Choice of basal insulin therapy is associated with weight and height development in type 1 diabetes: A multicenter analysis from the German/Austrian DPV registry in 10 338 children and adolescents
基础胰岛素治疗的选择与1型糖尿病患者的体重和身高发育有关:来自德国/奥地利DPV登记的10338名儿童和青少年的多中心分析
Heike Vollbach, Marie Auzanneau, Bettina Gohlke, and Reinhard W. Holl are the co-first and senior authors.
Funding information: Dr Bürger-Büsing Foundation; European Foundation for the study of diabetes; German center for Diabetes research, Grant/Award Number: 82DZD01402; German Diabetes Association; German Diabetes Foundation; German Ministry of Health
Abstract
enBackground
Available basal insulin regimes differ in pharmacokinetic profiles, which may be related to subsequent changes in anthropometry in patients with type 1 diabetes. This analysis elucidates the standardized height and body mass index development (height and BMI standard deviation score [height-SDS and BMI-SDS]) in pediatric type 1 diabetes patients depending on the choice of basal insulin.
Methods
Longitudinal data of 10 338 German/Austrian patients from the Diabetes Prospective Follow-up (DPV, Diabetes Patienten Verlaufsdokumentation) database were analyzed. Patients aged 5.0 to 16.9 years were treated exclusively with neutral protamine Hagedorn (NPH), insulin detemir (IDet), insulin glargine (IGla), or continuous subcutaneous insulin infusion (CSII) for at least 3 years. Population-based German reference data were used to calculate height-SDS and BMI-SDS. Multiple linear regression was conducted.
Results
BMI-SDS increased significantly in all regimes (NPH P = .0365; IDet P = .0003; IGla P < .0001; and CSII P < .0001). Direct comparison of the therapies revealed a favorable association only for NPH vs IGla. A rise in BMI-SDS was observed for all insulins in females, but only for IGla in males. BMI-SDS increment was not observed before 8 years of age.
Initially and at the end of the observation period, mean height was above the 50th percentile of the reference population. Across the cohort, height-SDS declined during the observation period, except for CSII. Apart from the 5.0- to 7.9-year-old subgroup, long-acting insulin analogues were associated with a significant loss of height-SDS.
Conclusions
Choice of basal insulin regimen might influence height development. CSII appeared to have a favorable effect on growth trajectories. All therapies were associated with an increase of BMI-SDS, most evident in females.
摘要
zh背景
现有的基础胰岛素方案在药代动力学方面存在差异, 这可能与1型糖尿病患者后续的人体测量学变化有关。这项分析阐明了儿童1型糖尿病患者基于基础胰岛素的选择的标准化身高和体重指数BMI(身高和BMI标准差评分[身高-SDS和BMI-SDS])的发展。
方法
对10338例德国/奥地利糖尿病进行前瞻性数据库中的纵向资料进行分析。年龄5.0~16.9岁的患者接受中性鱼精蛋白Hagedorn(NPH), 地特胰岛素(IDET), 甘精胰岛素(IGLA)或持续皮下胰岛素输注(CSII)治疗至少3年。以人群为基础的德国参考数据被用来计算身高-SDS和BMI-SDS。进行多元线性回归分析。
结果
BMI-SDS在所有方案中均显著升高(NPH P=.0365, IDET P=.0003, IGLA P<.0001, CSII P<.0001)。对这些疗法的直接比较显示, 仅对NPH和IGLA有有利的关联。在女性中观察到所有胰岛素的BMI-SDS升高, 但只在男性中观察到IGLA的升高。BMI-SDS在8岁前未见明显增加。
最初和在观察期结束时, 平均身高高于参考人口的第50个百分位数。在整个队列中, 除CSII外, 身高-SDS在观察期内均呈下降趋势。除了5.0到7.9岁的亚组外, 长效胰岛素类似物与身高-SDS显著降低相关。
结论
基础胰岛素方案的选择可能影响身高发育。CSII似乎对增长轨迹有有利的影响。所有的治疗都与BMI-SDS的增加有关, 这在女性中最为明显。
1 INTRODUCTION
Intensified insulin therapy (ICT) and continuous subcutaneous insulin infusion (CSII) are currently the prevailing therapy regimes in type 1 diabetes. Short-acting insulin analogues are predominantly utilized in CSII. In ICT, basal insulin requirements are covered with neutral protamine Hagedorn (NPH) insulin or alternatively long-acting insulin analogues like insulin detemir (IDet) and insulin glargine (IGla). The latter two are regarded to be advantageous over NPH insulin due to a reduced risk of hypoglycemia and a prolonged duration of action without pronounced peak activity. Other long-acting insulin analogues were not included in the analysis because of a lack of (IGla 300 IE/mL) or only recently received (insulin degludec) drug approval in pediatric patients (Figure 1).
