Long noncoding RNAs as potential diagnostic biomarkers for diabetes mellitus and complications: A systematic review and meta-analysis
Xuee Su and Huibin Huang contributed equally to this study.
Abstract
Aims
Long noncoding RNAs (lncRNAs) may be associated with the development of type 2 diabetes mellitus and its complications; however, the findings remain controversial. We aimed to synthesize the available data to assess the diagnostic utility of lncRNAs for identification of type 2 diabetes mellitus and its consequences.
Materials and Methods
We performed a systematic review and meta-analysis, searching PubMed, Embase, and Web of Science for articles published from September 11, 2015 to December 27, 2022. We evaluated human case–control or cohort studies on differential lncRNA expression in type 2 diabetes mellitus or its associated comorbidities. We excluded studies if they were non-peer reviewed or published in languages other than English. From 2387 identified studies, we included 17 (4685 participants).
Results
Analysis of the pooled data showed that lncRNAs had a diagnostic area under the curve (AUC) of 0.84 (95% CI: 0.80–0.87), with a sensitivity of 0.79 (95% CI: 0.74–0.83) and a specificity of 0.75 (95% CI: 0.69–0.80). LncRNAs had an AUC of 0.65 for the diagnosis of prediabetes, with 82% sensitivity and 65% specificity.
Conclusions
LncRNAs may be promising diagnostic markers for type 2 diabetes mellitus and its complications.
1 INTRODUCTION
Diabetes mellitus (DM) is a global health crisis and is associated with increased risk of heart disease and stroke; 90%–95% are type 2 diabetes mellitus (T2DM), which is characterized by increasing beta-cell loss and insulin resistance, predominantly caused by obesity.1 By 2045, 700 million individuals are expected to develop diabetes, with 463 million currently living with the disease worldwide.2 The disease is caused by multiple factors, including genetics, immune responses, oxidative stress, and endoplasmic reticulum stress.3 Persistently elevated blood glucose levels damage organs and tissues, leading to the development of diabetic complications, such as microvascular disease, diabetic retinopathy (DR), and diabetic nephropathy (DN).4, 5 DR is the most common manifestation of diabetic microangiopathy worldwide. Approximately 191 million people are expected to have DR by 2030, which potentially results in blindness and has a significant impact on the quality of life of those who are affected.6 DN is another common microvascular complication of diabetes, affecting approximately 3% of the population.7 The diagnosis and treatment of T2DM and its complications have made tremendous strides in recent years owing to the rapid improvement of healthcare systems; yet, the treatment outcomes for this chronic disease remain unsatisfactory. Thus, identifying preventive measures and new biomarkers for the early detection of disease through noninvasive tests is essential to prevent deterioration of beta-cell function and preserve remaining beta-cell mass.
Non-protein-coding RNA molecules with a length longer than 200 nucleotides are known as long noncoding RNAs (lncRNAs).8 With the rapid development in microarray technologies and high-throughput sequencing, the biological properties and functions of lncRNAs are gradually being recognized. The regulation of various cellular responses and diseases is significantly affected by lncRNAs. Nowadays, noncoding RNAs have emerged as a diagnostic and prognostic biomarker for various diseases, such as cancer, infectious diseases, and autoimmune disorders.9-11 According to early studies, diabetes development is linked to lncRNAs with distinct expression patterns that also play a role in other endocrine functions and diseases.12 For example, Wang et al13 found that a characteristic feature of T2DM is increased expression of HOX antisense intergenic RNA, a type of lncRNA. High serum HOX antisense intergenic RNA expression is a promising noninvasive diagnostic marker and an independent predictor of T2DM. Moreover, many lncRNAs are aberrantly expressed with T2DM complications, such as DN and DR, and are also closely related to their pathogenesis.14, 15
Because of their differential regulation, methylation, and other mechanisms,16 lncRNAs are anticipated to contribute to the early detection and treatment of T2DM and are attracting research attention. Thus, we aimed to assess lncRNAs as potential biomarkers for T2DM and associated comorbidities by conducting a systematic review and meta-analysis.
2 METHODS
2.1 Study design
This systematic review and meta-analysis was part of a thematic project conducted at the Second Affiliated Hospital of Fujian Medical University. The study protocol was registered in the International Prospective Register of Systematic Reviews database (registration number, 42022381403), following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines for the reporting of systematic reviews.17
2.2 Search strategy and selection criteria
We searched PubMed, Embase, and Web of Science from their inception until December 27, 2022. The reference lists of key reviews and meta-analyses were searched to supplement the identified citations. Our search strategy is available in Supplementary S3.
Eligible studies met the following inclusion criteria: (a) case–control or cohort studies on differential lncRNA expression in patients with T2DM or related complications; (b) included diabetic and nondiabetic patients, diabetes-related complications, and compared diabetic and nondiabetic control samples; (3) contained data on the total number of samples, area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, and specificity. We excluded studies unrelated to lncRNAs or T2DM, review papers, animal studies, case reports, those based on nonhuman subjects, or studies published in languages other than English.
