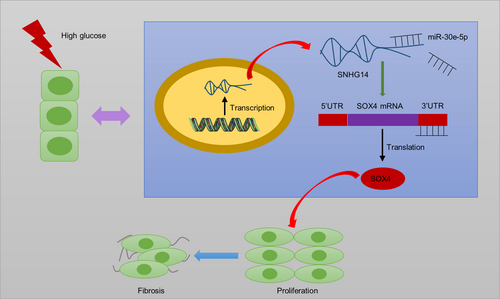
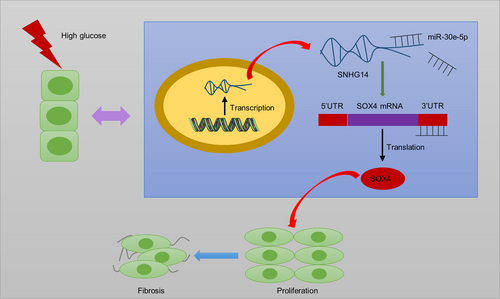
LncRNA SNHG14 silencing attenuates the progression of diabetic nephropathy via the miR-30e-5p/SOX4 axis
YunXia Wang, JiaJia Yang and Chun Wu are co-first authors. They contributed equally to the work.
Abstract
Background
Diabetic nephropathy (DN) is a diabetic complication. LncRNAs are reported to participate in the pathophysiology of DN. Here, the function and mechanism of lncRNA small nucleolar RNA host gene 14 (SNHG14) in DN were explored.
Methods
Streptozotocin (STZ)-induced DN mouse models and high glucose (HG)-treated human mesangial cells (MCs) were used to detect SNHG14 expression. SNHG14 silencing plasmids were applied to examine the function of SNHG14 on proliferation and fibrosis in HG-treated MCs. Potential targets of SNHG14 were predicted using bioinformatics tools and verified by luciferase reporter, RNA pulldown, and northern blotting assays. The functional role of SNHG14 in DN in vivo was detected by injection with adenoviral vector carrying sh-SNHG14 into DN mice. Serum creatinine, blood urea nitrogen, blood glucose, 24-h proteinuria, relative kidney weight, and renal pathological changes were examined in DN mice.
Results
SNHG14 expression was elevated in the kidneys of DN mice and HG-treated MCs. SNHG14 silencing inhibited proliferation and fibrosis of HG-stimulated MCs. SNHG14 bound to miR-30e-5p to upregulate SOX4 expression. In rescue assays, SOX4 elevation diminished the effects of SNHG14 silencing in HG-treated MCs, and SOX4 silencing reversed the effects of SNHG14 overexpression. In in vivo studies, SNHG14 downregulation significantly ameliorated renal injuries and renal interstitial fibrosis in DN mice.
Conclusions
SNHG14 silencing attenuates kidney injury in DN mice and reduces proliferation and fibrotic phenotype of HG-stimulated MCs via the miR-30e-5p/SOX4 axis.
1 INTRODUCTION
Diabetic nephropathy (DN), a serious microvascular complication of diabetes mellitus, affects approximately 50% of patients with both type I and type II diabetes.1, 2 DN is characterized by extracellular matrix accumulation, basement membrane thickening, and glomerular hypertrophy, which are related to glomerular mesangial cell proliferation.3 Hyperglycemic factors including terminal glycosylation products contribute to excess extracellular matrix and lead to renal fibrosis.4 Glomerular mesangial cells (MCs), the main constituents of the glomerulus, are involved in the process of glomerular fibrosis by inducing the synthesis of matrix proteins.5 Glomerular morphological change is the main pathological damage during DN progression. Thus, preventing the proliferation of MCs is considered as a promising strategy for treatment of DN.6 However, the molecular mechanisms related to the alterations in the renal microenvironment are not fully understood; therefore, identifying the underlying mechanisms to discover effective biomarkers for DN or renal fibrosis is of great importance for the treatment of DN.
