The mediating role of gestational diabetes mellitus in the associations of maternal prepregnancy body mass index with neonatal birth weight
妊娠期糖尿病在孕妇孕前体重指数与新生儿出生体重关系中的作用
Hao Hu, Pei Feng and Qian Yu contributed equally to this work.
Funding information: Natural Science Foundation of Jiangsu Province, Grant/Award Number: BK20181438; Maternal and Child Health Research Projects of Jiangsu Province, Grant/Award Number: F201830, F201847; the key technical (research) project for the prevention and control of major diseases and infectious diseases of Suzhou Municipal Commission of Health, Grant/Award Number: Gwzx201803
Abstract
enBackground
Both prepregnancy obesity and gestational diabetes mellitus (GDM) have been linked to adverse neonatal birth weight. However, the mediating role of GDM between prepregnancy obesity and neonatal birth weight is unclear.
Method
The cohort study included 17 260 singleton pregnant women and their newborns. Participants' demographic characteristics, disease history, family history of the disease, and the perinatal outcomes were recorded. The association between maternal prepregnancy body mass index (BMI) status and small for gestational age (SGA) or large for gestational age (LGA) neonates was analyzed using logistic regressions, before and after adjusting for covariates and GDM. The potential mediation of GDM on the association between prepregnancy BMI and adverse birth weight was examined.
Result
Multivariate logistic regression demonstrated that prepregnancy underweight women were more likely to deliver SGA neonates compared to those who had normal weights, whereas prepregnancy obese pregnant women were more likely to have LGA neonates. The RMediation analyses illustrated that the mediation effect of GDM on the maternal prepregnancy BMI (continuous variable) and the risk of SGA was not significant, whereas the association between prepregnancy BMI and LGA was statistically mediated by GDM (95%CI of a*b: 0.009-0.051). The Iacobacci (2012) method indicated that the impact of maternal prepregnancy overweight (Zmediation = 2.418, P = .015) and obesity (Zmediation = 2.165, P = .030) on LGA was partially mediated by GDM, with an indirect effect of 16.3% and 13.1%, respectively.
Conclusion
Prepregnancy BMI was observed to be associated with SGA and LGA. The association of prepregnancy overweight and obesity with LGA was found to be partially mediated by GDM.
摘要
zh背景
妊娠前肥胖和妊娠糖尿病(GDM)都与新生儿出生体重不良有关。然而, GDM在孕前肥胖和新生儿出生体重之间的作用尚不清楚。
方法
采用队列研究方法, 对17260名单胎孕妇及其新生儿进行调查。记录患者的人口学特征、病史、家族史及围产期结局。在校正协变量和GDM之前和之后, 采用logistic回归分析孕妇孕前BMI状态与小胎龄(SGA)和大胎龄(LGA)新生儿之间的关系。研究GDM在孕前BMI和不良出生体重之间的潜在作用。
结果
多因素logistic回归显示, 孕前体重偏轻的孕妇比正常体重的孕妇更容易分娩SGA新生儿, 而孕前肥胖孕妇更容易分娩LGA新生儿。RMediation分析显示, GDM对孕产妇孕前BMI和SGA风险的调节作用不显著, 而孕前BMI与LGA之间的关联是由GDM介导的(a*b的95%CI: 0.009-0.051)。Iacobacci's(2012)方法表明, 孕产妇孕前超重(Zmediation= 2.418, p=0.015)和肥胖(Zmediation= 2.165, p=0.030)对LGA的影响部分由GDM介导, 间接影响分别为16.3%和13.1%。
结论
孕前BMI与SGA、LGA相关。妊娠前超重和肥胖与LGA的关系部分由GDM介导。
1 INTRODUCTION
In women of reproductive age, the obesity epidemic has been a major contributing factor for obesity-related diseases1 and adverse outcomes of pregnancy. It is reported that prepregnancy obesity increased the risk of gestational diseases such as diabetes and hypertension in pregnant women.2 Moreover, maternal prepregnancy overweight/obesity was found to be related to higher risks of adverse outcomes in the fetus and chronic noncommunicable diseases like diabetes, hypertension, and coronary heart disease in adulthood of offspring.3 This suggests that early interventions on obesity in young women could put an end to the downward spiral of obesity across generations.