Insulin itself is a potent anabolic hormone. Accumulated evidence indicates that improved glycemic control in type 1 diabetes due to ICT provokes weight gain. This accompanying effect was already reported by the Diabetes Control and Complications Trial (DCCT) Research Group.1 Further predictors of weight gain such as low body mass index (BMI) at diabetes onset, pubertal diabetes onset, long diabetes duration, low glycosylated hemoglobin (HbA1c), higher basal/bolus insulin doses, and female sex were identified.2
In addition to altered pharmacokinetics following subcutaneous injection, potential explanations for this phenomenon are the anabolic effects of insulin and reduced glycosuria with consequently enhanced utilization of calories. Children and adolescents with type 1 diabetes are more likely to have a higher BMI than their peer group.3 Today, one-third of adolescent type 1 diabetes patients are overweight or obese.2 Apart from disease-related predictors and similar to the background population, the current obesogenic environment further contributes to a rising incidence of obesity and overweight in type 1 diabetes patients.
Impaired growth has frequently been reported in children with type 1 diabetes.4 Pathophysiology of the abnormality in growth trajectories in a chronic disease is complex. Hereby portal insulinopenia seems to be crucial for the dysregulation in the growth hormone–insulin-like growth factor 1–insulin-like growth factor-binding protein (GH-IGF1-IGFBP) axis.5 Well-characterized alterations include a decreased IGF1, IGFBP3, and GH-binding protein and an increase in GH and IGFBP1 persisting despite near-normal glycemic control.6 Additionally, and presumably most relevant, is a reduced stability of the IGF1 mRNA in the presence of portal insulinopenia.5 Due to its liver specificity, IDet might partially compensate portal insulinopenia and thereby GH hypersecretion. Recent reports suspected that this has a favorable impact on weight gain.7, 8
Little data are available on whether the choice of basal insulin regimes has an impact on observed changes in weight and height trajectories. Different pharmacokinetic profiles of short-acting insulin analogues, NPH insulin, IDet, and IGla, might exert a variable influence on the anthropometry of children and adolescents with type 1 diabetes. The aim of this analysis was to elucidate differences in longitudinal auxological development of height and BMI standard deviation scores (height-SDS and BMI-SDS) in children and adolescents with type 1 diabetes with four different insulin regimes. We therefore investigated a large cohort of German/Austrian patients from the Diabetes Prospective Follow-up (DPV, Diabetes Patienten Verlaufsdokumentation) database.
2 METHODS
As of September 2018, the DPV comprises data of 538 531 diabetic patients of 480 centers in Germany and Austria. The registry is used for quality management and scientific research.9 The participating centers enter their routine care data into an electronic health record. Anonymized data are transmitted every 6 months to the University of Ulm, Germany, for verification and central analysis. The collection and analysis of anonymized data from the DPV registry was approved by the ethics committee of Ulm University.
Based on the DPV registry, longitudinal data of 10 338 children and adolescents with type 1 diabetes aged 5.0 to 16.9 years were analyzed at the start of the observation period (2008-2018). For each patient, we investigated the five most recent treatment years, if available, and at least 3 years. Included patients were either treated with NPH insulin, IDet, or IGla within ICT or they received CSII treatment. Patients with a change or combination of therapy regimes were excluded. A minimum of 2 years of diabetes duration at the start of observation was a further inclusion criterion. Patients with migration background were excluded as anthropometric references may not apply.