LncRNAs are noncoding RNA molecules with over 200 nucleotides in length.7 Numerous studies have reported that lncRNAs participate in the pathogenesis of human diseases.8-10 LncRNAs have been demonstrated to play vital roles in DN progression. For instance, upregulated lncRNA ISG20 aggravates renal fibrosis in high glucose (HG)-treated MCs and DN mice.11 LncRNA NEAT1 modulates mitophagy to inhibit cell viability and increase reactive oxygen species production in renal tubular epithelial cells, thus accelerating damage of these cells in DN.12 LncRNA MALAT1 downregulation protects HG-stimulated podocytes from pyroptosis in DN development.13 The small nucleolar RNA host genes (SNHGs) are a special group of lncRNAs. They perform their function in the nucleus (epigenetic modulation and transcriptional regulation) and cytoplasm (translation regulation, miRNA sponging, and posttranscriptional modification), similar to other lncRNAs,14, 15 as well as influence small nucleolar RNAs (snoRNAs) at the intracellular level; in addition, they play important roles in cancer progression.16, 17 Several reports have shown the key roles of SNHG genes in the progression of DN. SNHG1 silencing alleviates hyperglycemia and kidney injury in DN mice.18 Suppression of SNHG5 protects against podocyte injury and progression of DN in mice.19 Silencing SNHG15 attenuates DN progression in pediatric patients.20 It is noteworthy that lncRNA SNHG14 is a newly found molecule mapping to 15q11.2 in humans21; it exerts diverse functions in types of human diseases.22, 23 Additionally, studies have suggested the function of SNHG14 in renal injuries. Yang et al and Shi et al indicated that SNHG14 was upregulated in patients with sepsis, and it promoted HK-2 cell apoptosis and inhibited cell growth under lipopolysaccharide stimulation.24, 25 Xue et al found that silencing SNHG14 protected against acute renal injury induced by ischemia/reperfusion in rats.26 These findings inspire us to investigate the function of SNHG14 in DN-associated renal injury. Interestingly, we found upregulation of SNHG14 in the kidneys of DN models in this study, implying the participant of SNHG14 in DN.
Here, streptozotocin (STZ)-induced DN mouse models and HG-treated human MCs were used to detect SNHG14 expression. We evaluated the role of SNHG14 in renal fibrosis in vitro and in vivo and investigated its underlying mechanism in DN, which may provide insight into the prevention and treatment of DN.
2 MATERIALS AND METHODS
2.1 Bioinformatic analysis
Potential miRNAs binding to SNHG14 were predicted in starBase (https://rnasysu.com/encori/index.php) (CLIP Data: ≥12 datasets). Possible target genes of miR-30e-5p were predicted in miRDB (https://mirdb.org/), targetScan (https://www.targetscan.org/vert_72/), and starBase. The upregulated genes in the glomerulus of DN patients were shown by the dataset GSE30122 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30122).
2.2 Cell culture and treatment
Human glomerular MCs were purchased from ScienCell Research Laboratories (Carlsbad, VA) and cultured in DMEM (Gibco, NY) containing 5% FBS (Gibco) at 37°C in with 5% CO2, followed by treatment with 5 mmol/L D-glucose negative control (NG), 25 mmol/L mannitol, or 25 mmol/L D-glucose (HG) for 24, 48, and 72 h.
2.3 Cell transfection
The shRNA targeting SNHG14/SOX4 (sh-SNHG14/sh-SOX4) was used to silence SNHG14/SOX4. MiR-30e-5p mimics were used to overexpress miR-30e-5p with NC mimics as negative controls. Coding region of SNHG14 or full-length SOX4 was planted into the pcDNA3.1 vectors to elevate the expression of SNHG14 or SOX4 with empty pcDNA3.1 vectors as NCs. All plasmids were purchased from GenePharma (Shanghai, China) and transfected into MCs using Lipofectamine 2000 (Invitrogen, USA).
2.4 Real-time quantitative polymerase chain reaction
Total RNAs from kidneys and MCs were isolated by TRIzol reagent (Invitrogen) and were reverse transcribed to complementary DNA using reverse transcription cDNA synthesis kit (Vazyme, China), and then SYBR Premix Ex TaqTM Kit (Think-Far Technology, Beijing, China) was used for real-time quantitative polymerase chain reaction (RT-qPCR) analysis on 7900HT Fast Real-Time PCR System (Applied Biosystems). GAPDH acted as internal control. Expression of RNA was calculated with the 2−∆∆Ct method.
2.5 Western blotting
Total proteins from MCs and homogenates of renal tissues were extracted by RIPA lysis buffer (Beyotime, Shanghai, China) and separated by 10% SDS-PAGE followed by transfer onto PVDF membranes. The PVDF membranes were incubated with primary antibodies at 4°C overnight and then with secondary antibody at room temperature for 2 h. Primary antibodies include anti-p-cadherin (ab242026; 1:1000; abcam), anti-ZO-1 (ab307799; 1:1000), anti-SOX4 (PA5-72852; 1 μg/mL, ThermoFisher Scientific, USA), anti-fibronectin (FN) (ab268020; 1:1000), anti-collagen IV (COl-4) (ab308360; 1:1000), anti-transforming growth factor beta1 (TGF-β1; ab215715; 1:1000), and GAPDH (ab9485; 1:2500). Protein bands were visualized with an ECL detection kit (Bio-Rad, Hercules, CA) and quantified with Quantity One software (Bio-Rad).