Gestational diabetes mellitus (GDM), as a common disease of pregnancy, increases the maternal and fetal risk of complications.4 Evidence from a meta-analysis5 has shown that prepregnancy obesity was a risk factor for GDM. The results of this study also demonstrated a dose-response relationship between prepregnancy obesity and GDM. A study6 of 33 973 pregnant women in China also reported that maternal prepregnancy body mass index (BMI) was positively associated with the risk of GDM, after adjusting for all confounding factors. Besides, maternal obesity and GDM usually coexist and share many metabolic features.7 So, GDM could be a causal link in the relationship between maternal overweight/obesity and adverse neonatal birth weight. However, to what extent GDM contributes to the effect of maternal obesity on neonatal birth weight remains unclear.
Hence, the relationship between maternal prepregnancy obesity and neonatal birth weight being mediated by GDM was hypothesized. The study was intended to explore the association between the degree of obesity, measured as BMI and adverse birth weight, besides testing if GDM mediates the relationship.
2 METHOD
2.1 Study design and participants
This registered cohort study used the data from the Women and Children Health Care Institution in Kunshan from 1 January 2013 to 31 December 2017. During this period, 106 130 pregnant women and their newborns were registered. Women with the first antenatal visit after 14 weeks (n = 30 644), multiple pregnancy or births (n = 1129), and stillbirth (n = 139) were excluded. Further, 56 947 participants who had missed second-trimester glucose tolerance test data or other missing values were excluded. Women diagnosed with diabetes history before pregnancy (n = 11) were also excluded from the analysis. Ultimately, 17 260 pregnant women and their newborns were included in the study. All participants signed informed consents and the ethics committee of The Women and Children Health Care Institution of Kunshan approved this study.
2.2 Variables and definition
Using a standard protocol, trained investigators collected demographic characteristics and clinical information. Maternal age (continuous variables), education levels (junior high school or less, senior high school, and college or more), parity (primiparity or multiparity), vaginal delivery (yes or no), history of diabetes and hypertension (yes or no), family history of hypertension and diabetes (yes or no), BMI and fasting blood glucose (FBG) level at the first prenatal visit (FBG, continuous variable), and FBG, 1-hour and 2-hour postprandial blood glucose level in 24-28 weeks of gestation, and neonatal sex, premature delivery (yes or no), birth weight, and gestational age were obtained in this study.
2.3 Definition of maternal prepregnancy obesity
Prepregnancy obesity, measured as pregnancy BMI, was calculated from the records of the weights and heights at the first prenatal care (usually at 12-14 gestation weeks). Prepregnancy BMI was categorized8 into underweight (BMI < 18.5 kg/m2), normal weight (18.5 kg/m2 ≤ BMI < 24 kg/m2), overweight (24 kg/m2 ≤ BMI < 28 kg/m2), and obese (BMI ≥28 kg/m2) according to the Chinese BMI classification standard.
2.4 Assessment of GDM
GDM screening was conducted by oral glucose tolerance tests between 24 and 28 weeks of gestation for participants after overnight fasting. According to the standard9 set by international diabetes and pregnancy research group, GDM is defined as FBG ≥5.1 mmol /L, or 1 hour plasma glucose ≥10.0 mmol /L or 2 hours plasma glucose ≥8.5 mmol /L.
2.5 Assessment of birth weight
Newborns were divided into three groups of small for gestational age (SGA), large for gestational age (LGA), and appropriate for gestational age (AGA) according to birth weight, gender, and gestational age, based on the criteria of the World Health Organization.10 LGA is defined as neonates with a birth weight of more than 90% by gestational age and gender. SGA is defined as neonates with a birth weight of less than 10% by gestational age and gender. AGA is defined as neonates with a birth weight between 10% and 90% by gestational age and gender.
