considerable heterogeneity were examined for possible sources of heterogeneity.
3 RESULTS
3.1 Study selection
Following a search of databases and search engines, 372 RCTs and 106 RWOS examining the management of MO in CRVO or BRVO were identified (Figure 1). Studies with less than 6 months follow up and/or less than 90 patients for RCTs and less than 100 patients for RWOS were excluded. A total of 21 RCT and 15 RWOS met the eligibility criteria to be included in this review (Tables 1–5).
| Authors | Acronym title of study | Title of paper with the results of the study | Intervention | No. of eyes | Mean letter change from baseline by treatment unless otherwise specified, letters (CI unless otherwise specified) | Mean CMT change as measured by OCT in microns | Ocular adverse events | Follow-up | Study outcome | Risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| CVOSG11 | CVOS | Evaluation of grid pattern photocoagulation for MO in CRVO: group M report | Macular Grid laser (one off treatment) vs. no treatment | 155 | Grid laser −4 Untreated −3 p = 0.78 |
Angiographic MO improved in 48% treated vs. 18% not treated p < 0.0001 CMT not available |
Not listed | 36 months | Macular laser did not improve VA. However, in younger patients there was a trend towards significant improvement. Macular laser reduced angiographic MO. |
Low |
| Ip et al.12 | SCORE | A randomised trial comparing the efficacy and safety of IVTA with observation to treat vision loss associated with MO secondary to CRVO | 1 mg IVTA vs. 4 mg IVTA vs. Sham (if required 4 monthly) | 271 | IVTA 1 mg −1.2 (−6.4to 4.1) IVTA 4 mg −1.2 (−6.3to 4.0) Sham −12.1 (−17.1 to −7.1) |
IVTA 1 mg −196 (−390 to −62) IVTA 4 mg −261 (−407 to −79) Sham −277 (−418 to −40) |
Incidences of cataract and raised IOP were similar for IVTA 1 mg and sham but higher for IVTA 4 mg | 12 months | IVTA 1 mg can be considered for treatment for MO due to CRVO, but not IVTA 4 mg, as although its effectiveness was similar to IVTA 1 mg, it was associated with higher rates of ocular adverse events. Early treatment is associated with better outcomes. |
Low |
Haller et al.13 |
GENEVA | Randomised, sham-controlled trial of dexamethasone IV implant in patients with MO due to RVO |
Dex-implant 0.7 mg vs Dex-implant 0.35 mg vs Sham (single treatment) |
436 | Dex-implant 0.7 mg Dex-implant 0.35 mg +2 Sham −2 p = 0.3 |
CRVO and BRVO combined. Significant reduction in CMT in treated eyes at 90 days but not 180 days. At 180 days: Dex-implant 0.7 mg −119 ± 203 Dex-implant 0.35 mg −123 ± 212 Sham −119+/188 p > 0.05 |
Incidence of IOP > =25 mmHg was higher in Dex-implanted eyes (16%) at 60 days compared to sham (0%). No difference than sham at 180 days. Incidence of cataract similar in sham and DEX-treated eyes. | 6 months |
Dex-implant improved vision and reduced the risk of vision loss. Shorter MO duration was associated with better vision outcomes in Dex treated eyes. Day 60 outcomes for Dex treated eyes were better than 6 months outcomes. CMT reduction was significantly different to sham at day 90 (p < 0.001) but not at 6 months |
Low |
Brown et al.14 |
CRUISE | Ranibizumab for MO following CRVO: six-month primary end point results of a phase III study | Ran 0.3 mg vs Ran 0.5 mg vs Sham (if required 6 monthly) |
392 | Ran 0.3 mg +12.7 (9.9–15.4) Ran 0.5 mg +14.9 (12.6–17.2) Sham +0.8 (−2.0–3.6) p < 0.0001, Ran vs. Sham |
Ran 0.3 mg −434 Ran 0.5 mg −452 Sham −168 p < 0.0001 Ran vs. Sham |
No difference in adverse events with different doses | 6 months | Ran is an effective treatment of MO due to CRVO. | Low |
Ogura et al.7 (Europe and Asia Pacific) |
GALILEO | Intravitreal aflibercept for MO due to CRVO | Aflb vs. sham with 5× 4-weekly loading doses, then PRN 4 weekly until week 48, then PRN 8 weekly | 177 | Aflb +13.7 Sham +6.2 p < 0.01 |
Aflb −423.5 Sham −219.3 p < 0.001 at 52 weeks |
Aflb: NVG in 7.8% Sham: NVG in 8.8% |
18 months | Aflb is an effective treatment of MO due to CRVO. Early treatment is associated with better outcomes. |
Low |
| Heier et al.15 | COPERNICUS | Intravitreal aflibercept for MO due to CRVO | Aflb vs. sham with 6× 4-weekly loading doses, then PRN 4 weekly until week 52 then PRN Aflb both arms 12 weekly | 188 | Aflb +17.3 Sham −4.0 p < 0.001 at 24 weeks Aflb +13.0 Sham +1.5 p < 0.001 at 100 weeks |
Aflb −457.2 Sham −144.8 p < 0.001 at 24 week Aflb + PRN −390.0 Sham + PRN −343.3 P value not provided |
Most ocular SAEs were related to the CRVO itself or the injection procedure. Incidence of NV was higher in the originally sham treated eyes (8.2% vs. 5.3%) | 24 months |
Aflb is an effective treatment of MO due to CRVO. Shorter MO duration associated with better outcomes. Visual and anatomic gains from 6 monthly loading doses maintained with 4 weekly PRN treatment. When PRN dosing was administered quarterly instead of monthly, visual, and anatomical improvements declined. |
Low |
| Hoerauf et al.16 | COMRADE C | Clinical efficacy and safety of ranibizumab vs. dexamethasone for CRVO | Monthly Ran ×3 then PRN vs. 1× Dex-implant | 243 | Ran +12.86 Dex-implant +2.96 p < 0.0001 |
Ran −376.7 Dex-implant −168.7 p < 0.001 |
IOP elevation: Ran 5.6% Dex-implant 31.9%. Cataract: Ran 0% Dex-implant 0.8% |
6 months | Ran monthly was more effective than Dex-implant 6 monthly at 6 months. There were more ocular adverse events in Dex-implant treated eyes. | Low |
| Li et al.17 | China Ozurdex in RVO | Safety and efficacy of dexamethasone for MO secondary to RVO in Chinese pts |
Dex-implant vs. sham at baseline and 6 months | 131 | Dex-implant +9.8 (11.0) Sham −0.6 (13.9) at 2 months VA in Dex-implant treated eyes returned to baseline at 6 months |
Dex-implant −487(203) Sham −41 (256) at 2 months. No difference at 6 months |
IOP rise >10 mmHg Dex-implant 27% Sham 1.5% Elevated IOP normalised by 4 months |
6 months | Dex-implant improved visual and anatomic outcomes 2 months after treatment, but this was not maintained to 6 months. Increase in IOP was common but normalised by 4 months. | Low |
| Scott et al.18 | SCORE 2 | Effect of bevacizumab vs. aflibercept on VA among patients with MO due to CRVO | Bev 4 weekly vs. Aflb 4 weekly |
305 | Bev +18.84 (16.01–21.67) Aflb +18.21 (15.71–20.72) |
Bev −413 (−457 to −369) Aflb −424 (−463 to −385) |
Bev 1 endophthalmitis. 2 IOP >35 mmHg. Aflb No cases of endophthalmitis or IOP >35 mmHg |
6 months | Bev given 4 weekly is noninferior to Aflb 4 weekly in eyes with MO due to CRVO. | Low |
| Vader et al.1 | BRVO | Comparing the efficacy of bevacizumab and ranibizumab in patients with RVO | Bev 4 weekly vs. Ran 4 weekly | 97 CRVO eyes and 47 hemi-CRVO | Bev +16.1 ± 14.3 Ran +17.1 ± 15.8 |
Bev −332.5 (246.5) Ran −398.3 (234.4) p = 0.58 |
No differences in AEs between the two groups p = 0.51 | 6 months | Bev given 4 weekly was noninferior to Ran 4 weekly. Anatomic and safety outcomes did not differ between treatment groups. | Low |
| Hykin et al.19 | LEAVO | Intravitreal ranibizumab vs. aflibercept vs. bevacizumab for MO due to CRVO |
Ran vs. Aflb vs. Bev given 4 weekly ×3, week 16 through 96, treatment given PRN |
463 | Ran +12.5 (SD 21.1) Aflb +15.1 (SD 18.7) Bev +9.8 (SD 21.4) |
Mean percentage persistently dry: Aflb 57% (48.7) Bev 28% (23.9) Ran 32% (27.4) More eyes treated with Bev had persistent MO than those treated with Aflb (18.5% vs. 5.2%; p < 0.001) or Ran (8% vs. 10.5%; p = 0.01) at week 100 |
No difference in side effect profile between agents. Conversion to ischaemic CRVO occurred in 5.4%. |
100 weeks | Ran was non-inferior to Aflb. Unable to demonstrate that Bev was non-inferior to Aflb or Ran (it may be worse or may not be worse). |
Low |
- Abbreviations: Aflb, aflibercept; Bev, bevacizumab; BRVO, branch retinal vein occlusion; cf, compared with; CMT, central macular thickness measured on optical coherence tomography, CRVO, central retinal vein occlusion; CVOS, central vein occlusion study; CVOSG, central retinal vein occlusion study group; Dex-implant, dexamethasone implant; ERM, epiretinal membrane; IOP, intraocular pressure; IV, intravitreal; MO, macula oedema; No., number; NV, neovascularisation; NVG, neovascular glaucoma; NVI, neovascularisation of the iris; OCT, optical coherence tomography; PRN, as required; pts, patients; Ran, ranibizumab; RVO, retinal vein occlusion, SD, standard deviation; VA, best corrected visual acuity; VEGF, vascular endothelial growth factor.