2.6 EdU
EdU staining was performed according to the manufacturer's protocol of EdU Kit (Ribobio, Guangzhou, China). MCs seeded in six-well plates at 2 × 105 cells/well were fixed with 3.7% paraformaldehyde and permeabilized with PBS-Tween-20 for 20 min, respectively, at 25°C. Then, each well was stained with 10 μM EdU and was cultured for 2 h. After 30 min of staining with DAPI (Sigma-Aldrich, MO), the images were observed under a Leica DM200 microscope (Leica, Solms, Germany) and analyzed with ImageJ software (National Institutes of Health, MD).
2.7 Immunofluorescence assay
In brief, the fixed MCs were permeabilized with 0.1% Triton X-100. After blocking with 5% goat serum solution., the slides were added with primary antibodies against FN (ab268020; 1:50), Col-4 (ab308360; 1:500), TGF-β1 (ab170874; 1:50), and SOX4 (ab243041; 2 μg/mL) at 4°C overnight and then with fluorescence-labeled secondary antibody (Proteintech, USA, 1:50) for 1 h. After nuclei were counterstained with DAPI, the intensity was recorded using a fluorescence microscope (Olympus, Japan).
2.8 FISH
As per the protocols of RiboTM lncRNA fluorescence in situ hybridization (FISH) Probe Mix (Green) (Ribobio), MCs in 24-well culture plates were fixed with 4% paraformaldehyde for 10 min, washed, and treated with 1 mL PBS containing 0.5% Triton X-100 for 5 min at 4°C. Then 200 μL prehybridization solution was added to each well for 30 min of blocking at 37°C. Cells were mixed with 250 μL hybridization solution overnight at 37°C in the dark. Cell nuclei were stained with DAPI. Five different fields of view were captured by a laser-scanning confocal microscope (ZEISS, Germany).
2.9 Luciferase reporter assay
Full-length SNHG14 or SOX4 3´UTR sequence with wildtype or mutant binding sites for miR-30e-5p was subcloned into the pmirGLO luciferase vector to construct SNHG14-Wt/Mut and SOX4 3´UTR-Wt/Mut vectors. The 3´UTR and coding sequences (CDS) of SOX4 were inserted into the pmirGLO luciferase vector, denoted as Luc-SOX4-3´UTR and Luc-SOX4-CDS, respectively. All plasmids were synthesized by GenePharma and transfected into MCs using Lipofectamine 2000 for 48 h. Luciferase activity was assessed using Luciferase Reporter Assay System (Promega).
2.10 Biotin-coupled probe RNA pulldown assay
A biotinylated SNHG14 probe (Bio-SNHG14) and NC probe (Bio-NC) were synthesized by GenePharma. The probes were incubated with M280 streptavidin-coupled Dynabeads (Invitrogen) at 25°C for 2 h to generate probe-coated beads, which were then incubated with the cell lysates at 4°C overnight. The beads were washed with wash buffer, and the RNA complexes were then purified with TRIzol reagent and subjected to PCR analysis.
2.11 Northern blotting
DIG Northern Starter kit (Roche, Switzerland) was used for northern blotting analysis. Total RNA samples were denatured in formaldehyde, resolved on a 1% agarose-formaldehyde gel, and then transferred to the nylon membranes (Beyotime). After crosslinking by ultraviolet irradiation (265 nm; 0.15 J/cm2), the membranes were hybridized with a biotin-labeled DNA probe. Finally, RNA signals were measured using a Chemiluminescent Nucleic Acid Detection Module (ThermoFisher Scientific).