2.3 Data analysis
2.3.1 Study selection
After removing duplicates using EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA), two team members (XES and JQL) further scanned the titles and abstracts independently, read the relevant full-text manuscripts, and extracted the study data from eligible studies. Any disagreements were settled through conversation and, if necessary, through adjudication by a third team member (YQH). Furthermore, the reasons for exclusion were recorded. Data from the screening process were fully documented, including author, country, year of publication, lncRNA type, disease type, participants, method of detection, lncRNA expression levels, diagnostic power, sensitivity, and specificity.
2.3.2 Data extraction and management
After consensus was reached, the data were entered into STATA statistical software 16.0 (Stata Corp., College Station, Texas, USA). We included the sample size, AUC, sensitivity, and specificity values from the original literature and calculated the following values: true positive, false positive, false negative, and true negative. An independent evaluation of the literature quality was conducted using the Newcastle-Ottawa Scale,18 and any uncertainties were discussed and highlighted. Each article received a score between 0 and 9, with scores of 6 and 9 denoting exceptional quality.
Data were transferred to STATA statistical software 16.0 for meta-analysis using the random-effects mode. Heterogeneity between trials was assessed using the I2 statistic, which describes between-study variation as a percentage of the total variation. If the I2 score was <50%, heterogeneity between the eligible studies was considered insignificant.19 For the pooled analysis, a fixed-effects model was employed; however, when heterogeneity was considerable, a random-effects model was applied. This was determined using a subgroup analysis of potential sources of survey heterogeneity. Deeks' funnel plot was the primary tool for assessment of publication bias, and a two-tailed p value of .05 was considered significant. The outcomes were expressed as the AUC, which can be used to determine the decision process for the best model and allows for a quantitative assessment of the accuracy and practical utility of marker classification prediction. In general, when 0.5 < AUC <1, the classifier used outperforms the random prediction, as the random prediction provides a good predictive value when a good threshold is set, indicating that the point on the ROC curve is remarkably close to the (0,1) point. An AUC of 0.5 signifies no distinction; 0.7–0.8 is regarded as fair; 0.8–0.9 is viewed as great; and 0.9 is considered remarkable.20
2.3.3 Measurement of the effects
The outcomes are presented as AUCs. The AUC values corresponding to lncRNA expression levels in diabetes and diabetes-related complications were calculated.
2.3.4 Missing data
Missing data were obtained by contacting relevant study authors. Studies with missing data were included when the specific data requested were sufficient for meta-analysis; otherwise, only the reported results were considered.
2.3.5 Subgroup analysis
We performed analyses to reduce heterogeneity and assess the effect of lncRNAs in patients with diabetes or its complications according to the following subgroups: (a) disease type (diabetes or prediabetes); (b) sample source, including serum, plasma, whole blood, and peripheral blood mononuclear cells (PBMCs); (c) lncRNA expression level (upregulated or downregulated); and (d) ethnicity of the included population.
2.3.6 Sensitivity analysis
To determine the reliability of the study findings, a sensitivity analysis of the sample size was conducted. The stability of the results was assessed using repeated meta-analyses to exclude publications that had already been included.
3 RESULTS
3.1 Literature search and study characteristics
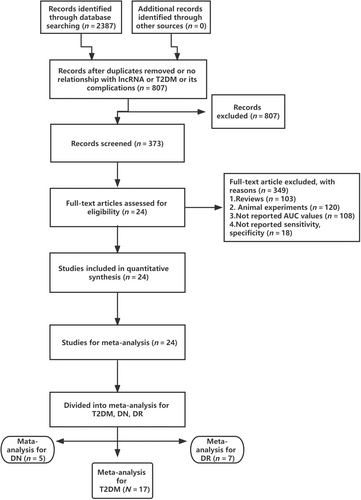
The methodology for selecting the studies is shown in Figure 1. A total of 2387 potentially relevant studies were identified in our online database, 807 of which were excluded owing to duplicate titles and abstract assessments or irrelevance to lncRNA, T2DM, or its complications. The remaining 373 articles were thoroughly evaluated. In addition to the 103 review articles, a total of 246 studies were removed for the following reasons: 120 were animal studies, 108 did not report AUC values, and 18 did not report numerical sensitivity or specificity results. In total, 24 studies ultimately met our eligibility criteria, of which T2DM, DN, and DR were analyzed in 17,21-34 5,5, 30, 35-37 and 7 studies,4, 28, 29, 32, 37-39 respectively (some articles included more than one of these outcomes). Table 1 provides the specifics of the survey characteristics and summarizes the quality assessment results. The detail scores of Newcastle-Ottawa Scale for quality of each included article are shown in Supplementary S4.