2.6 Statistical analyses
The descriptive analysis in this study was reported using medians (interquartile ranges) because all continuous variables didn't follow the normal distributions, and Kruskal-Wallis tests were applied to compare the difference among the three birth weight groups. The categorical variables were described as percentages and compared using the chi-square test or Fisherʼs exact test. Logistic regression was used to analyze the association between BMI and SGA and LGA respectively. Crude odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated in Model 1. Adjusted ORs and 95% CIs were computed in Model 2 by adding the demographic variables, disease history, and family disease history to Model 1, and in Model 3 by adding GDM to model 2.
According to the procedure recommended by Baron and Kenny (1986),11 the mediating effect of GDM on the relationship between prepregnancy BMI and adverse birth weight was examined (Figure 1). Briefly, the mediation model included the following procedures. First, a logistic regression model was constructed to test if prepregnancy BMI significantly affected the adverse birth weight without adjusting for GDM (c path). Second, another logistic regression model was established to estimate whether prepregnancy BMI was significantly related to GDM (a path). Third, the association between GDM and adverse birth weight (b path) was analyzed. And fourth, the mediator GDM was added to the c path, if the path between prepregnancy BMI and the adverse outcome of birth weight was no longer significant, it could be concluded that the relationship between prepregnancy BMI and the adverse birth weight was fully mediated by GDM (cʼ path). Otherwise, the relationship might be only partially mediated by GDM.
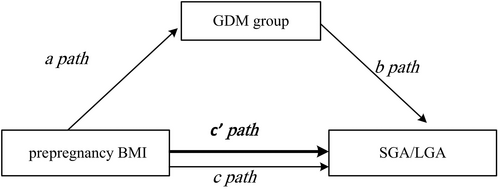
When the mediating effect of GDM on the association between prepregnancy BMI and the abnormal birth weight was analyzed, BMI was included in the logistic regression model as a continuous variable and a categorical variable, individually. When BMI was analyzed as a continuous variable, the product of regression coefficients of paths a and b (denoted as a*b) was used to test the mediation, namely, the standardized regression coefficients and standard errors (SE) of path a and path b were written into the R Mediation package12 to calculate the estimates of a*b and 95% CIs. The product of a*b indicates the magnitude of the mediating effect when the value of a*b is significant (the 95% CIs does not contain a zero). When the prepregnancy BMI was analyzed as a categorical variable, Iacobacciʼs (2012)13method was applied to examine the mediating effect of GDM. The mediating effect was measured by the regression coefficients of a, b, and cʼ paths, which was computed using the formula regression coefficients (a*b/c′)* 100%.The statistical analyses were performed using the SAS statistical software 9.4 and R software, and P < .05 was considered to be statistically significant.
3 RESULTS
The maternal median age (interquartile range) in this study was 27 (25-30) years old. According to the classification of prepregnancy BMI, 11 900 pregnant women (68.94%) were normal weight, 2571 (14.90%) were underweight, 2333 (13.52%) were overweight, and 456 (2.64%) were obese. Besides, 1355 (7.85%) pregnant women were diagnosed with GDM. Among 17 260 neonates, 8240 (47.74%) were male and 9020 (52.26%) were female. 10.42% (n = 1799) were LGA and 8.67% (n = 1497) were SGA.
