Combining glomerular basement membrane and tubular basement membrane assessment improves the prediction of diabetic end-stage renal disease
联合评估肾小球基底膜厚度和肾小管基底膜厚度可提高糖尿病肾病进展为终末期肾脏病的预测能力
Funding information: Applied Basic Research Project of Sichuan Science and Technology Department, Grant/Award Number: 2020YJ0063; Key Research and Development Project of Sichuan Science and Technology Department, Grant/Award Number: 19ZDYF1273; Popularization Project of the Science and Technology Project of the Sichuan Health Planning Committee, Grant/Award Number: 19PJ250; Postdoctoral Research Foundation in West China Hospital, Grant/Award Number: 2019HXBH101; West China Hospital; Research Foundation; Research and Development; National Natural Science Foundation of China, Grant/Award Numbers: 81670662, 81970626
Abstract
enBackground
To address the prognostic value of combining tubular basement membrane (TBM) and glomerular basement membrane (GBM) thickness in diabetic nephropathy (DN).
Methods
This retrospective study enrolled 110 patients with type 2 diabetes and biopsy-proven DN from 2011 to 2018. The pathological findings were confirmed according to the Renal Pathology Society classifications. GBM and TBM thicknesses were determined using the Haas' direct measurement/arithmetic mean method and orthogonal intercept method, respectively. Cox proportional hazard models were used to investigate the hazard ratios (HRs) for the influence of combined GBM and TBM thickness for predicting end-stage renal disease (ESRD).
Results
Patients were assigned to three groups according to the median GBM and TBM thickness: GBMloTBMlo (GBM < 681 nm and TBM < 1200 nm), GBMhiTBMlo/GBMloTBMhi (GBM ≥ 681 nm and TBM < 1200 nm, or GBM < 681 nm and TBM ≥ 1200 nm), and GBMhiTBMhi (GBM ≥ 681 nm and TBM ≥ 1200 nm). The GBMhiTBMlo/GBMloTBMhi and GBMhiTBMhi groups displayed poorer renal function, a more severe glomerular classification and interstitial inflammation, and poorer renal survival rates than the GBMloTBMlo group The GBMhiTBMlo/GBMloTBMhi and GBMhiTBMhi groups had adjusted HRs of 1.49 (95% confidence interval [CI], 1.21-9.75) and 3.07 (95% CI, 2.87-12.78), respectively, compared with the GBMloTBMlo group.
Conclusions
TBM thickness enhanced GBM thickness for renal prognosis in patients with type 2 diabetes.
摘要
zh背景
本研究旨在探讨联合评估肾小管基底膜(TBM)厚度和肾小球基底膜(GBM)厚度在糖尿病肾病预后判断中的预测能力。
方法
本回顾性研究纳入110例我中心2011年至2018年经肾活检证实为糖尿病肾病的2型糖尿病患者。采用肾脏病理学会分类评价糖尿病肾病患者肾脏病理改变,分别使用Haas直接测量法/算术平均法和正交截距法,在电镜下测定肾组织切片的GBM厚度和TBM厚度。采用Cox比例风险模型研究GBM厚度和TBM厚度联合评估预测终末期肾脏病的风险比。
结果
根据GBM厚度和TBM厚度的中位数值,将所有患者分为三组:GBMloTBMlo组(GBM <681 nm和TBM <1200 nm),GBMhiTBMlo/GBMloTBMhi组(GBM ≥681 nm和TBM <1200 nm,或GBM <681 nm和TBM ≥1200 nm),GBMhiTBMhi组(GBM ≥681 nm和TBM ≥1200 nm)。与GBMloTBMlo组患者比较,GBMhiTBMlo/GBMloTBMhi组和GBMhiTBMhi组患者肾活检时的基线肾功能更差,肾小球分级、间质炎症损伤更重,且肾脏生存率更低。在多因素Cox比例风险模型中,以GBMloTBMlo组作为参考组,GBMhiTBMlo/GBMloTBMhi组和GBMhiTBMhi组进展至终末期肾脏病的校正风险比分别为1.49(95%可信区间1.21–9.75)和3.07(95%可信区间2.87–12.78)。
结论
联合评估TBM厚度与GBM厚度可以显著提高对2型糖尿病患者肾脏预后的判断和预测。
1 INTRODUCTION
The global pandemic of diabetes mellitus is perhaps the largest epidemic in human history, with an estimated 463 million adults living with diabetes in 2019.1 It is the leading cause of end-stage renal disease (ESRD) in the Western world. Likewise, diabetic nephropathy (DN) has become the major cause of ESRD in China, because it develops in approximately 21.3% of patients with diabetes.2 Given the considerable healthcare burden of DN, there has been much interest in the search for more specific biomarkers. Although several new biomarkers for DN diagnosis, including β2-microglobulin, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin,3 β-trace protein,4 and α1-microglobulin,5 may play an important role in DN development and may be helpful for the clinician to evaluate the likelihood of DN in a given patient with type 2 diabetes and kidney impairment, the typical clinical symptoms and kidney biopsy remain the main diagnostic basis. More studies are needed to investigate the underlying mechanisms and molecular pathways for DN development to aid in its diagnosis and therapy.