| Study (ref) | Year | Country | LncRNA | Disease type | Sample size | Specimen | Method | Regulation | Diagnostic power | NOS score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Sen | Spe | AUC | |||||||||
| Omidvar et al | 2018 | Iran | lncRNA VIM-AS1 | T2DM | 100 | 100 | PBMCs | qRT-PCR | Down | 0.56 | 0.68 | 0.63 | 7 |
| Omidvar et al | 2018 | Iran | lncRNA CTBP1-AS2 | T2DM | 100 | 100 | PBMCs | qRT-PCR | Down | 0.59 | 0.75 | 0.68 | 7 |
| Saeidi et al | 2018 | Iran | lncRNA LY86-AS1 | T2DM | 100 | 98 | PBMCs | qRT-PCR | Down | 0.65 | 0.80 | 0.747 | 7 |
| Saeidi et al | 2018 | Iran | lncRNA HCG27_201 | T2DM | 100 | 98 | PBMCs | qRT-PCR | Down | 0.56 | 0.75 | 0.65 | 7 |
| Mansoori et al | 2018 | Iran | lncRNA LINC00523 | T2DM | 100 | 98 | PBMCs | qRT-PCR | Down | 0.81 | 0.61 | 0.74 | 7 |
| Mansoori et al | 2018 | Iran | lncRNA LINC00994 | T2DM | 100 | 98 | PBMCs | qRT-PCR | Down | 0.81 | 0.53 | 0.67 | 7 |
| Wang et al | 2018 | China | lncRNA CASC2 | T2DM | 296 | 56 | Blood | qRT-PCR | Down | 0.66 | 0.48 | 0.63 | 6 |
| Yin et al | 2017 | China | lncRNA GAS5 | T2DM | 10 | 30 | Plasma | qRT-PCR | Down | 0.7 | 0.6 | 0.74 | 7 |
| Carter et al | 2015 | United States | lncRNA GAS5 | T2DM | 47 | 49 | Blood | qRT-PCR | Down | 0.85 | 0.67 | 0.81 | 7 |
| Li et al | 2017 | China | lncRNA ENST00000550337.1 | T2DM | 64 | 60 | Blood | qRT-PCR | Up | 0.75 | 0.65 | 0.73 | 6 |
| Li et al | 2017 | China | lncRNA ENST 00000550337.1 | Prediabetes | 63 | 60 | Blood | qRT-PCR | Up | 0.75 | 0.65 | 0.71 | 6 |
| Li et al | 2017 | China | lncRNA TCONS_00007244 | T2DM | 20 | 20 | Blood | qRT-PCR | Up | 0.75 | 0.65 | 0.71 | 6 |
| Li et al | 2017 | China | lncRNA TCONS_00007244 | Prediabetes | 20 | 20 | Blood | qRT-PCR | Up | 0.65 | 0.75 | 0.69 | 6 |
| Li et al | 2017 | China | lncRNA TCONS_00000886 | T2DM | 20 | 20 | Blood | qRT-PCR | Up | 0.85 | 0.6 | 0.7 | 6 |
| Li et al | 2017 | China | lncRNA TCONS_00000886 | Prediabetes | 20 | 20 | Blood | qRT-PCR | Up | 0.9 | 0.5 | 0.69 | 6 |
| Shaker et al | 2018 | Egypt | LncRNA HOTAIR | T2DM | 30 | 81 | Serum | qRT-PCR | Up | 0.93 | 0.65 | 0.72 | 7 |
| Shaker et al | 2018 | Egypt | LncRNA MALAT1 | T2DM | 30 | 81 | Serum | qRT-PCR | Up | 0.57 | 0.69 | 0.64 | 7 |
| Toraih et al | 2019 | Egypt | lncRNA CDKN2B-AS1 | T2DM | 55 | 108 | Plasma | qRT-PCR | Up | 0.70 | 0.60 | 0.73 | 6 |
| Toraih et al | 2019 | Egypt | lncRNA PVT1 | T2DM | 55 | 108 | Plasma | qRT-PCR | Up | 0.83 | 0.60 | 0.74 | 6 |
| Cai et al | 2021 | China | lncRNA SNHG5 | T2DM | 62 | 60 | Serum | qRT-PCR | Up | 0.81 | 0.83 | 0.90 | 7 |
| Cao et al | 2020 | China | lncRNA LINC-P21 | T2DM | 108 | 68 | Serum | qRT-PCR | Up | 0.83 | 0.94 | 0.92 | 6 |
| Ji et al | 2022 | China | lncRNA NR2F1-AS1 | T2DM | 111 | 106 | Blood | qRT-PCR | Up | 0.7664 | 0.6667 | 0.789 | 7 |
| Su et al | 2022 | China | lncRNA TUG1 | T2DM | 105 | 105 | Serum | qRT-PCR | Up | 0.63 | 0.97 | 0.64 | 7 |