Table 1 illustrates the general demographic information and medical history of the study population by neonatal birth weight classification. As compared with AGA neonates, LGA neonates had a higher proportion of mothers with junior high school or below education level. Moreover, LGA neonates were more likely to have mothers who were prepregnancy overweight (22.57% vs 12.92%) and obese (6.17% vs 2.34%) and had a higher percentage of GDM than AGA neonates (10.67% vs 7.68%). However, SGA neonates were inclined to have mothers who were underweight (24.65% vs 14.94%) and had a higher percent of vaginal delivery (66.73% vs 60.89%) compared to AGA neonates.
| Variables | AGA n = 13 964 | SGA n = 1497 | LGA n = 1799 | H/χ2 | P value |
|---|---|---|---|---|---|
| N (%) | 13 964 (80.90%) | 1497 (8.67%) | 1799 (10.42%) | ||
| Maternal characteristics | |||||
| Age of delivery (y) | 27 (25-30) | 26 (24-29)a | 28 (25-31)a | 138.21 | <.001 |
| FBG of the first examination (mmol/L) | 4.62 (4.38-4.90) | 4.62 (4.33-4.86)a | 4.62 (4.40-4.94)a | 23.54 | <.001 |
| Education, n (%) | |||||
| Junior high school or below | 2706 (19.38%) | 260 (17.37%) | 396 (22.01%)a | 15.71 | .003 |
| Senior high school | 4276 (30.62%) | 476 (31.80%) | 572 (31.80%) | ||
| University or above | 6982 (50.00%) | 761 (50.84%) | 831 (46.19%) | ||
| Parity, n (%) | |||||
| Primiparity | 3214 (23.02%) | 427 (28.52%) | 318 (17.68%) | 54.64 | <.001 |
| Multiparity | 10 750 (76.98%) | 1070 (71.48%)a | 1481 (82.32%)a | ||
| Prepregnancy BMI (kg/m2) | 20.8 (19.2-22.9) | 19.8 (18.5-21.7)a | 24.3 (20.4-24.4)a | 3492.12 | <.001 |
| Prepregnancy BMI status | |||||
| <18.5 (underweight) | 2086 (14.94%) | 369 (24.65%)a | 116 (6.45%)a | 434.83 | <.001 |
| 18.5-23.9 (normal weight) | 9747 (69.80%) | 987 (65.93%) | 1166 (64.81%) | ||
| 24.0-27.9 (overweight) | 1804 (12.92%) | 123 (8.22%)a | 406 (22.57%)a | ||
| ≥28.0 (obesity) | 327 (2.34%) | 18 (1.20%) | 111 (6.17%)a | ||
| GDM, n (%) | 1072 (7.68%) | 91 (6.08%) | 192 (10.67%)a | 26.88 | <.001 |
| History of hypertension, n (%) | 11 (0.08%) | 0 (0.00%) | 1 (0.06%) | 0.12 | .8564 |
| Family history of hypertension, n (%) | 320 (2.29%) | 35 (2.34%) | 40 (2.22%) | 10.55 | .005 |
| Family history of diabetes, n (%) | 130 (0.93%) | 10 (0.67%) | 30 (1.67%) | 10.56 | .005 |
| Vaginal delivery, n (%) | 8503 (60.89%) | 999 (66.73%)a | 806 (44.80%)a | 208.58 | <.001 |
| Neonates characteristics | |||||
| Male sex, n (%) | 6887 (49.32%) | 696 (46.49%) | 657 (36.52%) | 105.66 | <.001 |
| Birth height (cm) | 50(50-50) | 50 (49-50)a | 50 (50-51)a | 2402.48 | <.001 |
| Birth weight (kg) | 3.36 (3.15-3.58) | 2.80 (2.65-2.94)a | 4.00 (3.75-4.20)a | 5963.29 | <.001 |
| Premature delivery, n (%) | 913 (6.54%) | 142 (9.48%)a | 75 (4.16%)a | 11.16 | .003 |
- Note: Continuous variables were described using medians (interquartile ranges), and Kruskal-Wallis tests were used to compare the difference among groups. Categorical variables were described as percentages and compared using the chi-square test or Fisherʼs exact test.
- Abbreviations: AGA, appropriate for gestational age; BMI, body mass index; FBG, fasting blood glucose; GDM, gestational diabetes mellitus; LGA, large for gestational age; SGA, small gestational age large.
- a SGA and LGA groups compared with AGA group individually, P < .05.