DN is morphologically characterized by a thickening of the glomerular basement membrane (GBM) underlying the podocytes, expansion of the extracellular matrix (ECM) surrounding mesangial cells, and interstitial fibrosis and tubular atrophy (IFTA).6 Our previous study reported that a thicker GBM was significantly associated with a lower estimated glomerular filtration rate (eGFR) and urinary proteinuria in a DN cohort.7 Other epidemiological studies revealed that GBM thickness was an independent risk factor for a reduced eGFR and DN progression in patients with biopsy-proven DN.8, 9 However, when DN develops, the tubular basement membrane (TBM) underlying proximal tubular epithelial cells displays thickening. Hyperglycemia induced cellular hypertrophy in tubular epithelial cells, stimulated their expression of type IV collagen in the TBM,10, 11 and induced the synthesis of interstitial type III collagen.10 Although most investigations have focused on the increased accumulation of ECM in the GBM and its relationship to glomerular function, little is known regarding the pathogenesis and significance of the thickened TBM.
Therefore, in the present study, we investigated the effect of TBM thickness combined with GBM thickness for predicting the renal outcome based on the new pathologic classification in 105 patients with type 2 diabetes and DN, who were followed up for a minimum of 1 year after renal biopsy.
2 METHODS
2.1 Patient selection and study design
Patients with diabetes who underwent renal biopsy at the West China Hospital of Sichuan University from January 2011 to October 2018 were retrospectively analyzed in this study. The indications for renal biopsy have been detailed described previously.12 They were type 2 diabetes mellitus (T2DM) patients with renal damage who lacked absolute contraindications. Especially T2DM patients with obvious glomerular hematuria and/or short diabetic duration, or with sudden onset overt proteinuria met the requirement of renal biopsy.13 We used the American Diabetes Association criteria for T2DM diagnosis.14 DN, which was defined based on the 2015 standard of An et al,15 was diagnosed by at least two renal pathologists according to the Renal Pathology Society (RPS) classification.6 Adult patients with T2DM and biopsy-confirmed DN who were followed up at our hospital for over 1 year were considered to be eligible for this study. Patients who met the exclusion criteria were excluded from this study: coexisting nondiabetic renal diseases, including membranous nephropathy and immunoglobulin A nephropathy; systemic disease such as antineutrophil cytoplasmic antibody associated vasculitis, anti-GBM disease, and lupus nephritis; entered into ESRD before renal biopsy; no glomeruli observed in electron micrographs; RPS glomerular class IV or global sclerosis; or other types of DM (Figure 1). In sum, a total of 110 patients with T2DM and associated DN and complete TBM/GBM data were enrolled in this study. Ten nondiabetic age-matched patients with minimal change disease (MCD) were retrospectively selected as controls for comparison. All patients provided informed consent and the institutional review boards of Sichuan University approved this study. This study was also in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
2.2 Clinical and laboratory information
Clinical and pathologic data were abstracted from electronic medical records at the time of renal biopsy; data included age, sex, body mass index, systolic or diastolic blood pressure, presence of diabetic retinopathy, and use of renin-angiotensin aldosterone system (RAAS) blockade. We evaluated the eGFR using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation.12 We defined hematuria as more than five erythrocytes per high-power field in at least two of three consecutive urine tests without urinary infection and no urinary tract malignancy or stone.16 Treatment was defined as the use of the RAAS blockade, glucose-lowering agents, and statins for more than half of the follow-up period. Patient follow-up examinations were performed 2-4 times per year based on the patient's individual condition.