| Su et al | 2022 | China | lncRNA MEG3 | T2DM | 105 | 105 | Serum | qRT-PCR | Up | 0.58 | 0.94 | 0.59 | 7 |
| Ma et al | 2021 | China | ENST00000588707.1 | T2DM | 127 | 130 | PBMCs | qRT-PCR | Down | 0.72 | 0.80 | 0.82 | 7 |
| Ma et al | 2021 | China | TCONS_00004187 | T2DM | 127 | 130 | PBMCs | qRT-PCR | Down | 0.82 | 0.61 | 0.83 | 7 |
| Wang et al | 2021 | China | lncRNA HOTAIR | T2DM | 96 | 82 | Serum | qRT-PCR | Up | 0.87 | 0.84 | 0.90 | 8 |
| Saleh et al | 2020 | Egypt | lncRNA HCG27_201 | T2DM | 70 | 70 | Plasma | qRT-PCR | Down | 0.96 | 0.83 | 0.96 | 7 |
| Saleh et al | 2020 | Egypt | lncRNA GAS5 | T2DM | 70 | 70 | Plasma | qRT-PCR | Down | 0.96 | 0.76 | 0.94 | 7 |
| Saleh et al | 2020 | Egypt | lncRNA LY86-AS1 | T2DM | 70 | 70 | Plasma | qRT-PCR | Down | 0.93 | 0.69 | 0.83 | 7 |
| Saleh et al | 2020 | Egypt | lncRNA HCG27_201 | Prediabetics | 65 | 70 | Plasma | qRT-PCR | Down | 0.91 | 0.64 | 0.756 | 7 |
| Saleh et al | 2020 | Egypt | lncRNA GAS5 | Prediabetics | 65 | 70 | Plasma | qRT-PCR | Down | 0.85 | 0.64 | 0.699 | 7 |
| Saleh et al | 2020 | Egypt | lncRNA LY86-AS1 | Prediabetics | 65 | 70 | Plasma | qRT-PCR | Down | 0.78 | 0.53 | 0.602 | 7 |
| Anbari et al | 2020 | Arabia | lncRNA-GHRL-3:2 | T2DM | 71 | 32 | Blood | qRT-PCR | Down | 0.93 | 0.84 | 0.93 | 7 |
| Anbari et al | 2020 | Arabia | lncRNA GHRL-3:3 | T2DM | 71 | 32 | Blood | qRT-PCR | Down | 0.88 | 0.91 | 0.90 | 7 |
| Zhou et al | 2020 | China | lncRNA MALAT1 | DN | 27 | 20 | PBMCs | qRT-PCR | Up | 0.80 | 0.68 | 0.79 | 6 |
| Cai et al | 2021 | China | lncRNA SNHG5 | DN | 58 | 62 | Serum | qRT-PCR | Up | 0.81 | 0.84 | 0.88 | 7 |
| Rajabinejad et al | 2022 | Iran | lncRNA MALAT1 | DN | 20 | 20 | Blood | qRT-PCR | Up | 1.00 | 0.71 | 0.92 | 7 |
| Rajabinejad et al | 2022 | Iran | lncRNA H19 | DN | 20 | 20 | Blood | qRT-PCR | Up | 0.84 | 0.88 | 0.92 | 7 |
| Zhu et al | 2022 | China | lncRNA ANRIL | DN | 66 | 20 | Blood | qRT-PCR | Up | 0.83 | 0.91 | 0.92 | 7 |
| Alfaifi et al | 2021 | Britain | lncRNA NKILA | DN | 200 | 200 | Serum | qRT-PCR | Up | 0.54 | 0.4 | 0.48 | 8 |
| Alfaifi et al | 2021 | Britain | lncRNA NEAT1 | DN | 200 | 200 | Serum | qRT-PCR | Up | 0.52 | 0.48 | 0.52 | 8 |
| Alfaifi et al | 2021 | Britain | lncRNA MALAT1 | DN | 200 | 200 | Serum | qRT-PCR | Up | 0.53 | 0.4 | 0.48 | 8 |
| Alfaifi et al | 2021 | Britain | lncRNA MIAT | DN | 200 | 200 | Serum | qRT-PCR | Up | 0.55 | 0.5 | 0.53 | 8 |
| Shaker et al | 2018 | Egypt | lncRNA HOTAIR | DR | 30 | 30 | Serum | qRT-PCR | Up | 0.76 | 0.8 | 0.78 | 7 |
| Shaker et al | 2018 | Egypt | lncRNA MALAT1 | DR | 30 | 30 | Serum | qRT-PCR | Up | 0.74 | 0.73 | 0.74 | 7 |
| Liu et al | 2021 | China | lncRNA ENST00000505731 | PDR | 30 | 30 | Blood | qRT-PCR | Up | 0.8 | 0.87 | 0.88 | 7 |
| Liu et al | 2021 | China | lncRNA NR-126161 | PDR | 30 | 30 | Blood | qRT-PCR | up | 0.50 | 0.97 | 0.79 | 7 |