| Authors | Country of study | Title of study | Intervention | No. of eyes | Mean letter change from baseline | Mean CMT change (microns) | Ocular adverse events | Follow-up | Study outcome | Risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Eter et al.20 | German Ozurdex RWSG | Dexamethasone IV implant in RVO. Real life data | Dex-implant given at discretion of doctor. |
206 | BRVO and CRVO combined +7.8 at week 12. Approximately +8 for CRVO at week 12 |
Largest reduction in CMT seen at week 6 ≈ 300 | Initiation IOP lowering medication 20.9%. Adjunctive anti-VEGF 6.8% Adjunctive laser 17.5% |
6 months | Dex-implant was effective in improving VA at 12 weeks. Best visual gains in eyes with short duration of MO. A significant proportion required treatment to reduce elevated IOP. Some eyes also required adjunctive anti-VEGF and laser. |
Low |
| Casselhom de Salles and Epstein 202121 | Sweden |
Real-life study of the use of anti-VEGF therapy vs. Dex-implant for treatment of MO in RVO | Anti-VEGF 3× monthly then PRN vs. Dex-implant PRN 3 monthly | 243 | Anti-VEGF +0.2 ± 27.6 Dex-implant −9.7 ± 32.6 p = 0.11 |
Anti-VEGF −310 ± 242 Dex-implant −185 ± 266 p < 0.05 |
NV occurred in 30% of all eyes. 72% of these eyes developed NVG |
Mean follow up 34 months | Anti-VEGF was more effective than Dex-implant in improving vision and reducing MO. | Low |
| Gale et al.22 | UK | A multi-centre study of 4626 patients to assess the effectiveness, safety, and burden of two categories of treatment of MO due to CRVO | Anti-VEGF vs. Dex-implant Rx at the discretion of the doctor. | Anti-VEGF 4619 Dex-implant4093 |
At 12 months Anti-VEGF (877 eyes) +10 Dex-implant (60 eyes) +8.4 36-months Anti-VEGF (219 eyes) +11.5 Dex-implant (9 eyes) +5.7 |
Not available | Endophthalmitis Anti-VEGF 0.01% Dex-implant 0.2% |
36 months |
Anti-VEGF was more effective than Dex-implant at improving vision in eyes, however treatment burden was higher. Dex-implant associated with higher rates of endophthalmitis. | Low |
| Hogg et al.23 | UK | Real-world visual and neovascularisation outcomes from anti-VEGF in CRVO | Ran or Aflb Rx at the discretion of the doctor. |
231 | Overall, +8.9 | Resolution of MO occurred in 41.2%. There was no difference between agents | NVI/NVG 21% PRP 10% | 6–48 months | Ran and Aflb were effective at improving vision and treating MO due to CRVO in a real-world UK setting. Despite treatment with anti-VEGF, 20% of eyes still developed NVI/NVG. | Low |
| Ciulla et al.24 | USA | VA outcomes and anti-VEGF therapy intensity in MO due to RVO: a RWA | Bev, Aflb or Ran Rx at the discretion of the doctor. | 6737 | Overall, +7.1 (95%CI +6.31 to +7.95) |
N/A | 9% received intravitreal corticosteroid rescue 12% received macular laser 3% received PRP |
12 months | All anti-VEGF agents were equally effective. Eyes with worse vision at baseline improved the most. VA outcomes were not as good as those seen in RCTs (10 letter difference). | Low |
| Lotery et al.25 | LUMINOUS | Effectiveness and safety open label | Ran Rx as per local Ran label. | 327 | At 1 year +10.8 |
−405 (95% CI, −450 to −360 μm) |
Ocular SAE occurred in 0.84% (endophthalmitis 0.14%; retinal haemorrhage 0.11%; retinal detachment 0.09%; cataract 0.07% and vitreous haemorrhage 0.07%) |
1 year (n = 144) 5 years for safety outcomes (n = 64) |
Ran is an effective treatment for eyes with MO due to CRVO. | Low |
| Wu et al.26 | USA PACORES |
5-year outcomes of Bev for treatment naïve eyes with MO secondary to CRVO | Bev monthly until the oedema resolved. | 102 | +10 (VA gain from mean 20/332 to 20/200) | −418 | No eyes developed endophthalmitis. NVG 4.9% |
60 months | Eyes with better baseline BCVA and shorter duration of symptoms were more likely to achieve better VA at 5 years. |
Low |
| Kim et al.27 | Korea Ozurdex | Dex-implant for the treatment of RVO | Dex-implant Rx as per local practice. |
146 | +10.6 at 2 months +20.9 (Rx naïve) c/w + 8.5 (non-Rx naïve) at 6 months |
No CMT data | IOP rise in 5.3%. 1 case of cataract. 1 case on non-penetrating filtration surgery 2 endophthalmitis |
Mean 101.5 days | Treatment naïve eyes gained more VA than non-treatment naïve eyes. | Low |
| Shimura et al.28 | Japan | Real-world data on aflibercept for secondary to CRVO | Aflb Rx at discretion of doctor. |
360 (Only 97 with BCVA data) |
−0.161 ± 0.446 LogMAR (95% CI −0.251 to −0.071) (n = 97) Approximately +9 letters |
−181.4 ± 0.487 (CI −259.0 to −103.8, n = 45 at 24 months) |
One eye progressed to ischemic CRVO. One eye developed NVG and cataract | 24 months | The visual gains in eyes treated with Aflb were not as great as those seen in RCTs which may be due to under treatment. | Low |
| Niedzwiechki et al.29 | FRB! |
12-month outcomes of Ran vs. Aflb for MO in CRVO | Ran vs. Aflb at discretion of doctor. | 296 | Ran +9.8 (5.5,14.1) Aflb +16.6 (12.9,20.4) p < 0.01 both cf baseline |
Ran −252 (−220, −282) Aflb −304 (−276, −333) p < 0.001both cf baseline |
NVG developed in 12 Ran and 4 Aflb eyes p = 0.01 |
12 months | Both Ran and Aflb are effective treatments, however eyes treated with Aflb had greater VA gains and reduction in CMT. | Low |
- Abbreviations: AE, adverse event; Aflb, aflibercept; Bev, bevacizumab; BRVO, branch retinal vein occlusion; cf, compared with; CMT, central macular thickness measured on optical coherence tomography, CRVO, central retinal vein occlusion; Dex-implant, dexamethasone implant; FFA, fundus fluorescein angiogram; FRB!, fighting retinal blindness registry; IVI, intravitreal injection; MO, macula oedema; N/A, not available; NVG, neovascular glaucoma; PANCORES, Pan-American Collaborative Retina Study group; PRP, laser panretinal photocoagulation; Ran, ranibizumab; RCT, randomised controlled trial; RWA, real world analysis; SAE, serious adverse event; SD, standard deviation; VA, best corrected visual acuity; VEGF, vascular endothelial growth factor.