2.3 Pathological classification
Renal biopsy tissues for light microscopy, immunofluorescence, and electron microscopy were prepared by standard procedures at West China Hospital and examined by expert nephropathologists. Sections for light microscopy were stained with hematoxylin-eosin, periodic acid-Schiff, Masson's trichrome, and periodic acid-Schiff silver methenamine. Light microscopy sections were assessed by two nephropathologists (L.L., H.X.) without the knowledge of clinical outcomes. Renal biopsy evaluation was scored according to the RPS DN classification.6
2.4 Measurement of GBM and TBM thickness
GBM and TBM thickness was determined by unbiased morphometry on electron microscope images.7 When determining GBM thickness, globally sclerotic glomeruli or collapsed glomeruli were excluded. One to two glomeruli of every kidney specimen were randomly selected and GBM thickness was evaluated by the Haas' direct measurement/arithmetic mean method, which was described in detail in our previous study.7
The TBM of the proximal tubules was selected for study because of the relative ease of identification of this structure, thus providing consistency of comparison within and between groups.17 Proximal tubular basement membrane thickness was determined from the orthogonal intercept, which was described previously.17, 18 In summary, electron micrographs with print magnifications of 7000-15 000 were obtained to measure the thickness of the proximal segment of the proximal tubule. A calibration grid was photographed at the same time to determine the precise magnification. Subsequently, a grid with perpendicular line segments was superimposed over each micrograph. We used a harmonic ruler on a log reciprocal scale to determine the orthogonal thickness of the TBM when a grid line intersected the TBM and the proximal tubular epithelial cell interface. To examine the entire section for the presence of a proximal TBM, the microscope stage controls were moved systematically by an equal distance. For each patient, 60-100 tubular profiles were finally assessed.
2.5 Renal outcomes
ESRD was the end point. ESRD was defined as an eGFR rate < 15 mL/min/1.73 m2, kidney transplantation, or the need for chronic renal replacement therapy.15, 19 All the patients were followed until April 2020.
2.6 Statistical analysis
Clinical and pathologic data and outcomes were expressed as counts and percentages for categorical variables. Continuous variables are expressed as the mean and SD when normally distributed, or as the median and interquartile range (IQR) when not. In patients with differing TBM and GBM thickness, differences in continuous variables were analyzed using one-way analysis of variance, followed by the Bonferroni or Tukey methods for multiple comparisons, or the Kruskal-Wallis H test, as appropriate. Categorical variables were analyzed using the chi-square test or Fisher's exact test.12
Survival curves for TBM and/or GBM thicknesses were obtained using the Kaplan-Meier method. Log-rank tests was used to determine if there was a statistically significant difference for renal survival rate among comparison groups. Data for hemoglobin A1c (HbA1c) were lacking for four patients (these four patients were included in multivariable analyses). First, the differences in clinical parameters between patients with or without missing values were examined to check whether the values were missed randomly. Then, multiple imputation methods were applied for multivariable models. The proportional hazard assumption in Cox models was tested to determine whether the dataset satisfied the basic assumptions of Cox analyses. The Cox proportional hazards model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for ESRD. GBM or TBM thickness was first analyzed as a continuous variable with HRs stemmed from per SD increment of natural log-transformed and then analyzed as a categorical variable with the GBMloTBMlo group regarded as reference. In the multivariable Cox proportional hazard models, each HR was adjusted for age, sex, eGFR, proteinuria, HbA1c, and pathological parameters (including RPS glomerular classification, IFTA, interstitial inflammation, arteriosclerosis, and arteriolar hyalinosis) at the time of renal biopsy. Age and sex were chosen on the basis of biological plausibility. HbA1c was selected to represent previous glycemic control and it was suggested in the literature to be associated with a higher risk of renal outcome.20 The pathological covariates evaluated by light microscopy were considered as potential confounders because they were associated with ESRD in previous studies.16, 21 Parameters with P < 0.05 in the multivariable Cox proportional hazard models were considered significant prognosis predictors. Correlations between clinical parameters and GBM or TBM thickness were evaluated using linear regression analyses.
All statistical analyses were performed using Stata version 14.0 (StataCorp LLC, College Station, TX, USA). Statistical tests were considered significant at P < 0.05.