2.12 DN mouse model establishment
Male C57BL/6 mice (18–22 g and 6-8 weeks) were obtained from Shanghai Laboratory Animal Company (Shanghai, China). Mice were maintained at 22–24°C under a 12:12 h light/dark cycle. All the protocols were conducted according to the guidelines approved by the Ethics Committee of Huai'an Rehabilitation Hospital (Huai'an, China). DN mouse models in this study were established as mentioned previously.27 Animals were intraperitoneally injected with 50 mg/kg STZ (S0130-100MG-1; LABLEAD, Beijing, China) dissolved in 0.1 M citrate buffer (pH 4.5) (Sigma Aldrich, MO, USA) daily for 5 consecutive days. The control mice received equal amounts of citrate buffer. Two weeks later, the blood glucose of mice was monitored and the animals with more than 300 mg/dL of blood glucose were considered as DN models. Four experimental groups were established: control, DN, DN + sh-NC, and DN + sh-SNHG14 (N = 8/group). Sh-NC or sh-SNHG14 (Vigene Biosciences, Shanghai) was inserted into the adenoviral vector (Life Technologies, Grand Island, NY, USA) to generate adenovirus solution of sh-NC or sh-SNHG14 as previously stated.26 After STZ injection for 2 weeks, adenovirus solution of sh-NC or sh-SNHG14 (20 μL, 107 particles/μL) was delivered into the mice via the tail vein.26 All animals were euthanized after 8 weeks of adenovirus injection.
2.13 Renal function measurement
The urine of mice was collected from metabolic cages to measure 24 h proteinuria using mouse albumin ELISA kits (Abcam, USA). Animals were weighed and anesthetized with 30 mg/kg sodium pentobarbital (Sigma Aldrich) followed by cervical dislocation. The blood taken from the abdominal aorta was centrifugated at 3000 × g for 15 min to obtain the serum. An automatic biochemistry analyzer (Abbott Labs, IL, USA) was employed to measure serum creatinine and blood urea nitrogen levels. The blood taken from the tail vein was used for blood glucose measurement by a glucose analyzer (Roche). The left kidney was weighed to calculate the ratio of kidney weight to body weight.
2.14 Pathological evaluation of kidneys
Kidney tissues of mice were fixed in 4% paraformaldehyde and embedded in paraffin. Sections (5 μm) were cut using Rotary Microtome (Leica, Frankfurt, Germany) and stained with hematoxylin–eosin solution (H&E) (Solarbio, Beijing) and Masson's trichrome (Solarbio) as per the manufacturer's instructions. Photos were obtained by DM5000B microscope (Leica Imaging Systems). The renal tubular injury was using a scoring system as published previously.28 Masson-stained areas were analyzed and quantified with ImageJ software.
2.15 Immunohistochemistry analysis
Renal tissue sections were treated with 0.1 M citrate buffer and boiled for 20 min in a microwave oven. After washing, samples were treated with 3% H2O2 followed by incubation in 5% BAS blocking solution for 30 min at 37°C. The rabbit anti-TGF-β1 (ab215715; 1:500) and rabbit anti-FN (ab268020; 1:2000) were added to incubate with sections in a wet box overnight at 4°C. After washing in PBS solution, sections were incubated with goat anti-rabbit IgG (ab6721; 1:1000) for 1 h and stained with avidin-biotin peroxidase complex (Solarbio) for 20 min at 37°C. Hematoxylin was used for counterstaining. The sections were photographed by an Axio Observer A1 microscope (ZEISS, Germany). Positive areas per high power field were analyzed with ImageJ software and expressed as percentage.
2.16 Statistical analysis
SPSS Software (Version 22.0, Chicago, IL, USA) was used to conduct statistical analyses. p < .05 was considered statistically significant. Statistical values are expressed as mean ± SD of five independent experiments. Student's t test was applied when two groups were compared. One-way analysis of variance was performed for multigroup comparisons.
3 RESULTS
3.1 Upregulated SNHG14 in renal tissues of DN mice and HG-treated MCs
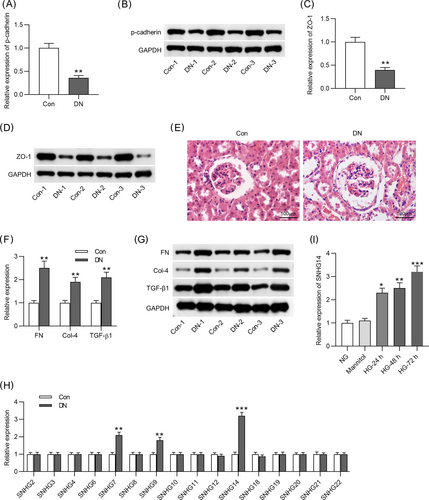
A DN model was established by STZ injection into mouse. Compared with the control group, the levels of p-cadherin and ZO-1 were significantly downregulated in the DN group (Figure 1A–D). Renal pathological alterations were observed by performing H&E staining. In the control mice, renal tissues showed normal glomeruli with clear tubular structure. However, dilated lumen of the renal tubules and enlarged glomeruli were detected in the DN group. There were significant interstitial inflammatory cell infiltration and vacuolarization of tubular epithelial cells (Figure 1E). Additionally, the mRNA and protein levels of fibrosis markers (FN, TGF-β1, and Col-4) were upregulated in DN mice compared with controls (Figure 1F,G). Studies have shown that there are 22 members of the SNHG family (SNHG1 to SNHG22).29 Notably, SNHG1,18 SNHG5,19 SNHG15,20 SNHG16,30 and SNHG1731 have been reported to participate in the development of DN. Therefore, we detected the expression levels of several SNHGs (SNHG2, SNHG3, SNHG4, SNHG6, SNHG7, SNHG8, SNHG9, SNHG10, SNHG11, SNHG12, SNHG14, SNHG18, SNHG19, SNHG20, SNHG21, and SNHG22) that have not been investigated in DN. As Figure 1H indicated, among these SNHGs, SNHG7, SNHG9, and SNHG14 were significantly upregulated in the kidneys of DN mice compared with the control group. In addition, SNHG14 was found upregulated in MCs by HG at 24, 48, and 72 h, compared to the control group (Figure 1I), suggesting that SNHG14 might be related to DN pathogenesis. However, the expression levels of SNHG7 and SNHG9 had no significant change in the control group and HG group (data not shown). Therefore, we investigated SNHG14 in the subsequent experiment.