| Authors | Acronym title of study | Title of paper with the results of the study | Intervention | No. of eyes | Mean letter change from baseline by treatment unless otherwise specified, letters (CI unless otherwise specified) | Mean CMT change as measured by OCT in microns | Ocular adverse events | Follow-up | Study outcome | Risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| BVOS study group 19849 | BVOS | Argon laser photocoagulation for macular edema in branch vein occlusion |
Argon laser applied in a grid over leaking area as demonstrated on fundus fluorescein angiography | 139 | Grid laser +1.33 No treatment +0.23 |
Not available | Perforation Bruch's membrane in one eye which did not affect VA. No other complications noted | 37 months | Eyes randomised to macular grid laser had greater visual acuity gains than untreated eyes. |
Unclear |
| Scott et al.30 | SCORE | A randomised trial comparing the efficacy and safety of IVTA with standard care to treat vision loss associated with MO secondary to BRVO: The standard care vs. corticosteroid for retinal vein occlusion (SCORE) study report 6 | Preservative free IVTA 1 mg vs. preservative free IVTA 4 mg vs. macular laser. Retreatment minimum 105 days from last treatment |
411 | IVTA 1 mg +4.4 (−1.1–9.9) IVTA 4 mg +8.0 (0.9–15.2) Macular laser +12.9 (7.4–18.4) |
IVTA 1 mg −293 IVTA 4 mg −286 Macular laser −330 |
IOP lowering medication initiated IVTA 1 mg 7% IVTA 4 mg 41% Macular laser 2% Trabeculectomy IVTA 4 mg 1 Cataract IVTA 1 mg 25% IVTA 4 mg 35% Macular laser 13% |
36 months | IVTA is not recommended for treating MO due to BRVO as it was no more effective than laser and associated with higher rate of adverse events. |
Low |
Haller et al.13 |
GENEVA | Randomised, sham-controlled trial of dexamethasone IV implant in patients with MO due to RVO | Dex-implant 0.7 mg vs. 0.35 mg vs. Sham (single treatment) | 436 | Dex-implant 0.7 mg +7.5 Dex-implant 0.35 mg +5 Sham +5 p = 0.3 |
CRVO/BRVO Dex-implant 0.7 mg 562 ± 188 (−119 ± 203) Dex-implant 0.35 mg 555 ± 204 (−123 ± 212) Sham 539 ± 186 (−119 ± 188) |
Ocular hypertension Dex-implant 0.7 mg 4.0% Dex-implant 0.35 mg 3.9% Sham 0.7% p = 0.002 Cataract Dex-implant 0.7 mg 7.3% Dex-implant 0.35 mg 4.1% Sham 4.5% p = 0.079 Retinal detachment 1 eye in each Dex-implant group |
6 months |
Dex-implant reduced the risk of vision loss and improved the speed and likelihood of visual improvement. Shorter MO duration associated with a greater improvement in BCVA in Dex-implant treated eyes. Day 60 outcomes better than 6-month outcomes suggesting treatment effect is less than 6 months. |
Low |
Campochiaro et al.31 |
VIBRANT | Intravitreal aflibercept for MO following BRVO: 24-week results of the VIBRANT study | Aflb 4 weekly vs. macular laser ± single rescue laser | 183 | Af1b 17.0 Laser 6.9 |
Aflb −280.5 Laser −128.0 p < 0.001 |
Aflb 1 traumatic cataract |
6 months | Monthly Aflb led to significantly greater visual and anatomic outcomes than macular laser | Low |
| Clark et al.32 | VIBRANT | Intravitreal aflibercept for MO following BRVO: 52-wk results of the VIBRANT study | Aflb 4 weekly to 24 weeks then 8 weekly vs. macular laser ± single rescue laser at 36 weeks. Eyes randomised to laser could receive rescue Aflb after 6 months. | 181 | Aflb 17.1 Laser 12.2 |
Aflb −283.9 Laser −249.3 p = 0.0218 |
12 months | Aflb given 8-weekly after monthly treatment for 6 months maintained visual and anatomic gains to 12 months. Eyes with delayed treatment still improved vision but gains less than those with shorter duration of MO. | Low | |
| Tadayoni et al.33, 34 | BRIGHTER | Individualised stabilisation criteria-driven ran versus laser in BRVO: Six-month results of BRIGHTER | Ran 0.5 mg vs. Ran 0.5 mg with laser vs. laser alone (randomised 2:2:1). Ran given monthly×3 then PRN, laser given 4 monthly if needed. | 455 | Ran +14.8 Ran with laser +14.8 Laser alone +6.0 p < 0.001 Ran vs. laser |
Ran −240.1 Ran with laser −223.3 Laser alone −89.8 p < 0.001 Ran vs. laser |
There were no new ocular or non-ocular safety events. |
6 months (primary outcome of a 24-month study) |
Ran with or without macular laser was superior to laser alone. Shorter duration of MO was associated with better visual outcomes. Presence of macula ischaemia at baseline did not influence VA gains in eyes treated with Ran. |
Unclear |
| Tadayoni et al.34 | BRIGHTER | Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion: 24-Month results of the BRIGHTER study |
Ran 0.5 mg vs Ran 0.5 mg with laser vs. laser alone (with rescue Ran 0.5 mg after month 6). After 3 monthly loading doses, eyes randomised to Ran with or without laser were assessed monthly and treated PRN with Ran. |
380 | Ran alone +15.5 Ran with laser +17.3 Laser alone +11.6 p < 0.001 Ran vs. Laser Ran with laser noninferior to Ran alone |
Ran alone −224.7 Ran with laser −248.9 Laser alone −197.5 |
There were no reports of neovascular glaucoma or iris neovascularisation. | 24 months | Ran with or without laser given PRN after 3 loading doses led to greater visual gains than macular laser alone. The addition of laser to Ran did not lower treatment need or improve outcomes. Eyes with delayed treatment with Ran still improved vision but gains less than those with shorter duration of MO. | Unclear |
| Vader et al.1 | BRVO | Comparing the efficacy of Bevacizumab and ranibizumab in RVO | Bev 4 weekly vs. Ran 4 weekly | 133 | Bev +14.2 ± 11.2 Ran + 14.0 ± 10.2 p = 1.0 |
Bev −232. ±199.9 Ran −214.3 ± 176.5 p = 0.513 |
Bev 7.1% Ran 9.2% p = 0.509 |
6 months | Bev given 4 weekly was noninferior to Ran given 4-weekly | Low |
| Li et al.17 | China Ozurdex RVOSG | Safety and efficacy of dexamethasone intravitreal implant for treatment of macular oedema secondary to retinal vein occlusion in Chinese patients: randomised, sham-controlled, multicentre study | Dex-implant 0.7 mg vs. sham. One off treatment. | 131 | 2 months Dex-implant +11.4 (SD 9.6) Sham +4 (SD 10) 6 months Dex-implant +6.7 Sham +6.7 |
2 months Dex-implant −323 (SD 189) Sham −83 (SD 187) 6 months Dex-implant −125 Sham −190 |
IOP rise >10 mmHg Dex-implant 27% Sham 1.5% |
6 months | Dex-implant improved visual and anatomic outcomes 2 months after injection, but this was not sustained for 6 months. Increase in IOP more common in Dex-implant treated eyes but resolved by 4 months. | Low |
| Wei et al.35 | BLOSSOM | Efficacy and safety of ranibizumab in Asian patients with branch retinal vein occlusion: Results from the randomised BLOSSOM study | Randomised 2:1 to receive Ran 0.5 mg or sham. Ran given monthly × 3 then PRN. Sham monthly × 5 then Ran 0.5 mg PRN from month 6 |
283 | Ran +12.5 Sham +5.0 |
Ran −280.0 (−291.6 to −268.4) Sham −269.7 (−286.2 to −253.1) |
No new safety concerns | 12 months | Ran is an effective treatment for MO due to BRVO in Asian patients when given PRN after 3 loading doses. Early visual gains maintained to 12 months. Better results seen in eyes with shorter duration of MO. | Low |
| Hattenbach et al.36 | COMRADE B |