3 RESULTS
3.1 Baseline demographics and clinical characteristics
A summary of the demographics and baseline characteristics of the 110 patients with DN enrolled in the study is presented in Table 1. The mean age of the participants was 51 years (SD, 11 years), and the cohort comprised 30 women (27.3%) and 80 men (72.7%). The median duration of T2DM was 60 months (IQR, 24-120 months), the median baseline eGFR was 91.9 mL/min/1.73 m2, and the median 24-hour proteinuria was 1.80 g/d.
| Characteristics | Total (n = 110) | GBMloTBMlo (n = 38) | GBMhiTBMlo/GBMloTBMhi (n = 40) | GBMhiTBMhi (n = 32) | P value |
|---|---|---|---|---|---|
| Age, mean (SD), y | 51 (11) | 51 (11) | 51 (11) | 50 (10) | 0.88 |
| Sex, male, n (%) | 80 (72.7%) | 23 (60.5%) | 31 (77.5%) | 26 (81.3%) | 0.11 |
| Smoking, Never/Ex/Current, n | 58/10/42 | 23/4/11 | 19/1/20 | 16/5/11 | 0.15 |
| History of hypertension, n (%) | 88 (80.0%) | 31 (81.6%) | 30 (75.0%) | 27 (84.4%) | 0.60 |
| BMI, mean (SD), kg/m2 | 26.05 (3.07) | 25.52 (3.48) | 25.78 (3.12) | 25.78 (3.12) | 0.35 |
| SBP, mean (SD), mm Hg | 139 (22) | 137 (20) | 144 (26) | 144 (26) | 0.38 |
| DBP, mean (SD), mm Hg | 86 (14) | 84 (13) | 87 (16) | 87 (16) | 0.33 |
| MAP, mean (SD), mm Hg | 104 (15) | 101 (13) | 106 (18) | 106 (18) | 0.45 |
| Duration of diabetes, median (IQR), months | 60 (24–120) | 60 (24-96) | 72 (24-132) | 72 (18-138) | 0.28 |
| History of DR, n (%) | 40 (36.4%) | 10 (26.3%) | 14 (35.0%) | 16 (50.0%) | 0.16 |
| HbA1c, median (IQR), % | 7.3 (6.3-8.4) | 7.3 (6.3–8) | 7.4 (6.5-8.6) | 7.04 (6-8.2) | 0.55 |
| FPG, median (IQR), mg/dL | 130 (102-163) | 133 (103-162) | 131 (111-168) | 120 (89-161) | 0.49 |
| Hemoglobin, mean (SD), g/L | 134.6 (26.1) | 134.3 (23.2) | 121.4 (22.4) | 121.4 (22.4) | <0.001 |
| Serum albumin, mean (SD), g/L | 38.4 (6.8) | 38.3 (6.9) | 35.5 (6) | 35.5 (6) | <0.01 |
| CKD stage, 1/2/3/4 (n) | 57/26/25/2 | 27/8/3/0 | 19/9/11/1 | 11/9/11/1 | 0.04 |
| BUN, median (IQR), mg/dL | 17.9 (14.5-23.5) | 15.8 (13.4-20.4) | 18.9 (14.9-24.8) | 19.9 (16.7-26.7) | 0.02 |
| Serum creatinine, median (IQR), mg/dL | 87 (68-121) | 74 (60.3-94.3) | 88.6 (72.5-132.5) | 116.5 (77.6-128.5) | <0.01 |
| eGFR, median (IQR), mL/min/1.73 m2 | 91.9 (60.1-108.6) | 97.9 (84.4-116.2) | 83.7 (54.9-97.6) | 65.7 (53.6-103.4) | <0.01 |
| Proteinuria, median (IQR), g/24 hours | 1.80 (0.84-4.79) | 1.15 (0.53-2.8) | 2.41 (1-4.36) | 3.5 (1.35-6.84) | <0.01 |
| ACR (IQR), mg/g | 1013(472-1606) | 685 (366-1394) | 1056 (472-1392) | 1477 (803-3067) | <0.01 |
| Hematuria, n (%) | 34 (30.9%) | 10 (26.3%) | 10 (25.0%) | 14 (43.8%) | 0.19 |
| UA, mean (SD), mg/dL | 6.52 (1.32) | 6.4 (1.4) | 6.5 (1.3) | 6.5 (1.3) | 0.55 |
| Triglyceride, mean (SD), mg/dL | 148.4 (107.2-224.9) | 151.5 (113.4-223.2) | 167.4 (106.7-248.4) | 113.8 (81-192.6) | 0.24 |
| Cholesterol, mean (SD), mg/dL | 183.5 (147.3-216.6) | 172.1 (133.4-200.7) | 199.3 (164.5-237) | 176.1 (146.4-210.4) | 0.09 |