The cohort was subsequently divided into age groups: 5.0 to 7.9 years, 8.0 to 13.9 years, and 14 to 16.9 years at the start of the observation period. This classification was chosen to allow the analysis of prepubertal, peripubertal, and nearly postpubertal/postpubertal type 1 diabetes patients. Because documentation of pubertal stage (“Tanner stage”) was not available for all individuals, we referred to the official endocrine definition of physiological prepubertal age as the age before 8.0 years in girls and 9.0 years in boys.10
All anthropometric parameters were collected during clinic visits. Subjects were examined in light pants, vest, and no shoes. Body weight was measured to the nearest 0.1 kg on a calibrated scale. Body height was measured with accuracy to the nearest 0.1 cm. BMI values were calculated as weight (in kilograms)/height (in square meters). Height-SDS and BMI-SDS were calculated using population-based German reference data.11
We used multiple linear regression models to estimate mean changes in height-SDS and BMI-SDS (adjusted mean estimates or least squares means [LSM]) associated with each basal insulin regimen. All models were adjusted for gender, age group, and height-SDS or BMI-SDS at the start of the observation period and HbA1c at the start and end of the observation period. Estimated changes in height-SDS and BMI-SDS were compared pairwise between basal insulin regimens (differences of LSM) using the Tukey-Kramer procedure for multiple comparison. All analyses were additionally stratified by age group and gender.
All statistical analyses were performed with the Statistical Analysis System (SAS) version 9.4 (SAS Institute Inc, Cary, North Carolina). P values were based on a general linear model. Statistical significance was inferred at a two-tailed P < .05.
3 RESULTS
3.1 Description of the cohort
Longitudinal data of 10 338 (52% males, age 5.0-19.9 years) pediatric and adolescent patients with type 1 diabetes documented since 2008 in the DPV registry were analyzed. Patients included in the present analysis were recorded in 280 centers in Germany and Austria. Descriptive data of the cohort are shown in Table 1.
| Study cohort (N = 10 338) | NPH (n = 723, 7.0%) | CSII (n = 6873, 66.5%) | Detemir (n = 1249, 12.1%) | Glargine (n = 1493, 14.4%) | |
|---|---|---|---|---|---|
| Age at start of observation (y) | 11.96/12.80 (10.00; 14.20) | 12.87/13.70 (11.40; 14.90) | 11.39/12.20 (9.10; 13.70) | 12.92/13.20 (11.90; 14.50) | 13.36/13.70 (12.50; 14.80) |
| Gender (males, %) | 52.3 | 61.6 | 49.3 | 56.6 | 58.2 |
| Observation period (y) | 4.21/4.50 (3.85; 4.65) | 4.01/4.30 (3.40; 4.60) | 4.27/4.50 (4.10; 4.65) | 4.11/4.40 (3.60; 4.60) | 4.09/4.40 (3.55; 4.60) |
| HbA1c_start of observation | 7.68/7.53 (6.91; 8.28) | 7.54/7.34 (6.70; 8.19) | 7.60/7.48 (6.90; 8.18) | 7.88/7.62 (6.92; 8.51) | 7.95/7.72 (7.08; 8.60) |
| HbA1c_end of observation | 8.09/7.82 (7.08; 8.75 | 7.89/7.62 (6.91; 8.59) | 8.05/7.79 (7.08; 8.69) | 8.09/7.77 (7.05; 8.79) | 8.38/8.08 (7.27; 9.16) |
| Change in BMI-SDS | 0.09/0.10 (−0.28; 0.47) | 0.05/0.09 (−0.25; 0.41) | 0.08/0.10 (−0.29; 0.47) | 0.10/0.10 (−0.31; 0.51) | 0.12/0.14 (−0.22; 0.48) |