3.2 SNHG14 silencing inhibits proliferation and fibrosis in HG-stimulated MCs
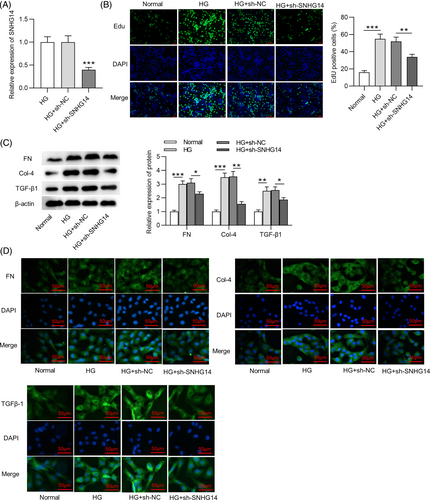
Next, the role of SNHG14 in DN in vitro was explored. RNA interference (RNAi) is a natural process through which expression of a targeted gene can be knocked down with high specificity and selectivity,32 and it enables sequence-specific gene silencing and can be employed to silence virtually any gene, including lncRNAs.33 Short hairpin RNA (shRNA) is an effective method mediating the RNAi effect.34 We used sh-SNHG14 to transfect into MCs. The RT-qPCR analysis showed that SNHG14 expression was reduced in HG-treated MCs after transfection with sh-SNHG14 (Figure 2A). According to EdU, SNHG14 silencing inhibited proliferation of MCs treated with HG (Figure 2B). In MCs with HG stimulation, FN, Col-4, and TGF-β1 protein levels were decreased after silencing SNHG14 (Figure 2C), suggesting that SNHG14 downregulation represses HG-induced fibrotic phenotype in MCs. Additionally, immunofluorescence staining yielded the same results (Figure 2D).
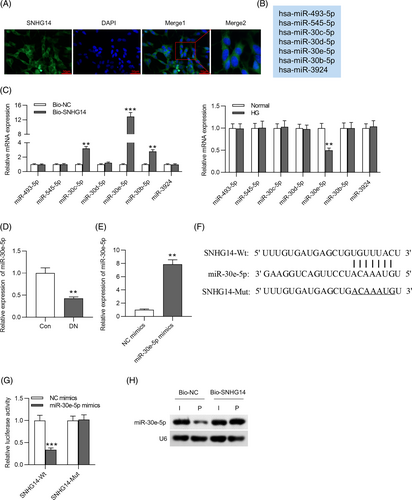
3.3 SNHG14 binds to miR-30e-5p
FISH showed that the majority of SNHG14 was in the cytoplasm in MCs (Figure 3A), suggesting that SNHG14 might exert functions at the posttranscriptional level. The starBase database shows thousands of miRNAs that have binding sites to SNHG14. We set the filter criteria as CLIP Data: ≥12 datasets and obtained seven miRNAs (miR-493-3p, miR-545-5p, miR-30c-5p, miR-30d-5p, miR-30e-5p, miR-30b-5p, and miR-3924) (Figure 3B). The exact RNA sequences of SNHG14 and potential binding miRNAs are shown in Supplementary Figure S1. To identify the miRNAs that can bind to SNHG14, we performed RNA pulldown assay and found that three miRNAs (miR-30c-5p, miR-30e-5p, and miR-30b-5p) showed binding capacity to SNHG14 (Figure 3C, left panel). However, only miR-30e-5p was significantly downregulated in HG-treated MCs among these candidates (Figure 3C, right panel). We thus selected miR-30e-5p as the research object. Additionally, miR-30e-5p was downregulated in DN mice compared with control (Figure 3D). To conduct luciferase reporter assay, we used miR-30e-5p mimics to upregulate the expression of miR-30e-5p in MCs. The overexpression efficiency of miR-30e-5p mimics was verified by RT-qPCR. The results showed that miR-30e-5p expression was significantly upregulated after transfection (Figure 3E). Binding site of miR-30e-5p to SNHG14 was predicted by starBase (Figure 3F). In luciferase reporter assay, miR-30e-5p mimics reduced the luciferase activity of SNHG14-Wt in MCs, and neither NC mimics nor miR-30e-5p mimics affected the luciferase activity of SNHG5-Mut (Figure 3G). Furthermore, the results of northern blot analysis showed that the Bio-SNHG14 probe could bind to miR-30e-5p in MCs (Figure 3H).