The ORs and 95% CIs of SGA and LGA associated with prepregnancy BMI levels are presented in Table 2. In the unadjusted Model 1, compared with mothers with normal weight, those with underweight had a significantly higher risk of SGA (OR = 1.75, 95% CI = 1.54-1.99). After adjusting for confounding factors (Model 2), the prepregnant underweight was still positively related to SGA. After additional adjusting for GDM, compared to the maternal normal-weight group, the underweight group had 1.64 (95% CI = 1.44-1.87) times of risk to have SGA neonates. However, overweight and obese mothers before pregnancy had elevated risks of LGA neonates with ORs of 1.88 (1.66-2.13) and 2.87 (2.29-3.59) in the unadjusted Model 1, respectively. After adjusting for confounding factors (Model 2), prepregnancy overweight and obese mothers still had higher risks to deliver LGA neonates. The overweight and obese groups had 1.79 (95% CI = 1.58-2.03),and 2.76 (2.20-3.46) times the risks of having LGA newborns after additional adjusting for GDM, respectively.
| Prepregnancy BMI status | SGA (OR, 95% CI) | LGA (OR, 95% CI) | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| <18.5 (underweight) | 1.75 (1.54-1.99)* | 1.65 (1.45-1.87)* | 1.64 (1.44-1.87)* | 0.46 (0.38-0.57)* | 0.48 (0.41-0.58)* | 0.48 (0.40-0.59)* |
| 18.5-23.9 (normal) | Reference | Reference | Reference | Reference | Reference | Reference |
| 24.0-27.9 (overweight) | 0.68 (0.56-0.87)* | 0.72(0.59-0.83)* | 0.72 (0.59-0.88)* | 1.88 (1.66-2.13)* | 1.80 (1.59-2.04)* | 1.79 (1.58-2.03)* |
| ≥28.0 (obesity) | 0.55 (0.34-0.89)* | 0.57(0.34-0.93)* | 0.58 (0.35-0.92)* | 2.87 (2.29-3.59)* | 2.79 (2.23-3.50)* | 2.76 (2.20-3.46)* |
- Note: Model 1: Crude OR and 95% CI. Model 2: Adjusted for maternal age, FBG of the first examination, educational level, parity, history of hypertension, and the family history of diabetes and hypertension, vaginal delivery and premature delivery. Model 3: Added GDM to model 2.
- Abbreviations: BMI, body mass index; CI, confidence interval; FBG, fasting blood glucose; GDM, gestational diabetes mellitus; LGA, large for gestational age; OR, odds ratio; SGA, small gestational age large.
- * P < .05.
Table 3 enumerates the mediating effect of GDM on the association between prepregnancy BMI (continuous variable) and SGA/LGA. The indirect effect of maternal prepregnancy BMI on the risk of SGA through GDM (95% CI of a*b = −0.046-0.016) was not found to be statistically significant (as it contained zero). Contrarily, the mediating effect of GDM on the association between BMI and LGA (95% CI of a*b = 0.009-0.0051) was found to be significant, with this effect being 8.7%.
| Dependent (Y) | Independent (X) | a path | b path | Mediation effecta | |
|---|---|---|---|---|---|
| Coefficient (SE) | Coefficient (SE) | a*b | 95% CI | ||
| SGA | Prepregnancy BMI | 0.055 (0.010) | −0.259 (0.334) | −0.014 | −0.046-0.016 |
| LGA | Prepregnancy BMI | 0.055 (0.010) | 0.525 (0.212) | 0.029 | 0.009-0.051 |
- Note: a path reports the standardized coefficient of the association between pre-pregnancy BMI and GDM. b path reports the standardized coefficient of the association between GDM and study outcomes.
- Abbreviations: BMI, body mass index; GDM, gestational diabetes mellitus; LGA, large for gestational age; SGA, small for gestational age.
- a the standardized coefficients of a and b paths mediated effect and asymmetric 95% CIs.