Head-to-head comparison of ranibizumab PRN versus single-dose dexamethasone for branch retinal vein occlusion (COMRADE-B) | Ran 0.5 mg monthly for 3 months followed by PRN vs. single Dex-implant 0.7 mg | 215 | Ran +14.9 (±9.86) Dex-implant +10.1 (±9.51) |
Ran −230.6 (±169.3) Dex-implant −112.3 (±172.1) |
Ocular hypertension Ran 0% Dex-implant 7% |
6 months | Ran given PRN after 3 loading doses resulted in greater VA gains at 6 months than eyes treated with a single-dose Dex-implant. Retreatment with Dex-implant may be required earlier than 6 months. | Low |
| Bandello et al.37 | A 12-month, multicentre, parallel group comparison of dexamethasone intravitreal implant versus ranibizumab in branch retinal vein occlusion |
Ran 0.5 mg monthly for 5 months, then PRN from month 6–11. Dex-implant 0.7 mg at baseline and month 5 with the option of retreatment at either month 10 or 11 |
307 | Ran +17.4 Dex-implant +7.4 p < 0.001. |
Ran −252 Dex-implant −227 p = 0.084 |
Eyes treated with Dex-implant had a higher risk of IOP elevation, cataract incidence and progression than eyes treated with Ran. The IOP elevation observed in Dex-implant treated eyes was transient but recurrent. | 12 months | Ran and Dex-implant both led to improved visual and anatomic outcomes. However Dex-implant was not ‘non-inferior’ than Ran. Dex-implant was associated with an increased risk of intraocular pressure elevation and cataract progression, but a lower injection burden, compared to Ran. |
Unclear |
- Abbreviations: Aflb, aflibercept; Bev, bevacizumab; BRVO, branch retinal vein occlusion; cf, compared with; CMT, central macular thickness measured on optical coherence tomography, CRVO, central retinal vein occlusion; CVOS, central vein occlusion study; CVOSG, Central retinal vein occlusion study group; Dex-implant, dexamethasone implant; ERM, epiretinal membrane; IOP, intraocular pressure; IV, intravitreal; MO, macula oedema; No., number; NV, neovascularisation; NVG, neovascular glaucoma; NVI, neovascularisation of the iris; OCT, optical coherence tomography; PRN, as required; Ran, ranibizumab; RVO, retinal vein occlusion, SD, standard deviation; VA, best corrected visual acuity; VEGF, vascular endothelial growth factor.
| Authors | Country of study | Title of study | Intervention | No. of pts | Mean letter change from baseline | Mean CMT change (microns) | Ocular adverse events | Follow-up | Study outcome | Risk of bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Eter et al.20 | Germany | Dexamethasone intravitreal implant in retinal vein occlusion: real life data from a prospective, multicentre clinical trial | Dex-implant given at discretion of doctor. |
366 | Dex-implant all BRVO eyes +6 Dex-implant MO <90 days +9 MO 90–180 days +3 MO > 180 days +3 |
−100 (SD 280) |
BRVO Laser trabeculoplasty 1.6% IOP lowering started 8.5% BRVO/CRVO 6.1% baseline phakic eyes underwent cataract extraction. 0.3% baseline phakic eyes cataract related ADR |
6 months | Dex-implant is effective in improving VA and reducing CMT in eyes with BRVO. The largest gains in VA occurred in patients with recent onset MO confirming benefit of early treatment. Best VA and anatomical outcomes at 6 weeks post treatment. |
Low |
| Korobelnik al 201614 | France | Two-year, prospective, multicentre study of the use of Dex-implant intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in the clinical setting in France (LOUVRE) |
Enrolment/Dex-implant day 0 (baseline); Further Rx at discretion of doctor. Primary outcome was change VA baseline to month 6 patients treated 0.7 mg Dex-implant | 202 | BRVO only 6 months +5.5 (19.6) 24 months +4.8 (20.5) BRVO/CRVO 6 months 1 Dex-implant 6.1 (2.3) >2 Dex-implants 6.0 (1.5) >1 Dex-implant +other RVO Rx 3.0 (1.8) |
CRVO/BRVO 6 months −190 24 months −180 BRVO/CRVO 6 months 1 Dex-implant 190 >2 Dex-implants 180 >1 Dex-implant +other RVO Rx 205 |
CRVO/BRVO IOP >5 mmHg 25.9% IOP > 30 mmHg 0.3% Cataract 23.9% |
24 months | Although Dex-implant was an effective treatment, it was associated with increased risk of cataract progression and increase in intraocular pressure. Patients switched from Dex-implant to other RVO treatments due to inadequate response did not have improved outcomes. |
Low |
| Casselhom de Salles and Epstein 202121 | Sweden | Real-life study of the use of anti-VEGF therapy vs. Dex-implant for treatment of MO in RVO | Anti-VEGF 3× monthly then PRN vs. Dex-implant PRN 3 monthly | 249 | Anti-VEGF + 9.8 ± 20.4 Dex-implant −2.1 ± 23.4 p < 0.05 |
Anti-VEGF −226 ± 174 Dex-implant −140 ± 243 p < 0.05 |
NV occurred in 14% of eyes. NV elsewhere (rather than on disc or iris) was most common location occurring in 71% of these patients. |
Mean follow up 34 months | Anti-VEGF was more effective than Dex-implant in treating eyes with MO due to BRVO. | Low |
| Vaz-Pereira 201738 | Portugal | Real-world outcomes of anti-VEGF treatment for RVO in Portugal | Ran vs. Bev. VA assessed at 3 time points: time of diagnosis, 6 months and 12 months after initiating treatment |
124 with BRVO | Ran and Bev 6 months +13 12 months +12 |
Ran and Bev 6 months −202 12 months −226 12 months Ran −274 Bev −270 |
Not available | 12 months | Both Ran and Bev are effective treatments. There were no significant differences between outcomes of eyes treated with Bev compared to Ran (although data were not provided separately in the manuscript). | Low |
| Ciulla et al.24 | USA | Visual acuity outcomes and anti-VEGF therapy intensity in MO due to RVO: a real-world analysis | Bev, Aflb or Ran Rx at discretion of doctor. | 8876 | All anti-VEGF + 9.4 (+8.94 to +9.78) |
Not available | Not available | 12 months | Although anti-VEGF was an effective treatment, the gains in vision in this RWOS were not as good as seen in RCTs, perhaps due to undertreatment. Mean change in VA correlated with treatment intensity. Patients with better VA at presentation were more susceptible to vision loss. | Low |
| Hunt et al.39 | FRB! | Twelve-month outcomes of ranibizumab vs. aflibercept for MO BRVO: data from the FRB! Registry | Ran or Aflb Rx at discretion of doctor. | 322 | Ran +10.8 (8.2–13.4) Aflb +10.9 (8.3–13.5) p = 0.59 |
Aflb −170 (−130 to −164), Ran −147 (−153 to −187) p = 0.001. |
Adverse event in 24 eyes (epiretinal membrane, macular hole, pigment clumping, atrophy). Vitreous haemorrhage occurred in two eyes. One eye developed endophthalmitis. |
12 months | Ran and Aflb are both effective treatments. Visual outcomes were similar for the 2 agents, despite a greater effect of Aflb on CMT and earlier time to first grading of inactivity. | Low |
- Abbreviations: Aflb, aflibercept; Bev, bevacizumab; BRVO, branch retinal vein occlusion; CMT, central macular thickness measured on optical coherence tomography, CRVO, central retinal vein occlusion; Dex-implant, dexamethasone implant; FRB!, fight retinal blindness registry; IVI, intravitreal injection; MO, macula oedema; NV, neovascularisation; pts, patients; Ran, ranibizumab; SD, standard deviation; VEGF, vascular endothelial growth factor; VA, best corrected visual acuity.