| Atef et al | 2022 | Egypt | lncRNA HIF1A-AS2 | NPDR | 15 | 15 | PBMCs | qRT-PCR | Up | 0.89 | 0.92 | 0.94 | 7 |
| Atef et al | 2022 | Egypt | lncRNA HIF1A-AS2 | PDR | 15 | 15 | PBMCs | qRT-PCR | Up | 0.96 | 0.93 | 0.97 | 7 |
| Toraih et al | 2019 | Egypt | lncRNA RNCR2 | DR | 108 | 55 | Plasma | qRT-PCR | Down | 0.70 | 0.70 | 0.74 | 6 |
| Toraih et al | 2019 | Egypt | lncRNA NEAT2 | DR | 108 | 55 | Plasma | qRT-PCR | Down | 0.62 | 0.56 | 0.62 | 6 |
| Ji et al | 2022 | China | lncRNA NR2F1-AS1 | DR | 158 | 106 | Serum | qRT-PCR | Up | 0.83 | 0.82 | 0.90 | 7 |
| Alfaifi et al | 2021 | Britain | lncRNA NKILA | DR | 200 | 200 | Serum | qRT-PCR | Up | 0.59 | 0.41 | 0.56 | 8 |
| Alfaifi et al | 2021 | Britain | lncRNA NEAT1 | DR | 200 | 200 | Serum | qRT-PCR | Up | 0.61 | 0.51 | 0.57 | 8 |
| Alfaifi et al | 2021 | Britain | lncRNA MALAT1 | DR | 200 | 200 | Serum | qRT-PCR | Up | 0.61 | 0.5 | 0.6 | 8 |
| Alfaifi et al | 2021 | Britain | lncRNA MIAT | DR | 200 | 200 | Serum | qRT-PCR | Up | 0.68 | 0.58 | 0.66 | 8 |
| Li et al | 2022 | China | lncRNA HIF1A-AS1 | PDR | 80 | 100 | Serum | qRT-PCR | Up | 0.66 | 0.79 | 0.77 | 7 |
- Abbreviations: AUC, area under the curve; DN, diabetic nephropathy; DR, diabetic retinopathy; NOS, Newcastle-Ottawa Scale; NPDR, nonproliferative diabetic retinopathy; PBMCs, peripheral blood mononuclear cells; PDR, proliferative diabetic retinopathy; qRT-PCR, quantitative reverse transcription polymerase chain reaction; Sen, sensitivity; Spe, specificity; T2DM, type 2 diabetes mellitus.
3.2 Diagnostic value of lncRNAs for type 2 diabetes mellitus
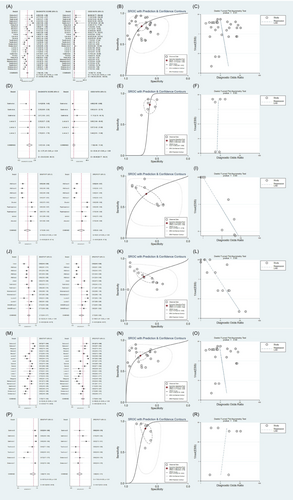
We identified 17 studies that compared patients with T2DM and healthy controls, involving a total of 4685 participants. We performed meta-analyses on the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) of lncRNAs in diagnosing T2DM. We also compiled the summary ROC (SROC) data from these analyses. The following results were obtained by using a random-effects model for the pooled lncRNA estimations: the AUC was 0.84 (95% CI: 0.80–0.87), and the sensitivity and specificity were 0.79 and 0.75, respectively; PLR was 3.1 (95% CI: 2.5–3.9), NLR was 0.28 (95% CI: 0.22–0.36), and DOR was 11 (95% CI: 7–16). In conclusion, lncRNA has an excellent diagnostic value for T2DM (Figure 2A,B).
3.3 Diagnostic value of lncRNAs for detecting prediabetes
For prediabetes, lncRNAs had the following values: AUC, 0.65 (95% CI: 0.61–0.69); sensitivity, 0.82 (95% CI: 0.75–0.87); specificity, 0.62 (95% CI: 0.56–0.67); PLR, 2.1 (95% CI: 1.8–2.5); NLR, 0.30 (95% CI: 0.21–0.42); and DOR, 7 (95% CI: 4–12). In summary, the diagnostic value of lncRNAs for prediabetes was relatively low and below the acceptable range, indicating that their diagnostic ability was unsatisfactory (Figure 2D,E).