Table 4 indicates the mediating effect of GDM on the relationship between prepregnancy BMI status and abnormal birth weight. Although maternal overweight and obesity were positively associated with GDM (standardized βs were 0.405 and 0.550, all P < .05), the mediating effect of GDM on the relationship between maternal prepregnancy overweight and obesity and SGA was not found to be significant statistically (P > .05). However, it was found that maternal overweight and obesity were positively associated with LGA (standardized βs were 0.578 and 0.981, all P < .05) after controlling for GDM and other confounding factors. Moreover, GDM partially mediated the effect of maternal overweight and obesity on LGA (Zmediation = 2.418, P < .05; Zmediation = 2.165, P < .05) with the indirect effect of 16.3% and 13.1%, respectively.
| Dependent (y) | Independent (x) | X → Y c path | X + M → Y cʼ path | X → M a path | M → Y b path | Z mediation | P value |
|---|---|---|---|---|---|---|---|
| BMI status | Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | |||
| SGA | <18.5 (underweight) | 0.496 (0.066)* | 0.502 (0.066)* | −0.004 (0.090) | −0.141 (0.115) | 0.031 | .975 |
| 24.0-27.9 (overweight) | −0.336 (0.010)* | −0.334 (0.010)* | 0.405 (0.075)* | −0.141 (0.115) | −1.176 | .239 | |
| ≥28.0 (obesity) | −0.602 (0.246)* | −0.598 (0.246)* | 0.550 (0.147)* | −0.141 (0.115) | −1.127 | .259 | |
| LGA | <18.5 (underweight) | −0.719(0.101)* | −0.720 (0.101)* | −0.004 (0.090) | 0.234 (0.085)* | 0.023 | .097 |
| 24.0-27.9 (overweight) | 0.587(0.064)* | 0.578 (0.064)* | 0.405 (0.075)* | 0.234 (0.085)* | 2.418a | .015* | |
| ≥28.0 (obesity) | 0.992 (0.116)* | 0.981 (0.116)* | 0.550 (0.147)* | 0.234 (0.085)* | 2.165a | .030* |
- Note: The table shows regression coefficients and SEs for each step of mediation analysis after controlling for maternal age, FBG of the first examination, educational level, parity, history of hypertension, and the family history of diabetes and hypertension, vaginal delivery, and premature delivery. c path reports regression coefficients of prepregnancy BMI status with SGA and LGA; c' path reports coefficients of prepregnancy BMI status to abnormal birth weight at term; a path reports coefficients of prepregnancy BMI status to GDM (the mediator under examination); b path reports coefficients of GDM (mediator) to abnormal birth weight at term.
- Abbreviations: BMI, body mass index; FBG, fasting blood glucose; GDM, gestational diabetes mellitus; LGA, large for gestational age; SGA, small for gestational age.
- a Zmediation statistics exceeding |1.96| were significant at .05 levels.
- * P < .05.
4 DISCUSSION
First, the association between prepregnancy obesity and neonatal birth weight was examined, where it was found that the higher the maternal prepregnancy BMI, the greater the risk of LGA compared to AGA. Contrarily, the lower the maternal prepregnancy BMI, the greater the risk of SGA compared to the AGA group. Furthermore, we explored the mediated effect of GDM on this association. Overall, the mediation analysis indicated that the relationship between prepregnancy BMI and LGA was partially mediated by GDM.