| Intervention | Effects | |
|---|---|---|
| CRVO | Anti-VEGF | All anti-VEGF agents studied better than sham.7, 14, 15 Real world results not as good as clinical trials.24, 28 Better than Dex-implant in real-world setting.21, 22 Most studies found no difference in outcomes when comparing different anti-VEGF agents23, 24 except the registry study FRB! which reported better visual and anatomical outcomes compared with ranibizumab29 |
| Intravitreal bevacizumab | Non-inferior to ranibizumab when given monthly.1, 2 Non-inferior to aflibercept when each given monthly1, 18 but when given monthly for three injections followed by PRN it was not non-inferior19 | |
| Intravitreal ranibizumab | Is an effective treatment for MO from CRVO with both 0.3 and 0.5 mg14 and is safer and more effective than 6 monthly Dex-implant16 | |
| Intravitreal aflibercept | Efficacious when given monthly less effective when switched to PRN with three monthly assessments14 however was effective when given PRN with monthly reviews.19 Non-inferior to ranibizumab7, 15 more effective than ranibizumab in real-world setting40 | |
| Dex-implant | Better than sham.13 Treatment effect approximately 3–4 months.16, 22 More ocular adverse events than anti-VEGF16, 22 but with fewer injections required22 | |
| IVTA | Better than sham. 1 mg is as effective as 4 mg but is associated with lower risk of ocular adverse events, thus 1 mg dose recommended12 | |
| Macular laser | Does not improve visual outcomes compared to observation11 | |
| Treatment timing | Early treatment (shorter duration of MO) associated with better visual outcomes7, 12, 15 When switching to PRN dosing, monthly monitoring prevents decline in visual and anatomical gains15, 41 |
|
| BRVO | Anti-VEGF | No difference between anti-VEGF agents when given 4-weekly.23, 24 In a RWOS, aflibercept had greater effect on anatomy and time to resolution of MO but not vision39 Real world outcomes not as good as clinical trial outcomes24, 28, 42 |
| Intravitreal bevacizumab | As effective as ranibizumab38 | |
| Intravitreal ranibizumab | Better with or without laser, than laser alone34 | |
| Intravitreal aflibercept | Better than laser33 | |
| Dex-implant | Improvement in visual and anatomical outcome best at 6 weeks to 3 months post injection20 Less effective than anti-VEGF and with higher incidence of ocular adverse events43 |
|
| IVTA | No better than macular laser but with higher rates of adverse events12 | |
| Macular laser | Better than observation43 | |
| Treatment timing | Early treatment (shorted duration of MO) associated with better visual outcomes20, 33 |
- Abbreviations: BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; Dex, dexamethasone; FRB!, fight retinal blindness registry; IVTA, intravitreal triamcinolone acetonide; MO, macula oedema; RON, Radial optic neurotomy, RWOS, real world outcome studies, VEGF, vascular endothelial growth factor.
3.2 Studies
3.2.1 Summary of evidence for CRVO
There were 185 RCTs identified which examined treatment of MO due to CRVO after duplicates were removed. After exclusion of studies with less than 90 eyes and/or less than 6 months follow up, or failure to meet inclusion criteria (studies required visual and/or anatomical outcomes), there were 11 studies which met the inclusion criteria (Figure 1). Of the 11 included RCTs (Table 1), one compared observation to grid pattern laser (CVOS; Central retinal Vein Occlusion Study),11 one compared two doses of preservative free triamcinolone acetonide to sham (SCORE Standard Care vs. cOrticosteroids for Retina vein occlusions),25 two compared intravitreal dexamethasone implant (Dex-implant) to sham13, 17 and one compared dexamethasone implant (Dex-implant) to ranibizumab (COMARADE C).16 One study compared ranibizumab to sham (CRUISE),7 two studies compared aflibercept to sham (COPERNICUS, GALILEO),15 and three compared two or three of the commonly used anti-VEGF agents; bevacizumab ranibizumab and aflibercept (SCORE 2,18 BRVO, bevacizumab ranibizumab for retinal vein occlusion,1 LEAVO, lucentis eylea avastin retinal vein occlusion19).
3.2.2 Intravitreal corticosteroids
The first intravitreal agent used in the management of MO due to CRVO was triamcinolone acetonide. The SCORE study compared triamcinolone acetonide at doses 1 and 4 mg to sham/no treatment which was the standard of care at the time. Macular grid photocoagulation had been studied in the Central Vein Occlusion Study, but no significant difference in visual outcome between eyes treated with macular laser and those observed was found at any follow up time point, except there was a trend towards significance in younger patients.14 In the SCORE study, one intravitreal injection of triamcinolone acetonide improved vision compared to observation at 12 months.12 The ocular adverse events of raised intraocular pressure (IOP) and cataract progression were less in the triamcinolone acetonide 1 mg group than the 4 mg group leading to the recommendation that triamcinolone acetonide 1 mg dose be used instead of 4 mg. Later the GENEVA (Global Evaluation of implaNtable dExamethasone in retinal Vein occlusion with macular edema) study group compared the use of Dex-implant 0.7 mg and Dex-implant 0.35 mg to sham. At both doses there was a reduction in the risk of vision loss and improved speed and incidence of improvement in vision.13 The GENEVA studies found that earlier treatment of MO was associated with better visual outcomes which peaked around 60 days after treatment. A Chinese RCT comparing the Dex-implant with sham also reported better anatomical and visual outcomes with Dex-implant compared to sham treatment. Anatomical and visual improvement peaked at 3–4 months after injection. Ocular adverse events included an increase in IOP which normalised by 4 months after each injection of DEX-implant, even with repeat treatments.17
3.2.3 Intravitreal VEGF-inhibitors
The first large RCT using anti-VEGF was the CRUISE study (Central Retinal vein occlUSIon Study).14 The study compared two doses of ranibizumab (0.3 and 0.5 mg) to sham in eyes with MO secondary to CRVO. There were significantly better visual and anatomical outcomes in eyes treated with ranibizumab at 6 months compared eyes treated with sham.14 Following this study, the same group designed a RCT comparing monthly ranibizumab for 7 months followed by PRN treatment (with monthly visits) to fixed monthly injections of ranibizumab for 15 months (SHORE, The Study evaluating dosing regimens for treatment with intravitreal ranibizumab injections in subjects with MO following retinal vein occlusions). Visual and anatomical outcomes did not differ significantly between the two treatment regimens.
The GALILEO and COPERNICUS studies compared aflibercept to sham in eyes with MO due to CRVO7, 15 (VEGF TRAP-EYE: investigation of efficacy and safety in CRVO). Both studies reported better visual and anatomical outcomes in eyes treated with aflibercept compared to sham, particularly when treatment was started early. However, when the patients were switched to PRN treatment and less frequent reviews (three monthly), the effect of treatment diminished.
SCORE 2 study compared the effect of bevacizumab to aflibercept given every 4 weeks for 6 months to eyes with MO due to CRVO and found that bevacizumab was a non-inferior treatment to aflibercept when given monthly.18 The LEAVO (Lucentis, Eylea, Avastin in Vein Occlusion) study compared three anti-VEGF agents: ranibizumab, aflibercept and bevacizumab. Three monthly doses were given followed by PRN dosing for 100 weeks.19 Bevacizumab was non-inferior to ranibizumab but was not non-inferior to aflibercept with regards to the mean change in vision. However, bevacizumab was less effective in resolving MO than ranibizumab or aflibercept.