| HDL-C, mean (SD), mg/dL | 43.7 (36.0-54.1) | 43.1 (37.5-58.4) | 41.8 (36-56.1) | 46.8 (37.3-53.9) | 0.74 |
| LDL-C, mean (SD), mg/dL | 99.2 (72.3-125.7) | 98.2 (63.4-114.1) | 100.7 (72.3-139.6) | 98.2 (77.1-116.2) | 0.58 |
| RAAS inhibitor, n (%) | 98 (89.1%) | 35 (92.1%) | 34 (85.0%) | 29 (90.6%) | 0.64 |
| OHA therapy, n (%) | 68 (61.8%) | 25 (65.8%) | 27 (67.5%) | 16 (50.0%) | 0.27 |
| Insulin therapy, n (%) | 64 (58.2%) | 20 (52.6%) | 22 (55.0%) | 22 (68.8%) | 0.36 |
| Statins, n (%) | 64 (58.2%) | 23 (60.5%) | 25 (62.5%) | 16 (50.0%) | 0.55 |
| No. of hypertensive drugs, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–1) | 1 (0–2) | 0.23 |
- Note: Data are presented as the mean (SD) for continuous variables with a normal distribution, median (25th-75th percentile) for continuous variables without a normal distribution, or percentages for categorical variables. †CKD stage1: eGFR≥90 mL/min/1.73 m2; stage 2: eGFR 60-89 mL/min/1.73 m2; stage 3: eGFR 30-59 mL/min/1.73 m2; stage 4: eGFR 15-29 mL/min/1.73 m2.
- Abbreviations: ACR, albumin/creatinine ratio; BMI, body mass index; BUN, blood urea nitrogen; CKD, chronic kidney disease; DBP, diastolic blood pressure; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GBM, glomerular basement membrane; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein-cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein-cholesterol; MAP, mean arterial pressure; OHA, oral hypoglycemic drugs; RAAS, renin-angiotensin-aldosterone system; SBP, systolic blood pressure; TBM, glomerular basement membrane; UA, uric acid.
The median GBM and TBM thickness in the patients with DN of 681 nm (Figure 2) and 1200 nm, respectively, were significantly increased compared with that in the nondiabetic controls with MCD of 380 and 574 nm, respectively. According to the median membrane thickness, 54 patients had a GBM thickness < 681 nm, and 56 patients had a GBM thickness ≥ 681 nm. In total, 53 patients had a TBM thickness < 1200 nm, and 57 patients had a TBM thickness ≥ 1200 nm. Consequently, the patients were assigned to three groups: GBMloTBMlo group (GBM <681 nm and TBM <1200 nm, n = 38), GBMhiTBMlo/GBMloTBMhi group (GBM ≥681 nm and TBM <1200 nm, or GBM <681 nm and TBM ≥1200 nm, n = 40), and GBMhiTBMhi group (GBM ≥681 nm and TBM ≥1200 nm, n = 32). Compared with the patients in the GBMloTBMlo group, those in the GBMhiTBMlo/GBMloTBMhi and GBMhiTBMhi groups had more severe proteinuria, albumin/creatinine ratio (ACR), and greater serum creatinine but lower hemoglobin, serum albumin, and eGFR (P < 0.05). Patients in the GBMhiTBMhi group had the lowest hemoglobin and serum albumin concentrations but the greatest proteinuria among the three groups. There were no significant differences in blood pressure or the prevalence of diabetic retinopathy among the three groups. The usage of hypoglycemic agents, RAAS inhibitors, or statins did not significantly differ among the three groups.