In our study, around 8.7% of neonates were SGA and 10.4% were LGA. A multicenter retrospective cohort study14 conducted in Chinese urban women reported 6.21% and 10.32% of neonates born SGA and LGA, respectively. Similarly, a research in Japan15 indicated that the incidence rate of SGA was 9.3% and LGA was 10.13%. Data from the Pregnancy Risk Assessment Monitoring System (PRAMS)16 in the United States revealed that the proportion of SGA was 16.60%, and LGA was 9.55%. These discrepancies could be due to racial differences.17
Results from this study were consistent with most of the earlier researches in which the prepregnancy weight out of the normal bound was found to be associated with increased risks of adverse birth weight. A meta-analysis18 showed that compared to the normal weight mothers, overweight mothers had 1.45(95% CI = 1.29-1.63) times risk to deliver LGA neonates, whereas mothers who were obese had a higher risk of 1.88 times (95% CI = 1.67-2.11) to deliver LGA neonates. In China, prepregnancy obese women were found to be more vulnerable to have LGA neonates (OR = 2.03, 95% CI = 1.90-2.18).19 An elevated birth weight affected the weight status trajectory of offspring during childhood and adolescence,20 whereas the children having higher birth weight were also prone to be obese as adults.21
Furthermore, it was observed that GDM mediated the relationships between prepregnancy overweight, obesity, and LGA. Previous literature had reported certain mediators affecting the relationship between maternal and obesity in children. A birth cohort study demonstrated22 that the placental weight partially mediated the effects of prepregnancy BMI on fetal growth. A longitudinal birth cohort study23 including 2267 participants indicated that the mediation effect of excess gestational weight between maternal obesity and offspring obesity at 4-year-olds was 8.1%. Contrarily, the mediation of GDM on the prepregnancy obesity condition and SGA was not found to be significant in our study. Several studies showed that maternal prepregnancy obesity and GDM were strong determinants of LGA.24, 25 In this study, we did not exclude participants whose offspring were SGA, as SGA was an important risk factor for the neonatal complications or death among infants born to mothers with GDM.26 Other research27 indicated SGA neonates born to mothers with GDM had a higher risk of long-term cardiovascular hospitalizations compared to SGA neonates born to mothers without GDM.
Previous studies have also observed that prepregnancy BMI was a risk factor for the development of GDM.16, 28 However, the mechanism of how BMI affects fetal growth through GDM remains unclear. The hypothesis of fetal overgrowth and systemic inflammation could account for this. First, inflammation has been often associated with obesity because of the secretion of proinflammatory cytokines by adipocytes. Hence, the abundance of adipocytes in obese women could produce excessive markers of inflammation, which in turn could lead to the development of GDM.29-31 Second, maternal hyperglycemia leads to fetal overgrowth. Among pregnant women with GDM, the pancreatic beta cells failed to compensate sufficiently32 and gluconeogenesis was affected, resulting in a hyperglycemic metabolic environment for the mother, as the glucose shifted down the placental concentration gradient. Thus, this maternal hyperglycemia could lead to fetal hyperglycemia,33, 34 which in turn stimulated fetal pancreas to increase the production of insulin. Because insulin is an important fetal growth hormone, the resulting hyperinsulinemia could lead to fetal overgrowth.
The strength of our study included the large sample size and a large collection of maternal health information. This study had several potential limitations that need to be considered. First, we had not considered the mediation of GDM when we analyzed the mediating effect of GDM on the relationship between prepregnancy BMI and neonatal birth weight, which could lead to an overestimation of the mediating effect. Second, the maternal diet and physical activity levels were not collected; thus, the effect of those factors on neonatal birth weight could not be analyzed.
5 CONCLUSION
In conclusion, maternal prepregnancy BMI status is associated with abnormal fetal birth weight and the association is mediated by GDM. It suggests that screening and management of maternal prepregnancy obesity and GDM could restrict the future epidemic of obesity in childhood.
ACKNOWLEDGEMENTS
We thank all members of our study at the Maternal and Child Health Bureau of Kunshan and the School of Public Health, Medical College of Soochow University. This work was supported by the Natural Science Foundation of Jiangsu Province (Grant No. BK20181438), the Maternal and Child Health Research Projects of Jiangsu Province (Grant No.F201830, No.F201847) and the key technical (research) project for the prevention and control of major diseases and infectious diseases of Suzhou Municipal Commission of Health (Grant No. Gwzx201803).
CONFLICT OF INTERESTS
The authors do not have any disclosures and/or conflict of interest.