3.4 Diagnostic value of lncRNAs for DN and DR
Our search results yielded 437 studies that distinguished between healthy controls and patients with DN and DR. Regarding the DN and DR studies, five eligible trials with 1913 participants and seven eligible trials with 2580 participants, respectively, were included. Summary estimates of lncRNAs were as follows: DN: AUC, 0.75; sensitivity, 71%; and specificity, 67% (Figure 2G,H), and DR: AUC, 0.7; sensitivity, 71%; and specificity, 71% (Figure 2J,K). Therefore, the diagnostic values of lncRNAs for DN and DR are acceptable.
3.5 Systematic review of the diagnostic value of lncRNAs for diabetic cardiomyopathy
The development and progression of diabetes lead to several problems that affect most of the major organs of the body. Diabetic cardiomyopathy (DCM) has a significant negative impact on health. We thoroughly searched all the literature on the value of lncRNAs for detection of DCM and found 25 studies, 23 of which were reviews or animal studies, and one study did not report diagnostic results.40 Ultimately, only one study met the inclusion criteria; thus, we were unable to perform a meta-analysis. Regarding the diagnosis of DCM, the AUC values for terminal differentiation-induced ncRNA expression in myocardial biopsy and serum samples were 0.95 (95% CI: 0.89–1.0) and 0.93 (95% CI: 0.85–1.0), respectively. Therefore, downregulation of terminal differentiation-induced ncRNA expression may be a potential diagnostic biomarker for DCM.41
3.6 Subgroup analysis based on ethnic derivation
The validity of lncRNAs as diagnostic tools may vary according to dietary differences across ethnicities. We also performed a subgroup analysis of the lncRNA expression levels in various ethnic groups. The random-effects model yielded an AUC of 0.82 (95% CI: 0.78–0.85), sensitivity of 0.75 (95% CI: 0.70–0.80), and specificity of 0.77 (95% CI: 0.69–0.83) among Asian participants (Figure 2M,N). For participants from Africa, these values were 0.78 (95% CI: 0.74–0.81), 0.88 (95% CI: 0.76–0.94), and 0.69 (95% CI: 0.63–0.74), respectively (Figure 2P,Q).
3.7 Subgroup analysis based on lncRNA levels
Using the data on the differential expression of lncRNA, all included studies were divided into two groups based on the abnormal expression of lncRNAs, including nine downregulated and eight upregulated lncRNAs. As for the downregulated lncRNAs, the AUC of the SROC curve was 0.81 (95% CI: 0.77–0.84), with a sensitivity of 0.81 (95% CI: 0.72–0.87) and a specificity of 0.71 (95% CI: 0.66–0.77; Supplementary S1A,B). Regarding the upregulated lncRNAs, the AUC of the SROC curve was 0.83 (95% CI: 0.80–0.86), with a sensitivity of 0.77 (95% CI: 0.71–0.82) and a specificity of 0.78 (95% CI: 0.68–0.86; Supplementary S1D,E).
3.8 Subgroup analysis based on the sample type
To examine the sources of heterogeneity in the different diagnostic values of lncRNAs for the detection of T2DM, we performed a subgroup analysis based on the source of specimens tested for lncRNA expression levels.
According to the sample type, all included studies were classified into four groups: PBMCs, plasma, serum, and blood. For the PBMC group, the results of the random-effects model were as follows: an AUC of 0.76 (95% CI: 0.72–0.79), sensitivity of 0.70 (95% CI: 0.62–0.77), and specificity of 0.70 (95% CI: 0.63–0.76; Supplementary S2A,B). For the blood group, the results showed an AUC of 0.87 (95% CI: 0.83–0.89), sensitivity of 0.82 (95% CI: 0.76–0.87), and specificity of 0.77 (95% CI: 0.69–0.83; Supplementary S2D,E). For the plasma group, the results reported an AUC of 0.81 (95% CI: 0.77–0.95), sensitivity of 0.89 (95% CI: 0.77–0.95), and specificity of 0.68 (95% CI: 0.60–0.76; Supplementary S2G,H). For the serum group, the results showed an AUC of 0.89 (95% CI: 0.86–0.91), sensitivity of 0.77 (95% CI: 0.66–0.85), and specificity of 0.87 (95% CI: 0.77–0.94); (Supplementary S2J,K).
3.9 Risk of bias and sensitivity analysis
Funnel plots showed no evidence of publication bias in our meta-analysis. Moreover, we performed a sensitivity analysis to assess the reliability of our meta-analysis results by excluding one study at a time, and no outliers were found. Investigating the source of heterogeneity is challenging, and this may be the cause of all detected heterogeneity.
4 DISCUSSION
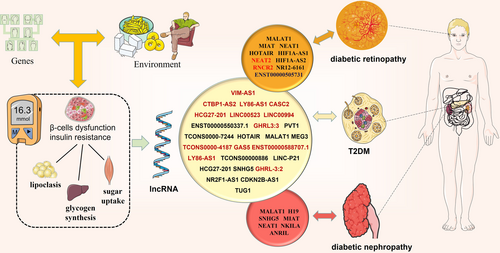
We conducted a systematic review and meta-analysis of case–control and cohort studies on the association between lncRNA expression and diagnostic outcomes in patients with T2DM or its associated complications (see the schema in Figure 3).
