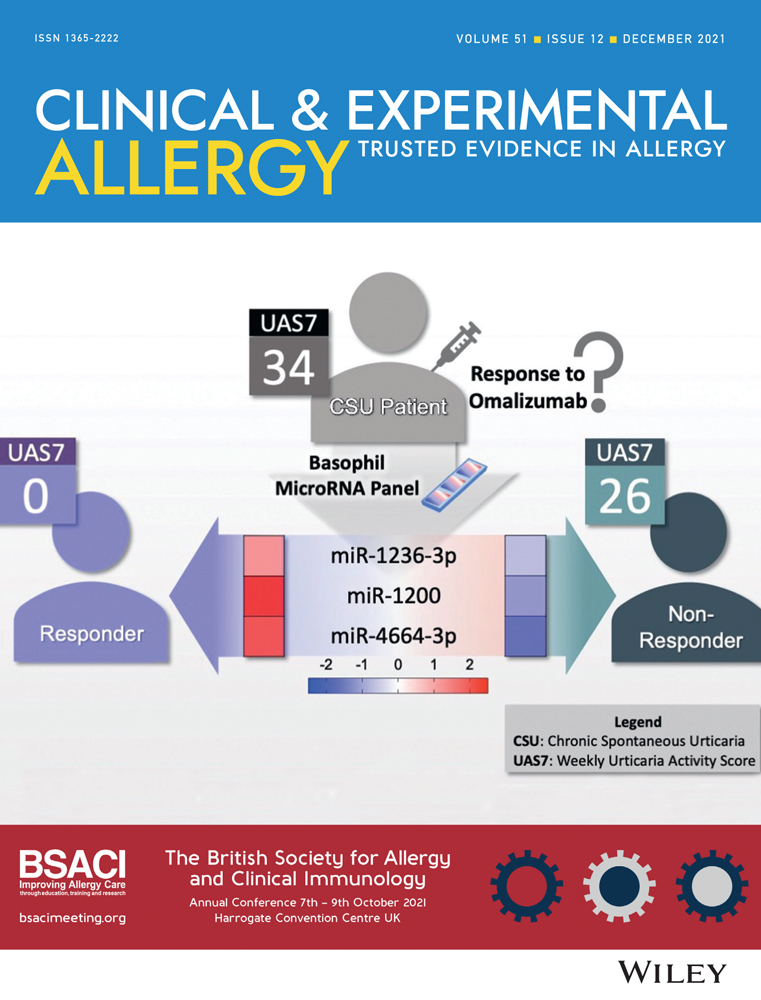
Unique basophil microRNA signature in chronic spontaneous urticaria patients who respond to omalizumab
Funding information
Funding for this investigator-initiated study was provided by Novartis Pharmaceuticals Corporation (grant number CIGE025EUS44T to W.R.H.).

CONFLICTS OF INTEREST
T.A.-S. has patents MicroRNAs as Predictors of Response to Anti-IgE Therapies in Chronic Spontaneous Urticaria and Methods of Treatment using Omalizumab and Ligelizumab pending. J.W.M. reports grant from Novartis Pharmaceuticals Corporation, during the conduct of the study; In addition, J.W.M. has patents MicroRNAs as Predictors of Response to Anti-IgE Therapies in Chronic Spontaneous Urticaria and Methods of Treatment using Omalizumab and Ligelizumab pending. T.K.B. reports grant from Novartis Pharmaceuticals Corporation, during the conduct of the study; In addition, T.K.B. has patents MicroRNAs as Predictors of Response to Anti-IgE Therapies in Chronic Spontaneous Urticaria and Methods of Treatment using Omalizumab and Ligelizumab pending. M.C.A. reports personal fees from Regeneron, outside the submitted work. A.G.A. has nothing to disclose. D.H.P. reports grant from Novartis Pharmaceuticals Corporation, during the conduct of the study; grants from Genentech, DBV Technologies, Aimmune, Regeneron, and NIH/NIAID (Immune Tolerance Network), outside the submitted work. S.A.T. reports grant from Novartis Pharmaceuticals Corporation, during the conduct of the study; grants from Genentech, DBV Technologies, Aimmune, Regeneron, NIH/NIAID (Immune Tolerance Network), and personal fees from Aimmune, outside the submitted work. W.R.H. reports grant from Novartis Pharmaceuticals Corporation, during the conduct of the study; In addition, W.R.H. has patents MicroRNAs as Predictors of Response to Anti-IgE Therapies in Chronic Spontaneous Urticaria and Methods of Treatment using Omalizumab and Ligelizumab pending.