| Change in height-SDS | −0.03/−0.05 (−0.37; 0.30) | −0.03/−0.05 (−0.35; 0.30) | 0.00/−0.02 (−0.34; 0.33) | −0.12/−0.12 (−0.46; 0.19) | −0.09/−0.09 (−0.43; 0.23) |
| BMI-SDS_start of observation | 0.23/0.24 (−0.29; 0.79) | 0.26/0.25 (−0.32; 0.79) | 0.24/0.26 (−0.27; 0.79) | 0.12/0.15 (−0.46; 0.71) | 0.29/0.29 (−0.23; 0.86) |
| BMI-SDS_end of observation | 0.32/0.35 (−0.24; 0.92) | 0.31/0.32 (−0.23; 0.94) | 0.32/0.35 (−0.23; 0.91) | 0.21/0.25 (−0.38; 0.85) | 0.41/0.45 (−0.16; 0.99) |
| Height-SDS_start of observation | 0.21/0.21 (−0.46; 0.89) | 0.18/0.17 (−0.51; 0.89) | 0.23/0.22 (−0.44; 0.89) | 0.16/0.22 (−0.53; 0.86) | 0.20/0.19 (−0.47; 0.89) |
| Height-SDS_end of observation | 0.18/0.18 (−0.52; 0.86) | 0.15/0.17 (−0.58; 0.89) | 0.22/0.22 (−0,47; 0.89) | 0.05/0.04 (−0.66; 0.74) | 0.11/0.11 (−0.58; 0.77) |
| 5.0-7.9 years (n = 1335) | 8.0-13.9 years (n = 6069) | 14.0-16.9 years (n = 2934) | Females (n = 4929) | Males (n = 5409) | |
| Age at start of observation (y) | 6.50/6.50 (5.80; 7.20) | 11.71/12.20 (10.40; 13.10) | 14.96/14.90 (14.50; 15.20) | 11.94/12.80 (10.00; 14.10) | 11.99/12.80 (10.00; 14.30) |
| Gender (males, %) | 53.70 | 51.10 | 54.2 | ||
| Observation period (y) | 4.15/4.45 (3.70; 4.60) | 4.33/4.55 (4.20; 4.65) | 3.99/4.30 (3.30; 4.60) | 4.22/4.50 (3.90; 4.65) | 4.20/4.50 (3.80; 4.65) |
| HbA1c_start of observation | 7.37/7.31 (6.82; 7.89) | 7.62/7.48 (6.89; 8.21) | 7.95/7.72 (7.01; 8.60) | 7.69/7.53 (6.93; 8.26) | 7.67/7.53 (6.89; 8.28) |
| HBA1c_end of observation | 7.60/7.47 (6.92; 8.10) | 8.15/7.90 (7.15; 8.86) | 8.20/7.86 (7.09; 8.95) | 8.10/7.83 (7.12; 8.79) | 8.08/7.81 (7.07; 8.74) |
| Change in BMI-SDS | −0.24/−0.26 (−0.61; 0.08) | 0.14/0.16 (−0.22; 0.53) | 0.12/0.15 (−0.20; 0.46) | 0.17/0.19 (−0.20; 0.56) | 0.01/0.02 (−0.35; 0.39) |
| Change in Height-SDS | −0.04/−0.04 (−0.30; 0.22) | −0.07/−0.09 (−0.49; 0.36) | 0.05/−0.02 (−0.21; 0.23) | 0.00/−0.03 (−0.30; 0.27) | −0.06/−0.07 (−0.45; 0.32) |
| BMI-SDS_start of observation | 0.45/0.47 (−0.05; 0.99) | 0.16/0.17 (−0.37; 0.72) | 0.29/0.30 (−0.20; 0.84) | 0.31/0.34 (−0.21; 0.88) | 0.16/0.17 (−0.36; 0.70) |
| BMI-SDS_end of observation | 0.21/0.20 (−0.31; 0.74) | 0.30/0.33 (−0.26; 0.91) | 0.41/0.45 (−0.14; 1.02) | 0.49/0.53 (−0.03; 1.07) | 0.17/0.18 (−0.39; 0.77) |
| Height-SDS_start of observation | 0.22/0.22 (−0.48; 0.94) | 0.23/0.23 (−0.44; 0.90) | 0.18/0.19 (−0.51; 0.83) | 0.21/0.23 (−0.48; 0.88) | 0.21/0.20 (−0.44; 0.89) |
| Height-SDS_end of observation | 0.19/0.19 (−0.50; 0.84) | 0.15/0.16 (−0.54; 0.84) | 0.23/0.20 (−0.47; 0.93) | 0.22/0.20 (−0.48; 0.88) | 0.15/0.14 (−0.56; 0.83) |
| Patients using NPH (%) | 4.7 | 5.3 | 11.5 | 5.6 | 8.2 |
| Patients using CSII (%) | 89.6 | 69.1 | 50.6 | 70.7 | 62.6 |
| Patients using Detemir (%) | 3.4 | 12.6 | 15 | 11 | 13.1 |
| Patients using Glargine (%) | 2.3 | 13 | 22.9 | 12.7 | 16.1 |
- Note: Data are means/medians (lower/upper quartile) or percentages, unless otherwise indicated.
- Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusion; HbA1c, glycosylated hemoglobin; NPH, neutral protamine Hagedorn; SDS, standard deviation score.