3.4 MiR-30e-5p targets SOX4
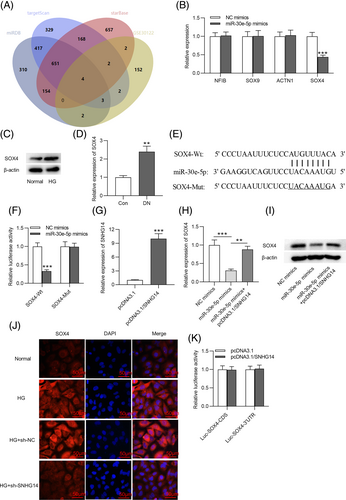
To further investigate the ceRNA network involving SNHG14, the target gene of miR-30e-5p was also explored. The miRDB, targetScan, and starBase databases are common online tools used to predict the target genes of miRNA.35 The GSE30122 dataset shows the differentially expressed genes in kidney tissues from patients in the DN group and normal controls.36 To obtain the genes that are upregulated in DN and can bind to miR-30e-5p, we used Venn diagram to screen the overlapping genes between the miRDB, targetScan, and starBase databases and the GSE30122 dataset. Four genes (NFIB, SOX9, ACTN1, and SOX4) were obtained, as shown in Figure 4A. Subsequently, we detected the expression of these four genes in MCs transfected with miR-30e-5p mimics. We found that SOX4 was notably downregulated in MCs transfected with miR-30e-5p mimics, and the other genes (NFIB, SOX9, and ACTN1) had no detectable change (Figure 4B). Additionally, the protein expression of SOX4 was elevated in MCs stimulated with HG and in DN mice (Figure 4C,D). We searched the miRDB, targetScan, and starBase databases to identify the binding site of 3´UTR SOX4 to miR-30e-5p (Figure 4E). As Figure 4F revealed, the luciferase activity of SOX4-Wt was decreased by miR-30e-5p mimics, but that of SOX4-Mut was unchanged. The pcDNA3.1/SNHG14 vector was transfected into MCs to overexpress SNHG14 (Figure 4G). A decrease in the mRNA and protein expression of SOX4 induced by miR-30e-5p overexpression was rescued by SNHG14 elevation (Figure 4H,I). Immunofluorescence further demonstrated that SNHG14 silencing reduced SOX4 expression in HG-treated MCs (Figure 4J). SNHG14 has the same binding site to 3´UTR of SOX4 (UGUUUAC) as miR-30e-5p. Additionally, we found the luciferase activity of Luc-SOX4-3´UTR or Luc-SOX4-CDS was unaffected in MCs with SNHG14 (Figure 4K). This result excluded the binding of SNHG14 to 3´UTR of SOX4 and suggested that SNHG14 enhances SOX4 expression by miR-30e-5p.