A single Dex-implant given at baseline was compared to ranibizumab given as three 4-weekly loading doses followed by PRN treatment in the COMRADE C trial.16 At 6 months, vision and anatomical outcomes were better for eyes randomised to ranibizumab than eyes randomised to Dex-implant. There were more ocular adverse events in Dex-implant treated eyes, including cataract, increased IOP and the development retinal neovascularisation.
3.2.4 Results of meta-analysis of visual outcomes of RCTs for management of MO due to CRVO
A total of nine RCTs reported change in BCVA at 6 months encompassing 3512 eyes. Seven studies included an anti-VEGF arm with a mean improvement in BCVA at 6 months of 15.56 letters (95%CI 13.18–17.93, p < 0.01). The eyes treated with anti-VEGF had an improvement in mean vision at 6 months [15.55 (95%CI 13.18–17.93, p < 0.01)] in contrast to eyes treated with the Dex-implant [4.55 (95%CI −1.43 to 10.54, p = 0.14)] or sham [−0.61 (95%CI −2.79 to 1.57, p = 0.58)] (2.1 in Figure 2). All nine studies favoured intravitreal treatment compared to sham or no treatment (2.1 in Figure 2). The pooled SMD of the nine studies was 0.97 letters (95% CI 0.40–1.54). The forest plot resulted in considerable heterogeneity between the included studies (I2 = 96.3%, p < 0.01), a reflection of the different therapies indicated for CRVO.
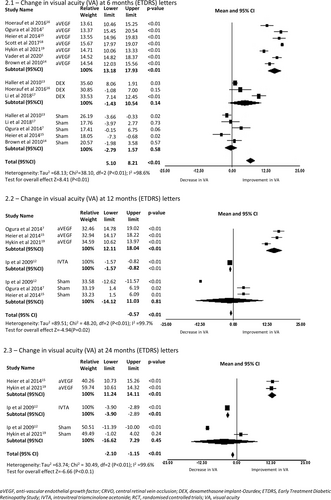
In the three studies with 12 months of follow-up (2.2 in Figure 2) the BCVA gains in the anti-VEGF arms, were maintained, with a mean improvement of 15.07 letters (95% CI 12.11–18.04, p < 0.01) from baseline. The IVTA and sham arms trended lower with a mean change of −1.2 letters (95% CI −1.57 to −8.23, p < 0.01) from baseline for IVTA, and −1.54 letters (95%CI −14.12 to 11.03, p = 0.81), for sham. This trend continued at 24 months (2.3 in Figure 2) with two anti-VEGF studies reporting a mean gain of 12.68 letters (95%CI 11.24–14.11, p < 0.01), whereas IVTA treated eyes lost −3.4 letters (95%CI −3.90 to −2.89, p < 0.01), perhaps a result of cataract progression in the IVTA group. However, any intravitreal treatment with anti-VEGF or corticosteroid resulted in significantly better vision than sham or no treatment at 12 months with a SMD of 2.22 letters (95% CI 0.44–4.01, p < 0.01) (2.2 in Figure 2).
3.2.5 Real-world evidence (Table 2)
A UK retrospective cohort study of 231 patients with MO secondary to CRVO receiving ranibizumab or aflibercept reported on treatment outcomes. Both drugs were effective in improving vision.23 However, because only 14% of the cohort received ranibizumab there was inadequate power to compare the relative efficacy between the two agents. The FRB! registry reported outcomes of 296 eyes with MO due to CRVO. Again, it was reported that both ranibizumab and aflibercept were effective treatments.29 However, there were greater anatomical and visual outcomes seen in eyes treated with aflibercept at 12 months compared to ranibizumab. It is also important to note that despite receiving anti-VEGF, neovascular complications still occurred in the anterior segment (n = 16 eyes) and posterior segment (n = 17 eyes) and were associated with poorer visual outcomes. Laser pan-retinal photocoagulation was performed in 83 eyes. Rubeotic glaucoma more frequently developed in ranibizumab-treated eyes than in aflibercept-treated eyes (12 vs. 4 aflibercept p = 0.01). However the number of injections, irrespective of the agent were also strongly associated with rubeotic glaucoma (p < 0.001).29
Ciulla et al.24 reviewed the USA Vestrum Health Retina Database which included 6737 eyes treated with bevacizumab, aflibercept or ranibizumab as management of MO due to CRVO. Eyes gained an average of 7.1 letters (CI +6.31 to +7.95, p < 0.001) at 12 months with no differences found between the anti-VEGF agents. The Pan-American Collaborate Retinal Study group (PACORES) performed a multicentre retrospective case series of 102 eyes treated with bevacizumab for MO due to CRVO and reported a mean gain of 0.22 LogMAR letters (approximately nine letters) at 5 years. Eyes with better baseline BCVA and shorter duration of symptoms were more likely to achieve better BCVA.26 The LUMINOUS study25 reported visual improvement in CRVO eyes treated with ranibizumab at 1 year in routine clinical practice (+10.8 letters; SD19.7). Anti-VEGF real world data in eyes with MO due to CRVO have not demonstrated unexpected non-ocular safety outcomes.
The UK EMR database users' group22 retrospectively reviewed 4619 files of patients with MO secondary to CRVO and found visual improvements were greater in eyes treated with anti-VEGF than those treated with Dex-implant, although with a larger treatment burden. The endophthalmitis rate was also higher in the Dex-implant treated eyes (0.01% anti-VEGF vs. 0.2% Dex-implant). The rate of endophthalmitis following Dex-implant in another prospective observational study was similar at 0.25% (2/790 injections),27 whereas a German Dex-implant study reported no cases of endophthalmitis among 573 patients who were followed for 6 months.20 Both these prospective, observational studies treating eyes with Dex-implants for MO found better visual outcomes in treatment naive eyes with a shorter duration of MO.
3.2.6 Results of meta-analysis of visual outcomes of real-world studies of eyes with MO due to CRVO
A total of 3 RWOS reported change in BCVA at 6 months encompassing 479 eyes. There was a mean improvement of 12.14 letters (95%CI 7.34–16.94, p < 0.01) (4.1 in Figure 4) in the anti-VEGF arms, and 14.70 letters (95% CI 12.43–16.97, p < 0.01) in the Dex-implant arms. The forest plot resulted in considerable heterogeneity between the included studies (I2 = 86.3%, p < 0.01), a reflection of the different study designs and intravitreal therapy used (Figure 3).

Six RWOS reported 12 months of follow-up in eyes with CRVO (4.2 in Figure 4). There was a mean improvement in BCVA of 11.35 letters (95% CI 8.86–13.84, p < 0.01) from baseline in the anti-VEGF treatment arms. Two studies reported visual outcomes at 24 months (4.3 in Figure 4). Eyes with CRVO treated with ant-VEGF reported a mean gain of vision of 9.29 letters (95%CI −1.00 to 19.58, p = 0.08). However, visual gains declined at 36 months with 2 studies reporting a mean of 3.79 letters (95% CI −2.34 to 9.92, p = 0.02). Eyes receiving anti-VEGF therapy did better on average than those receiving Dex-implant with a SMD 0.37 (95% CI 0.16–0.59, p < 0.01) at 36 months (4.4 in Figure 4).
3.3 Summary of evidence for BRVO
There were 187 RCTs identified which examined treatment of MO due to BRVO after duplicates were removed. After exclusion of studies with less than 90 eyes and/or less than 6 months follow up, or failure to meet inclusion criteria (studies required visual and/or anatomical outcomes), 13 RCTs were included (Figure 1 and Table 3). One RCT compared macular grid laser to observation (Branch Vein Occlusion Study or BVOS9), two compared aflibercept to macula laser (VIBRANT,31, 32 BRIGHTER33), one compared intravitreal steroid to laser (SCORE30), one compared intravitreal steroid to sham (GENEVA13), one compared Dex-implant to sham (China Ozurdex RVOSG17), three compared Dex-implant to ranibizumab (COMRADE,44 COMRADE B,36 Bandello et al.37). One study compared bevacizumab to ranibizumab (BRVO1). Three studies compared ranibizumab to sham (BRAVO,40 HORIZON,45 BLOSSOM35).