3.2 Body mass index SDS
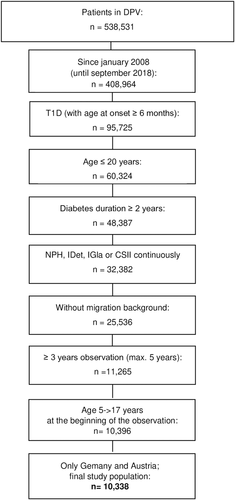
Initial mean BMI-SDS of the whole cohort was above average of the reference population (Table 1). The same applied to all analyzed therapy regimes separately. During the observation period, BMI-SDS increased independently of the used insulin (Δ BMI-SDS [means]: NPH P = .0365; CSII P < .0001; IDet P = .0003; IGla P < .0001) (Figure 2A). It is noteworthy that direct comparison of the Δ BMI-SDS of the four therapy regimes revealed a significant difference only for NPH vs IGla (Figure 2A).
Analysis stratified by gender uncovered different patterns of Δ BMI-SDS for boys and girls (Figure 2B). Female patients showed a significant rise in BMI-SDS in all therapy regimes (Δ BMI-SDS [means]: NPH P = .0006; CSII P < .0001; IDet P = .0001; IGla P < .0001), whereas in males only the treatment with IGla was associated with an increase in BMI-SDS (Δ BMI-SDS [means]: NPH P = .2227; CSII P = .6441; IDet P = .5881; IGla P = .0215) (Figure 2B).
In females, direct comparison of the insulin regimes suggested that the use of IDet was associated with a more favorable BMI-SDS development compared with CSII. In males, no differences were found (Figure 2B).
The age-stratified analysis is shown in Figure 2c. Different templates of BMI-SDS development were found in the subgroups. A significantly lower BMI-SDS increase with NPH compared with CSII and IGla was observed in peripubertal patients (8.0-13.9 years old).
3.3 Height-SDS
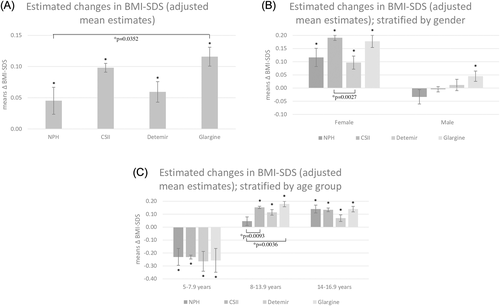
Regarding the whole cohort and the subgroups of different therapy regimes, the initial height ranged above the 50th percentile of the German reference population (Table 1). Concerning the whole cohort, height-SDS decreased during the observation period, except for CSII. The decline reached statistical significance for NPH and was highly significant for IDet and IGla (Δ change [means]: NPH P = .0241; CSII P = .5739; IDet P < .0001; IGla P < .0001). In the direct comparison of the entire data, CSII treatment was linked to no significant change of height-SDS in contrast to all other therapeutic regimens where height-SDS decreased significantly. In direct comparison, the extent of Δ height-SDS differed between CSII and long-acting insulin analogues as well as NPH insulin and IDet (Figure 3A).
Results were concordant for long-acting insulin analogues but not for CSII and NPH in females and males (females: Δ height-SDS [means]: NPH P = .1504; CSII P = .0030; IDet P < .0001; IGla P = .0156; males: Δ height-SDS [means]: NPH P = .0359; CSII P = .0881, IDet P < .0001; IGla P < .0001). Direct comparison showed that Δ height-SDS development was consistently favorable in CSII regimen for both genders (Figure 3B).
Data stratified by age group are shown in Figure 3C (5.0- to 7.9-year-olds: Δ height-SDS [means]: NPH P = .3978; CSII P = .0007; IDet P = .1826; IGla P = .9589) (8.0- to 13.9-year-olds: Δ height-SDS [means]: NPH P = .0005; CSII P = .0062; IDet P < .0001; IGla P < .0001) (14.0- to 16.9-year-olds: Δ height-SDS [means]: NPH P = .0768; CSII P = <.0001; IDet P = .6244; IGla P = .3906). Direct comparison revealed that CSII treatment was connected with a beneficial change in Δ height-SDS in 8.0- to 16.9-year-olds.
The numbers for analyses stratified by age group and gender were too small for valid conclusions, but descriptive data showed a decrease of BMI-SDS at an age between 5.0 to 7.9 years for boys and girls (data are shown in the supplement). Interestingly, after the age of 8.0 years, a gender specific pattern of BMI-SDS development became obvious: While girls experienced a noticeable increase, it was less striking in boys. The loss of height-SDS was more pronounced in boys than in girls before the age of 14 years. Beyond this age an increase in growth was noticed for all therapies except for IDet in males (data are shown in the supplement).