3.2 Baseline pathological characteristics
Regarding pathological parameters, the severity of the RPS glomerular class, IFTA, and interstitial inflammation were increased in patients in the GBMhiTBMhi group compared with those in the GBMloTBMlo and GBMhiTBMlo/GBMloTBMhi groups (P < 0.01 and 0.04, respectively). Conversely, there were no significant differences in the degree of arteriolar hyalinosis or arteriosclerosis among the three groups (Table 2).
| Characteristics | Total (n = 110) | GBMloTBMlo (n = 38) | GBMhiTBMlo /GBMloTBMhi (n = 40) | GBMhiTBMhi (n = 32) | P value |
|---|---|---|---|---|---|
| RPS classificationa, n (%) | <0.01 | ||||
| Class I | 14 (12.7%) | 10 (26.3%) | 4 (10.0%) | 0 (0%) | |
| Class IIa | 53 (48.2%) | 19 (50.0%) | 21 (52.5%) | 13 (40.6%) | |
| Class IIb | 15 (13.6%) | 5 (13.2%) | 4 (10.0%) | 6 (18.8%) | |
| Class III | 28 (25.5%) | 4 (10.5%) | 11 (27.5%) | 13 (40.6%) | |
| IFTAa, n (%) | 0.52 | ||||
| Score 0 | 6 (5.5%) | 4 (10.5%) | 1 (2.5%) | 1 (3.1%) | |
| Score 1 | 74 (67.3%) | 26 (68.4%) | 28 (70.0%) | 20 (62.5%) | |
| Score 2 | 28 (25.4%) | 7 (18.4%) | 10 (25.0%) | 11 (34.4%) | |
| Score 3 | 2 (1.8%) | 1 (2.6%) | 1 (2.5%) | 0 (0%) | |
| Interstitial inflammationa, n (%) | 0.04 | ||||
| Score 0 | 7 (6.4%) | 6 (15.8%) | 1 (2.5%) | 0 (0%) | |
| Score 1 | 85 (77.3%) | 28 (73.7%) | 32 (80.0%) | 25 (78.1%) | |
| Score 2 | 18 (16.3%) | 4 (10.5%) | 7 (17.5%) | 7 (21.9%) | |
| Arteriolar hyalinosisa, n (%) | 0.07 | ||||
| Score 0 | 18 (16.4%) | 11 (28.9%) | 3 (7.5%) | 4 (12.5%) | |
| Score 1 | 28 (25.5%) | 10 (26.3%) | 12 (30.0%) | 6 (18.8%) | |
| Score 2 | 64 (58.1%) | 17 (44.7%) | 25 (62.5%) | 22 (68.8%) | |
| Arteriosclerosisa, n (%) | 0.06 | ||||
| Score 0 | 25 (22.7%) | 14 (36.8%) | 8 (20.0%) | 3 (9.4%) | |
| Score 1 | 50 (45.5%) | 12 (31.6%) | 19 (47.5%) | 19 (59.4%) | |
| Score 2 | 35 (31.8%) | 12 (31.6%) | 13 (32.5%) | 10 (31.3%) | |
| TBM, median (IQR), nm | 1200 (938-1588) | 974 (725-1149) | 1220 (990-1527) | 1708 (1493-1890) | <0.01 |
| GBM, median (IQR), nm | 681 (523-863) | 502 (454-576) | 699 (615-821) | 908 (785-1126) | <0.01 |
- a Defined by the Renal Pathology Society diabetic nephropathy classification.
- Note: Data are presented as medians (25th–75th percentiles) for continuous variables without a normal distribution and as percentages for categorical variables.
- Abbreviations: GBM, glomerular basement membrane; IFTA, interstitial fibrosis and tubular atrophy; IQR, interquartile range; RPS, Renal Pathology Society; TBM, tubular basement membrane.
3.3 Correlations between GBM/TBM thickness and clinicopathological findings
Liner regression analysis revealed that TBM thickness was negatively associated with the eGFR (R2 = 0.15, standard β = −0.38, P < 0.001), serum albumin concentration (R2 = 0.10, standard β = −0.30, P < 0.01), and hemoglobin concentration (R2 = 0.13, standard β = −0.34, P < 0.001) but was positively associated with proteinuria (R2 = 0.10, standard β = 0.30, P < 0.01, Figure 3A–D), and ACR (R2 = 0.12, standard β = 0.34, P < 0.01). Similarly, GBM thickness was negatively associated with the eGFR (R2 = 0.07, standard β = −0.26, P < 0.01) but positively associated with proteinuria (R2 = 0.07, standard β = 0.24, P = 0.02). However, the duration of diabetes was not significantly associated with TBM or GBM thickness.