3.3.1 Macular laser
Macular laser photocoagulation was the first proven treatment to improve vision in eyes with MO due to BRVO in the BVOS.9 The results of the BVOS9 led to macular laser becoming the standard of care for eyes with visual impairment due to MO secondary to BRVO. The BVOS9 was conducted in the mid-1980s, and patients waited 3 months before enrolment to allow for spontaneous resolution of MO before receiving laser. Eyes without macular ischaemia and vision between 20/40 and 20/200, had a 65% chance of gaining two lines of visual acuity when treated with macular laser versus 37% of control eyes.9
3.3.2 Intravitreal corticosteroids
After the BVOS, the SCORE (Standard Care vs. cOrticosteroids for REtina vein occlusion) study compared macular laser to intravitreal triamcinolone acetonide.30 Compared to macular laser, intravitreal triamcinolone acetonide did not demonstrate a greater visual benefit but was linked to increased incidence of ocular adverse events.30
Later, the effect of another corticosteroid formulated for extended-delivery, Dex-implant, was assessed in the GENEVA study, a randomised, sham-controlled trial which included eyes with BRVO and CRVO. BRVO eyes randomised to Dex-implant 0.7 mg were more likely to improve vision than those randomised to sham, however, Dex-implant treated eyes were more likely to develop ocular adverse events such as cataract and elevated IOP. The treatment effect appeared to be close to 3–4 months, however, eyes were only eligible to receive a single Dex-implant at baseline with the primary outcome being at 6 months. The China Ozurdex study also compared a single injection of Dex-implant 0.7 mg to sham at 6 months with similar outcomes.17
The COMRADE B37 study compared ranibizumab 0.5 mg monthly for 3 months followed by ranibizumab PRN to a single Dex-implant 0.7 mg in eyes with MO due to BRVO. At 6 months, ranibizumab treated eyes had greater mean BCVA gains than Dex-implant treated eyes. This difference was more evident at 1 year in the COMRADE extension study40 (even with a second Dex-implant allowed in the second 6 months). Ranibizumab also had a better safety profile. Bandello et al.37 compared the same agents although retreatment with Dex-implant was at 5 months. Although the study was underpowered, the authors concluded that Dex-implant was not ‘non-inferior’ to ranibizumab. Dex-implant treated eyes also had an increased risk of IOP elevation and cataract progression compared to ranibizumab treated eyes.
3.3.3 Intravitreal VEGF-inhibitors
The first large RCT using anti-VEGF in eyes with MO due to BRVO was the BRAVO40 study (Branch RetinAl Vein Occlusion Study). The study compared two doses of ranibizumab (0.3 and 0.5 mg) to sham, in eyes with MO secondary to BRVO. Eyes treated with ranibizumab had significantly better visual and anatomical outcomes at 6 months than eyes randomised to receive sham.14 The BRIGHTER study found that ranibizumab with or without macular laser was superior to laser alone.33, 34
The VIBRANT study compared intravitreal aflibercept to macular laser in eyes with MO due to BRVO at 24 and 52 weeks. Monthly aflibercept led to significant greater visual and anatomic outcomes than macula laser. In the study, intravitreal aflibercept was given 4 weekly for 6 months, then 8-weekly. Visual and anatomic improvements seen at 6 months were maintained to 1 year.14, 31, 32
The BRAVO,40 HORIZON45 and BLOSSOM35 studies compared injections of aflibercept to sham in eyes with MO due to BRVO. As with CRVO eyes, better visual and anatomic outcomes were seen in eyes treated with aflibercept compared to sham and the treatment effect was greater when treatment was started early.33, 34 Although there are few data on long term outcomes of eyes with RVO receiving anti-VEGF injections,46 VA appears to be better maintained compared to eyes receiving anti-VEGF for neovascular age related macular degeneration.
3.3.4 Results of meta-analysis of visual outcomes of RCTs in eyes with MO due to BRVO
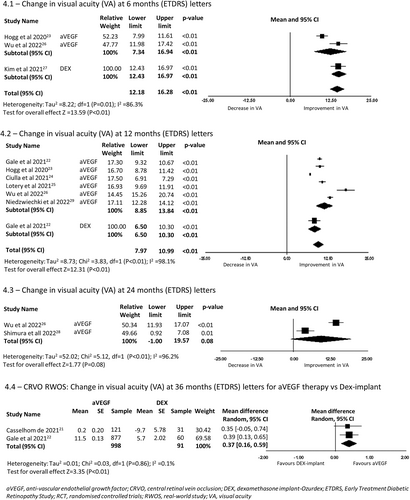
A total of eight RCTs of BRVO reported change in BCVA at 6 months encompassing 2623 eyes. There was a mean improvement in vision of 14.54 letters (95%CI −13.2 to 15.88, p < 0.01) (5.1 in Figure 5) in the anti-VEGF arms, 6.29 letters (95%CI 5.51–7.08, p < 0.01) in the Dex-implant arms, and 4.65 letters (95%CI 3.99–5.31, p < 0.01) in the IVTA arms. All eight studies favoured intravitreal treatment compared to laser or sham (3.3 in Figure 3). The pooled SMD of six studies comparing treatments was 0.5 letters (95% CI 0.21–0.78). The forest plot resulted in considerable heterogeneity between the included studies (I2 = 87.5%, p < 0.01).
In the five RCTs of eyes with BRVO and 12-month outcomes (5.2 in Figure 5), the mean BCVA improved by 15.91 letters (95% CI 14.22–17.59, p < 0.01) from baseline in eyes treated with anti-VEGF, 7.4 letters (95% CI 5.68–9.12, p < 0.01) in eyes treated with the Dex-implant, and 4.95 letters (95%CI 4.20–5.70, p < 0.01) in eyes treated with IVTA. Three RCTs reported 12-month outcomes after macular laser in eyes with BRVO. In these studies, there was a mean gain in vision of 8.53 letters (95%CI 2.84–14.20, p < 0.01) from baseline to 12 months.
In the 2 RCTs of eyes with BRVO reporting outcomes at 24 months (5.3 in Figure 5), one study reported that eyes treated with anti-VEGF gained a mean of 15.5 letters (95% CI 13.38–17.62, p < 0.01), eyes treated with anti-VEGF and laser gained 17.40 letters (95%CI 15.27–19.52, p < 0.01), eyes treated with IVTA 5.13 letters (95%CI 4.09–6.16, p < 0.01) and eyes receiving macular laser gained 11.19 letters (95%CI 10.09–12.28, p < 0.01).
Intravitreal therapy was significantly better than laser or sham at 12 months with a SMD of 0.51 letters (95% CI 0.23–0.79, p < 0.01) (3.4 in Figure 3). This effect was lost at month 24 with a SMD of −0.23 (95% CI −1.35 to 0.90, p = 0.69) (3.5 in Figure 3).
3.3.5 Real-world evidence (Table 4)
The visual acuity gains seen in RCTs have not been replicated in RWOS.24, 42 However, they have still confirmed the primary role of anti-VEGF for the treatment of MO associated with BRVO. From the six included studies, two examined outcomes using Dex-implant,20, 47 one compared anti-VEGF to Dex-implant,21 one compared ranibizumab to aflibercept,23 one ranibizumab to bevacizumab,38 and one examined outcomes using any anti VEGF agent.24 As seen in RCTs, early treatment was associated with better visual gains.20
The two non-comparative RWOS20, 48 which assessed outcomes of eyes treated with Dex-implant reported that Dex-implant was an effective treatment of MO due to BRVO. Best visual and anatomical outcomes were seen 6 weeks post treatment.20 However, treatment was associated with an increased risk of cataract and elevated IOP.
All anti-VEGF agents (bevacizumab, ranibizumab and aflibercept) were found to be effective treatments of MO associated with BRVO in RWOS.24, 38, 39 Anti-VEGF was found to be more effective than Dex-implant in improving vision and reducing MO.21
Ang et al.42 performed a meta-analysis of real-world evidence on outcomes of treatment of MO due to BRVO. The review included 2530 eyes followed for 12 months. Although visual and anatomical gains were achieved in eyes treated with anti-VEGF in RWOS, they were not as impressive as seen in the seminal RCTs. This is likely due to multiple factors including poorer treatment compliance, fewer injections in RWOS and differences in patient baseline characteristics.