Our study revealed a significant correlation between the expression of abnormal lncRNAs and the presence of T2DM, which is consistent with the findings of previous studies.42 In our meta-analysis, we included 10 studies more than the seven included in a previous study.42 We discovered that lncRNAs are highly accurate in diagnosing T2DM or prediabetes. In addition, we analyzed five and seven lncRNA studies on DN and DR, respectively, and our findings revealed that lncRNAs have some diagnostic power for the detection of DN and DR. We performed a regression analysis to investigate the causes of heterogeneity and did not identify any outliers. Using subgroup analysis, we found that lncRNAs had a unique diagnostic predictive value for T2DM across sample sources and ethnic differences, with high diagnostic value in serum samples and in samples from Asian populations.
Inflammation and dysregulated metabolism affect multiple cells and organs, such as beta cells, and liver, skeletal muscle, kidney, brain, and adipose tissue, gradually resulting in T2DM.43 Cao et al31 suggested that serum expression of LINC-P21 was elevated in patients with T2DM, related to its targeting of miR-766-3p to upregulate NR3C2, resulting in insulin secretion and proliferation in pancreatic beta cells. Elevated serum LINC-P21 and decreased serum miR-766-3p levels are candidate diagnostic biomarkers in patients with T2DM. Studies investigating the upregulation or inhibition of lncRNA in T2DM revealed an association with functional impairment of INS-1 cells or increased hepatic glycogen synthesis.44 In general, these studies suggest that lncRNAs have the potential as direct targets for therapeutic interventions in diabetes. Thus, lncRNAs have emerged as important regulators of glucose and lipid metabolism.
Moreover, the role of lncRNAs in diabetes-related complications should not be overlooked. One of the most serious microvascular complications of DM is DN due to mesangial cell proliferation and changes in the renal microenvironment.45 In an animal study, the lncRNA SOX2OT was significantly downregulated in the mesangial cells by different pathways, whereas overexpression thereof significantly inhibited fibrosis of the mesangial cells.46 In a study by Cai et al, SNHG5 expression was substantially elevated in DN, confirming its potential as a novel biomarker for the diagnosis of DN that may interact with miR-26a-5p. DN is characterized by an accumulation of extracellular matrix, hypertrophy, and fibrosis in the glomeruli and renal tubular cells. Additionally, growing evidence suggests that these DN features are closely linked to ncRNA regulation.47
Ogurtsova et al48 reported that lncRNAs have unique expression profiles and play an important role in the development of DR. One study found that lncRNA H19 blocked endothelial-mesenchymal transition (EndMT) in DR.49 It inhibited transforming growth factor-1 and its signaling pathway by blocking the MAPK-ERK1/2 signaling pathway that controlled EndMT when glucose levels are elevated. A similar mechanistic study of lncRNAs in DR was reported, in which overexpression of the lncRNA SNHG7 inhibited EndMT and tube formation.50
Our study demonstrated the applicability of lncRNAs as diagnostic biomarkers not only for T2DM but also for its complications, such as DN and DR.
We conducted a meta-regression and subgroup analysis based on ethnic origin, sample origin, and lncRNA levels. To improve the search for biomarkers for early diagnosis of type 2 diabetes, future studies should include subgroup analyses based on specific types of lncRNA, sex, and age. Fortunately, obtaining patient blood samples and determining the levels of lncRNA expression make this investigation easy and practical. Furthermore, this diagnostic analysis showed no asymmetry in the Deeks' funnel plot, indicating no publication bias. The high incidence and prevalence of T2DM poses challenges to the effective treatment of patients. Therefore, early detection of diabetes and prevention of its complications are particularly important for high-risk groups.
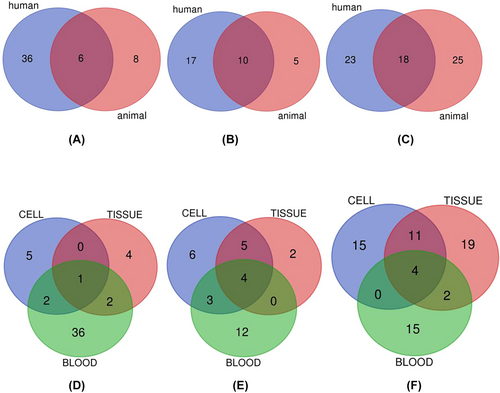
A large number of lncRNAs have been tested in animal models but not in human tissues. This may be attributed to the lower expense of animal experiments compared with that of clinical studies. Owing to the limited resources available for clinical studies, we aimed to validate lncRNA biomarkers that are prevalent in animals and humans by using Venn diagram statistics. As shown in Figure 4, different biomarkers have been detected in humans and animals or in blood, tissues, and cells. Our Venn diagrams show that MALAT1 and TUG1 are examples of the lncRNAs validated in animals and humans, whereas MALAT1 and NEAT1 are examples of those validated in diabetic cells, tissues, and blood. These findings may serve as a solid starting point for future studies. Table 2 provides specific information on the characteristics of the overlapping lncRNAs. To the best of our knowledge, this is the first meta-analysis to explore the diagnostic value of lncRNAs in T2DM, DN, and DR.