3.5 SNHG14 silencing inhibits MC proliferation and fibrosis by inhibiting SOX4
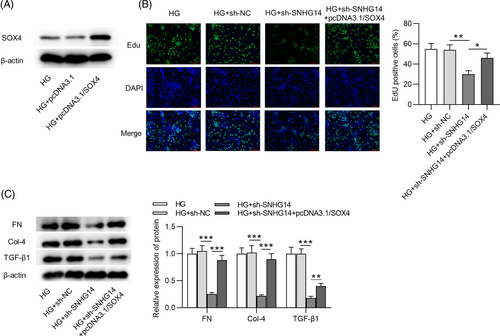
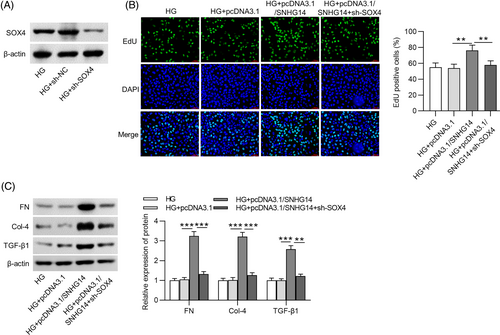
In rescue assays, after transfection with pcDNA3.1/SOX4, the protein expression of SOX4 in HG-stimulated MCs was elevated (Figure 5A). EdU showed that SOX4 overexpression attenuated the effects of SNHG14 deficiency on the proliferation of HG-treated MCs (Figure 5B). Meanwhile, after MCs were stimulated by HG, the fibrosis-associated protein levels decreased by SNHG14 depletion were restored by SOX4 upregulation (Figure 5C). Furthermore, we performed rescue experiments for shRNA–mediated silencing of SOX4. The protein expression of SOX4 in HG-stimulated MCs was decreased after sh-SOX4 transfection (Figure 6A). EdU showed that SNHG14 overexpression increased the proliferation of HG-treated MCs, which was reversed by SOX4 silencing (Figure 6B). Additionally, the fibrosis-associated protein levels upregulated by SNHG14 overexpression were reduced by SOX4 silencing (Figure 6C).
3.6 SNHG14 downregulation ameliorates renal injury in DN mice
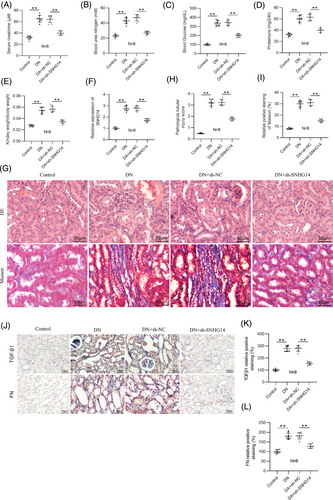
To investigate the function of SNHG14 in vivo, we silenced SNHG14 in DN mice by injection with adenoviral vector carrying sh-SNHG14 into DN mice. Serum creatinine, blood urea nitrogen, blood glucose, 24-h proteinuria, and relative kidney weight were then examined in DN mice (Figure 7A–E). We found that the levels of these indicators were significantly higher in DN mice compared with control mice. However, their levels were notably reduced by SNHG14 silencing. RT-qPCR analysis verified upregulation of SNHG14 in the mouse kidneys of DN, and sh-SNHG14 induced a marked reduction in its expression (Figure 7F). H&E and Masson staining showed that sh-SNHG14 injection ameliorated renal pathological changes, including interstitial inflammatory cell infiltration and tubular vacuolar degeneration as well as interstitial fibrosis in DN mice (Figure 7G–I). Immunohistochemistry analysis further verified that silencing SNHG14 downregulated TGF-β1 and FN in the renal interstitium of DN mice (Figure 7J–L).
4 DISCUSSION
To understand the pathogenesis of DN, an in vivo DN model was established using STZ-administrated mice. DN mouse models exhibited an increase in DN markers (p-cadherin and ZO-1) and fibrosis markers (FN, Col-4, and TGF-β1) in the kidneys. Additionally, interstitial inflammatory cell infiltration and vacuolarization of tubular epithelial cells were observed in DN mice. All these pathological changes suggested a successful DN animal model. MCs are the main constituents of the glomerulus; the proliferation of MCs has been considered key contributor to renal fibrosis. Therefore, preventing the proliferation of MCs is suggested as a promising strategy for treatment of DN. Our results showed that HG stimulation promoted cell proliferative ability and upregulated fibrosis markers in MCs, suggesting that HG induced fibrotic phenotype in MCs.