4 DISCUSSION
Considering the rising prevalence of overweight and obesity among pediatric type 1 diabetes patients, it seems crucial to better understand the auxological development during the course of the disease. It is important to identify patients early who are at a higher risk for imbalanced weight gain, especially when aggravated by impaired growth. In the present DPV database analysis, differences in BMI-SDS and height-SDS development in pediatric type 1 diabetes patients were identified, comparing different basal insulin regimens. Data for the change in height-SDS and BMI-SDS were adjusted for gender, age group, and height-SDS or BMI-SDS at the start of the observation period and HbA1c at the start and end of the observation period to rule out potential confounders.
Analyzing the entire data, we found that in accordance with the literature1 all regimes were accompanied by a relevant increase in BMI-SDS. Like in previous reports, we did not observe differences in regard to BMI-SDS development between CSII and a therapy of multiple daily injections (MDI) using either NPH insulin, IGla, or IDet as basal insulin regimes.12 In addition to obesogenic dietary factors, reversal of metabolic distress, and insulin deficiency, subcutaneous insulin administration itself contributes to a disproportional increase of weight. This is mediated by anabolic effects like promoting lipogenesis and impairing proteolysis such as GH-stimulated insulin resistance.6 In females, IDet was related to a more favorable BMI development in comparison with CSII. In the literature, higher doses of IDet in comparison with IGla are needed to achieve comparable glycemic control and fasting plasma glucose levels.13 Nevertheless, beneficial effects on weight development have been reported with IDet.13 These observations were attributed to a centrally mediated reduction of energy intake in combination with an at least partially conserved physiological portal/peripheral insulin gradient.7, 14
Comparing basal insulin therapies in the whole cohort revealed NPH to be advantageous over IGla concerning BMI-SDS development. This effect was also observed in the subgroup of patients aged 8.0 to 13.9 years, but without consistency in the other subgroups.
Especially female patients showed a gain of BMI-SDS independent of the administered basal insulin regimen. Except in CSII, female patients exhibited a concurrent loss of height-SDS leading to a disproportional weight gain. In contrast, in male patients we found a relevant BMI-SDS incline only if IGla was administered, but not with NPH, CSII, or IDet. Consistent with the existing literature, we found girls with type 1 diabetes to be vulnerable to weight gain and BMI-SDS increase.15 This observation included CSII treatment. A larger fat mass16 and less physical activity17 and a hereby suggested increased insulin resistance might promote the pronounced weight gain in diabetic females. Furthermore, and in particular in girls, insulin sensitivity is lowered physiologically with the onset of puberty.18 In contrast to pediatric type 1 diabetes patients, longitudinal surveillance in adult patients found a relevant weight gain irrespective of gender. To summarize, gender differences in BMI trajectories already seem to manifest during childhood, whereas, according to the literature, in adulthood both genders seem to be at a comparable risk for weight gain.
A consistent BMI-SDS decrease in all regimes was shown for the youngest patients in our cohort (5.0-7.9 years old). As inclusion criteria a diabetes duration of at least 2 years was required to rule out initial weight changes due to disease manifestation. One can speculate that the observed BMI-SDS decrease might be due to restrictively controlled carbohydrate and energy intake by parents. In contrast, an excessive BMI-SDS increase becomes obvious in the older age groups. This strengthens the need for a stringent focus on BMI development from early puberty onward to counteract disproportional weight gain, especially in diabetic girls.
The initial height of the whole cohort and in all subgroups was above population reference. Independent of the chosen therapy regime, height-SDS remained positive during the observation period. Nevertheless, across the cohort and without identifiable gender differences, a significant decrease of height-SDS was observed with NPH, and with IDet and IGla. Noteworthy was the almost unaffected height-SDS development in CSII treatment. In females with CSII, even a slight increase of height-SDS was detected. Except for the subgroup of patients aged 5.0 to 7.9 years, height-SDS development was advantageous in favor of CSII treatment compared with the regimes with long-acting insulin analogues (IDet or IGla) in all analyzed cohorts. In adolescents, the catch-up growth seemed to be diminished when using IDet or IGla in comparison to CSII treatment. Results of additional analysis without adjustment for HbA1c (data not shown) did not vary substantially. Therefore, the adverse effects of long-acting insulins on height did not seem to be associated with glycation control.