| Item | Names | Total | Elements |
|---|---|---|---|
| T2DM | Human ∩ animal | 6 | EPB41L4A-AS1 MALAT1 TUG1 DRAIR MIAT Kcnq1ot1 |
| DR | Human ∩ animal | 10 | NEAT1 MALAT1 HOTAIR H19 XIST ZFAS1 MEG3 ANRIL TUG1 AQP4-AS1 |
| DN | Human ∩ animal | 18 | CYP4B1-PS1–001 SOX2OT HOXB3OS NEAT1 GAS5 XIST Lnc-ISG20 CASC2 PVT1 OIP5–AS1 H19 MEG3 NONHSAG053901 TUG1 CES1P1 MIAT ANRIL MALAT1 |
| T2DM | Cell ∩ tissue ∩ blood | 1 | MALAT1 |
| DR | Cell ∩ tissue ∩ blood | 4 | HOTAIR ZFAS1 MEG3 ANRIL |
| DN | Cell ∩ tissue ∩ blood | 4 | NEAT1 CASC2 MALAT1 ANRIL |
- Abbreviations: DN, diabetic nephropathy; DR, diabetic retinopathy; lncRNA, long noncoding RNA; T2DM, type 2 diabetes mellitus.
5 LIMITATIONS AND FUTURE RECOMMENDATIONS
Our meta-analysis has some limitations. First, some heterogeneity was detected, and subgroup analysis based on the lncRNA type could not fully explore the main sources of heterogeneity owing to the limited number of studies. Second, the sample size of studies on the diagnostic value of lncRNAs in DN and DR was relatively small, which may have led to insufficient statistical power. Third, the sample size of the lncRNA studies on other diabetic complications was insufficient for a meta-analysis, allowing only systematic assessment; thus, their associated diagnostic value requires further study. Fourth, although a large number of lncRNAs have been tested in humans, the experimental results did not provide specific AUCs, sensitivities, or specificities. Despite our attempts to obtain these data by email, the results were not available; therefore, we were unable to obtain additional data for the meta-analysis.
Recent studies have suggested that lncRNAs, circular RNAs, and mRNAs compete with each other through miRNA response elements to modulate the progression of T2DM and may function as miRNA sponges.51 Thus, future research may focus on constructing a competing endogenous RNA network to further understand the biological effects of lncRNAs in patients with T2DM and its complications. Furthermore, lncRNAs may compete for shared miRNAs to regulate other RNA transcripts, thereby influencing the pathogenesis of T2DM and its complications. Finally, with a large number of lncRNAs studies in the diagnosis of diabetes, we may focus on the diagnostic value of a specific LncRNA for diabetes and related complications.
6 CONCLUSION
Our findings suggest that lncRNAs are promising biomarkers and therapeutic targets for T2DM, DN, and DR. They may be extremely beneficial in the early detection and diagnosis of these conditions. The biological functions of these markers require further investigation to help determine the molecular mechanisms underlying the pathogenesis of T2DM.
AUTHORS CONTRIBUTIONS
Xuee Su, Huibin Huang, Yinqiong Huang, and Shu Lin were involved in the conception of the article. Xuee Su and Jinqing Lai independently examined studies that might be eligible for inclusion, and any disagreements were resolved by a third review author (Yinqiong Huang), if necessary. Xuee Su and Jinqing Lai obtained datasets for research based on a standardized form. After the consensus was reached, the data were entered into STATA statistical software. and any uncertainties were discussed and highlighted (Yinqiong Huang and Shu Lin). Xuee Su and Huibin Huang drafted the manuscript. Yinqiong Huang and Shu Lin contribute to project supervision, and study design. All authors have read and approved the final manuscript. Xuee Su and Huibin Huang contributed equally to this work.
FUNDING INFORMATION
This work was supported by the Science and Technology Bureau of Quanzhou (grant number 2020CT003), the Nursery Fund Project of the Second Affiliated Hospital of Fujian Medical University (2021MP25), Key Young Talents Health Training Project of Fujian Province (2020GGA057), Natural Science Foundation of Fujian Province (2020J01221), and National Natural Science Foundation of China (82200871). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
DISCLOSURE
The authors declare no competing fnancial interests.
Open Research
DATA AVAILABILITY STATEMENT
This article (and its supplementary information files) contains all data generated or analyzed during this study.