3.3.6 Results of a meta-analysis of visual outcomes of real-world studies of BRVO
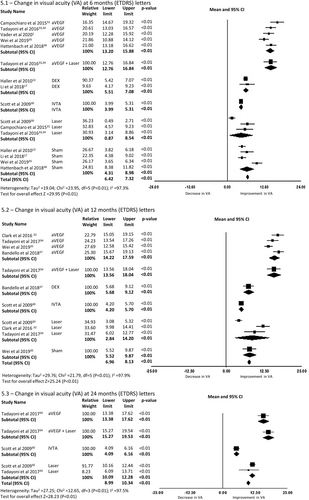
A total of three RWOS reported change in VA at 6 months encompassing 692 eyes. There was a mean improvement in BCVA of 13.00 letters (95%CI 11.31–14.69, p < 0.01) in the one study reporting outcomes for eyes treated with anti-VEGF and 5.82 letters (95% CI 5.03–6.61, p < 0.01) in the two studies reporting outcomes for eyes treated with Dex-implant (6.1 in Figure 6).
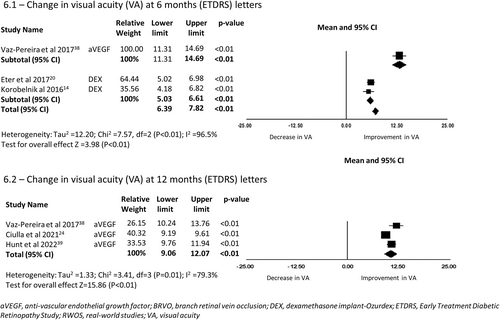
Three RWOS including eyes with BRVO reported VA change at 12 months, encompassing 9322 eyes. There was a mean improvement of 10.57 letters (95% CI 9.06–12.07, p < 0.01) in BCVA from baseline for eyes receiving anti-VEGF therapy (6.2 in Figure 6).
4 DISCUSSION
4.1 Clinical recommendations for CRVO
Intravitreal treatment with either anti-VEGF agents or corticosteroids in eyes with MO due to CRVO is more effective than the natural history. Our results support previous meta-analyses despite having different inclusion criteria.8, 49 Anti-VEGF agents are considered first line treatment due to fewer ocular adverse events than intravitreal corticosteroid and better visual outcomes when corticosteroids are given 6-monthly. Prompt treatment is associated with better visual outcomes.7, 12, 13, 15 The choice of VEGF inhibitor does not appear to be important with similar visual and anatomic outcomes, as long as it is given as often as required, and patients kept under close observation when treatment is given PRN.7, 15 Ocular adverse events appear comparable between the different anti-VEGF agents.
Intravitreal corticosteroids have been used for treatment of MO50 for decades. The risks of cataract and raised IOP12, 13, 16, 17, 36, 51 mean that they should be cautiously used, especially in the setting of younger phakic patients. For this reason, they are considered second line treatments for MO due to CRVO. There is a need for more long-term studies using corticosteroid with more frequent treatment than 6-monthly to properly assess their maximal effectiveness when ocular adverse events are managed carefully. Macular laser is not recommended as a treatment for eyes with MO due to CRVO.11
Data from RWOS remind clinicians of the risk of eyes developing neovascular glaucoma, and retinal neovascularisation, which is more common in eyes that receive fewer injections.29 Although one RWOS suggested that aflibercept was associated with better visual and anatomical outcomes at 12 months than ranibizumab or bevacizumab, a large USA RWOS did not find any difference between any of the anti-VEGF agents.24 Overall, the visual outcomes in RWOS are not as good as those of RCTs. This is likely to be due to undertreatment but may be due to a difference in inclusion and exclusion criteria. RWOS tend to include all patients receiving treatment for the condition, whereas RCTs exclude many patients due to concurrent eye or systemic disease, (which may limit visual potential or be associated with poorer compliance).
4.2 Clinical recommendations for BRVO
As in eyes with CRVO, anti-VEGF agents are considered first line treatment for eyes with MO due to BRVO, supporting previous meta-analyses despite different inclusion criteria.8, 46, 49 Again, prompt treatment is paramount in order to gain maximum visual outcomes.20, 32, 35 The anti-VEGF agent used does not appear to influence outcomes. Ideally intravitreal injections should be given monthly until stability is reached, followed by close monitoring if a PRN regime is used.
Intravitreal triamcinolone acetonide was no more effective than laser in eyes with MO due to BRVO but was associated with a greater risk of ocular adverse events9 and is therefore not recommended. Although intravitreal dexamethasone implant was more effective than observation, it is generally reserved as a second line treatment due to higher rates of ocular adverse events that anti-VEGF therapy (progression of cataract and raised IOP)20-22, 27, 37, 44, 47 and inferior vision acuity outcomes when given 6 monthly.
Laser treatment for MO secondary to BRVO has been proven to be more effective than observation, however it is less effective than anti-VEGF and is therefore also reserved as a second-line or adjunctive treatment. Although not a focus of this review, laser pan retinal photocoagulation remains the standard of care for the treatment of neovascular complications associated with RVO (CVOS11 BVOS9). Of note, a significant number of eyes receiving anti-VEGF still developed neovascular complications.21, 23, 29
4.3 What is next?
A current phase III, randomised, clinical trial is investigating faricimab, a bispecific anti-VEGF, ang-2 inhibitor for treatment of MO due to CRVO (COMINO, ClinicalTrials.gov Identifier NCT04740931, estimated to be completed in late 2023). A similar study is also investigating faricimab for MO secondary to BRVO (BALATON; ClinicalTrials.gov Identifier NCT04740905). The RAPTOR (ClinicalTrials.gov Identifier NCT03802630) and RAVEN (ClinicalTrials.gov Identifier NCT03810313) studies investigated brolucizumab for the treatment of MO due to RVO, but these trials were ceased prematurely due to the concern of increased risk of retinal vasculitis. RIPPLE-1 is a phase II, single-masked dose-ranging study evaluating IBE-815 (a slow-release low dose dexamethasone implant; ClinicalTrials.gov Identifier NCT04576689) for MO due to diabetes or RVO.
4.4 Summary
Anti-VEGF agents should be considered as first line therapy for eyes with MO due to CRVO or BRVO. Visual loss from MO due to CRVO or BRVO is more likely to recover when intravitreal treatment is started early, regardless of which anti-VEGF agent is used. Monthly injections of anti-VEGF agents are required until stability of VA and MO is achieved, after which treatment can be given PRN with assessments 1–2 monthly. Although an alternative is to use a treat and extend approach, there are few data on its outcomes. Second line treatment with intravitreal Dex-implant in eyes with CRVO and BRVO still offers potential for visual improvement with fewer treatment visits, although non treatment visits for evaluation of cataract and IOP are still required. Triamcinolone acetonide 1 mg can be considered for eyes with MO due to CRVO, but not BRVO, as in BRVO eyes, it was no more effective than laser but associated with a greater risk of ocular adverse events. Macular laser is more effective than observation in eyes with MO due to BRVO, but it is less effective than anti-VEGF and thus is used as a second line or adjunctive therapy. Macular laser is no more likely to improve vision in eyes with CRVO than its natural history. A significant number of eyes with CRVO still require laser panretinal photocoagulation to manage neovascular complications.
Clinical trial outcomes of treatment of CRVO and BRVO are superior compared to RWOS. This is likely to represent undertreatment but also several other factors, including a difference in eyes and patients included in RWOS compared to RCTs, and more frequent treatment and better patient compliance seen in patients enrolled in RCTs.
ACKNOWLEDGEMENT
Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
Samantha Fraser-Bell, Sophia L. Zagora, and Elisa E. Cornish have received speaker fees from Novartis, Bayer, Allergan, and Roche. Kimberley Spooner is an employee of Roche but Roche had no input into this manuscript.