Evidence has suggested the functional roles of lncRNAs in renal fibrosis prevention and DN progression. For example, lncRNA PVT1 promotes cell migration and fibrosis of HG-stimulated MCs to participate in DN development.37 LncRNA-NR_033515 facilitates epithelial-to-mesenchymal transition, fibrosis, and proliferation of mouse MCs.38 SNHG14 is involved in various biological processes, such as regulating neuronal cell apoptosis and inflammation,39 obesity-induced endoplasmic reticulum stress in adipocyte,40 mesenchymal stem cell osteogenesis,41 cancer cell proliferation, and epithelial-mesenchymal transition.42 The literature suggests the research value of SNHG14 in human diseases. Additionally, SNHG14 is abnormally expressed in obesity mouse models induced by high-fat diet40 and in rats with renal injury.26 Similarly, our study showed that SNHG14 was upregulated in DN animal models and HG-induced human MCs. In this study, through cell experiments, we observed that SNHG14 downregulation markedly inhibited cell proliferation and reversed fibrotic phenotype in HG-treated MCs by decreasing Col-4, FN, and TGF-β1 expression. SNHG14 overexpression exerted an opposite result. Consistent with our findings, SNHG14 have been reported to promote the proliferation of various cells, such as tumor cells,43 trophoblast cells,44 and atherosclerosis cells.45 Moreover, by adenoviral vector delivery, we demonstrated that inhibiting SNHG14 expression also improved renal function and ameliorated interstitial fibrosis in mouse models of DN. These findings suggested a protective function for SNHG14 silencing against DN. SNHG1 knockdown inhibits HG-induced ferroptosis of HK-2 cells18; SNHG5 knockdown alleviates podocyte injury19; silencing of SNHG15 suppresses the inflammation in HG-induced human glomerular mesangial cells20; SNHG16 depletion inhibits the proliferation of mice mesangial cells30; and SNHG17 knockdown reduces the apoptosis of podocytes.31 Our study was the first to show the effects of the lncRNA SNHG family on DN via the regulation of fibrosis in vitro and in vivo.
To explore the ceRNA network, potential miRNAs of SNHG14 were predicted by online tools. The results from FISH showed that SNHG14 was mainly localized in the cytoplasm of MCs, suggesting that SNHG14 may function at the posttranscriptional level. Numerous studies have demonstrated the involvement of miRNAs in the pathogenesis of DN through the ceRNA network.46-48 By luciferase reporter assay, SNHG14 was found to bind to miR-30e-5p in MCs. Research shows that miR-30e-5p restrains hypertrophic phenotypes induced by angiotensin II in cardiomyocytes.49 Exosomal miR-30e-5p reduces HG-stimulated pyroptosis in human renal proximal tubular cells.50 Moreover, miR-30e-5p expression was found reduced in the urine and plasma of patients with severe diabetic kidney disease compared with type 1 diabetes controls,51 and its expression was revealed to be related to the proteinuria level in DN patients.52 These studies have associated dysregulation of miR-30e-5p with diabetes-related kidney disease. We also showed reduced miR-30e-5p expression in the kidneys of DN mice and HG-treated MCs. We concluded that SNHG14 may exert its function by miR-30e-5p at the posttranscriptional level. Nevertheless, the exact role and clinical relevance of miR-30e-5p in DN remain unknown and should be evaluated in the future.
To further investigate ceRNA mechanism involving in our study, the target gene of miR-30e-5p was identified. Here, we found upregulated SOX4 in the glomerulus of DN patients from the dataset GSE30122 and demonstrated the binding of miR-30e-5p to SOX4. The SOX family is a critical group of transcriptional regulators implicated in various biological processes.53 As a well-known transcriptional factor, SOX4 is necessary for endocrine pancreas development.54 Collins et al indicated that upregulation of the diabetes gene SOX4 suppressed insulin secretion and elevated the risk of diabetes.55 Additionally, SOX4 was found to promote angiogenesis and inflammation in HG-stimulated retinal endothelial cells, which might serve as a promising target for diabetic retinopathy.56 Here, our study showed that SOX4 was upregulated in DN animal models and HG-induced human MCs. In rescue assays, SOX4 overexpression reversed the effects of SNHG14 deficiency on proliferation, and fibrosis of HG-stimulated MCs. All these findings indicated that SOX4 was involved in the regulatory action of SNHG14 in DN development.
There are limitations to the current work. The upstream and downstream associations between SNHG14 and miR-30e-5p remain unknown, and the effects of the SNHG14/miR-30e-5p/SOX4 axis in renal tubular compartment should be further investigated. Moreover, the mechanisms of diabetic complications are complex and are affected by environmental and genetic factors, and these need further investigations in follow-up research.
Collectively, SNHG14 acts as ceRNA to elevate SOX4 expression by sponging miR-30e-5p (Figure 8). In DN progression, SNHG14 silencing reduces fibrosis and proliferation of HG-stimulated MCs as well as improves renal function and ameliorates interstitial fibrosis in mouse models of DN. The present study revealed that SNHG14 may be a potential biomarker and therapeutic target for further DN clinical application. However, a limitation in this research is that we did not pinpoint in which cell type SNHG14 specifically exerts its function within the animal level. Further investigations need be conducted in animal models to address this issue.