Among the investigated insulins, in in vitro analysis IGla was most potent to activate the IGF1 receptor as compared with human insulin or IDet.19 In vivo a single dose of IDet in relation to NPH or IGla was found to cause higher levels of bioactive IGF1 but not total IGF1. This finding was accompanied by a larger suppression of IGFBP1 with consecutive lower GH concentrations.8 Other studies reported no differences between IDet and NPH or IDet and IGla regarding the GH-IGF1-IGFBP axis.20, 21 The assumed enhanced catabolic metabolism of IDet, as mentioned above, might also interfere with the GH-IGF1 axis and could explain the impact found here on growth patterns. Ghrelin, for example, which increases GH secretion, was able to antagonize central effects of IDet. Additionally, portal insulinopenia in type 1 diabetes causes diminished IGF1 levels and elevated IGFBP1 levels, hence provoking GH hypersecretion. IDet seems to partially normalize these abnormalities by its increased hepatic selectivity. Lower GH levels might enhance GH-induced insulin resistance, but long-term effects on growth remain unclear.
However, growth is regulated in a complex way. Moreover, daily insulin requirements, which were not investigated in this study, can also directly influence growth patterns in patients with pediatric type 1 diabetes. CSII treatment probably provides the most physiological pattern of insulin dispensation, which might explain the favorable effect on height development described in this report. Blair et al. compared BMI- and height-SDS in children and adolescents between CSII and MDI during the first year following type 1 diabetes diagnosis and found no difference in BMI- or height-SDS.12 We speculate that this rather short observation period might be not long enough to notice significant differences. Further studies on the influence of basal insulin regimes are needed to investigate long-term height development.
Several authors report that at the time of type 1 diabetes onset, children were taller than their healthy peers, but they lose height-SDS throughout the course of the disease due to impaired growth velocity.9 Our data confirmed a decrease in height-SDS until mid-puberty associated with insulin intake. Later on, a significant increase of height-SDS is only seen for CSII. Since Tanner stages were not available for all individuals, height development could not be interpreted in correlation with pubertal development.
Our study profits from its multicenter approach which was made possible by the DPV database. The long-term standardized follow-up of a large number of children and adolescents with type 1 diabetes allows a longitudinal analysis of well-standardized parameters such as height-SDS, BMI-SDS, and insulin therapy. Duration of diabetes was required to be at least 2 years in order to diminish auxological changes due to disease manifestation. Our observation period was restricted to 2008 to 2018 to avoid dietary changes due to secular trends and to have a comparable diabetes management.
This study has some limitations. First, our analyses did not include daily insulin dose and Tanner stages as important parameters for interpreting auxological development. Second, the number of patients treated with NPH was relatively small (n = 723). In our study, a relatively high percentage of patients was treated with CSII (66.5%). Especially in younger children, CSII is currently the standard regimen for treating type 1 diabetes in Germany and Austria. Since a minimum duration of 2 years of diabetes at the start of the observation period was an inclusion criterion, many patients in the age group of 8.0 to 13.9 years were under 6 years old at the time of diabetes manifestation. With respect to this consideration, our data are comparable with the literature (Table 1).22 We did not evaluate the data for a possible impact of socioeconomic status.
Data showed an association between the type of basal insulin regimen and height development. CSII appeared to have the most favorable effect on growth trajectories. When expressed in absolute figures, the alterations of height-SDS are less distinguished compared with changes in BMI-SDS. Therefore, the clinical impact of the observed effects was moderate, especially for height-SDS. The influence of the findings in view of therapeutic decision-making processes remains unclear. Previous findings of the positive association between insulin and increased weight gain were confirmed in our analysis. All basal insulin regimens increased BMI-SDS, but there were no consistent findings to support the use of one therapy regimen over another in children and adolescents with type 1 diabetes.
ACKNOWLEDGEMENT
For a full list of the centers participating in the DPV initiative and the contribution of their data to this study, please see the online supplementary file on our website. The DPV initiative is financially supported by the German Ministry of Health, the German Diabetes Foundation, the German Diabetes Association, the German Centre for Diabetes Research (DZD, Deutsches Zentrum für Diabetesforschung; grant no. [FKZ, Förderungskennzeichen]: 82DZD01402), the Dr Bürger-Büsing Foundation, and the European Foundation for the Study of Diabetes (EFSD).
CONFLICT OF INTEREST
The authors have nothing to disclose.