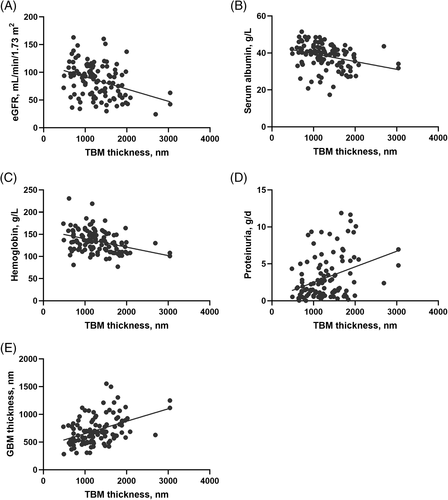
TBM thickness was significantly positively correlated with GBM thickness (R2 = 0.20, standard β = 0.45, P < 0.001, Figure 3E). TBM thickness also displayed a positive correlation with glomerular classification (R2 = 0.13, standard β = 0.36, P < 0.01) and the IFTA score (R2 = 0.06, standard β = 0.23, P = 0.01). Furthermore, GBM thickness was positively associated with the RPS glomerular classification, IFTA, and interstitial inflammation. However, arteriosclerosis and arteriolar hyalinosis were not significantly associated with TBM or GBM thickness.
3.4 Effect of combining TBM thickness with GBM thickness assessment for the prognosis of renal outcomes
During a median follow-up of 27 months (range 6-94 months), 23 (21.9%) patients progressed to ESRD. Five patients were lost to follow-up and only had baseline clinical and pathologic data. When stratified by GBM thickness, the Kaplan-Meier survival analysis showed that patients with greater GBM thickness (≥681 nm) had poorer renal survival rates than those with lesser GBM thickness (<681 nm; log-rank test P = 0.01, Figure 4A). Additionally, the Kaplan-Meier survival analysis showed that patients with greater TBM thickness (≥1200 nm) displayed poorer renal survival rates than those with lesser TGM thickness (<1200 nm; log-rank test P = 0.03, Figure 4B).
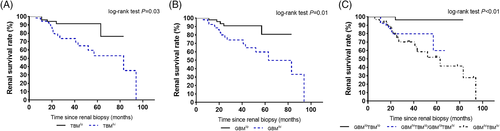
The percentages of patients in the GBMloTBMlo, GBMhiTBMlo/GBMloTBMhi, and GBMhiTBMhi groups who progressed to ESRD were 4.3%, 34.8%, and 60.9%, respectively (P = 0.01). The survival curves of the three groups, indicating the time to ESRD, are presented in Figure 4C. The Kaplan-Meier survival analysis showed that patients in the GBMhiTBMhi group had poorer renal survival rates than those in the GBMloTBMlo and GBMhiTBMlo/GBMloTBMhi groups (log-rank test P < 0.01).
Univariate and multivariable adjusted HRs for ESRD are presented in Table 3 according to the baseline GBM and TBM thickness, expressed as a continuous variable. Univariate Cox proportional hazard analysis showed that greater GBM thickness was associated with progression to ESRD (HR, per 1 SD of natural log-transformed GBM thickness, 1.08; 95% CI, 1.02-1.84), whereas greater TBM thickness was significantly associated with progression to ESRD (HR, per 1 SD of natural log-transformed TBM thickness, 1.89; 95% CI, 1.18-3.04). Additionally, patients in the GBMhiTBMlo/GBMloTBMhi and GBMhiTBMhi groups had higher HRs of 1.26 (95% CI, 1.03-8.55) and 1.98 (95% CI, 1.52-11.65) compared with the GBMloTBMlo group as the reference, respectively. Following adjustment for age, sex, baseline eGFR, proteinuria, HbA1c, and pathological parameters, including RPS glomerular classification, IFTA, interstitial inflammation, arteriosclerosis, and arteriolar hyalinosis, in multivariable Cox models, greater GBM (HR, per 1 SD of natural log-transformed GBM thickness, 1.21; 95% CI, 1.17-2.38) and TBM thickness (HR, per 1 SD of natural log-transformed TBM thickness, 1.90, 95% CI, 1.18-2.87) remained significantly associated with ESRD. When GBM and TBM thickness were combined, patients in the GBMhiTBMlo/GBMloTBMhi and GBMhiTBMhi groups had adjusted HRs of 1.49 (95% CI, 1.21-9.75) and 3.07 (95% CI, 2.87-12.78) compared with the GBMloTBMlo group, respectively. Their HRs for progressing to ESRD increased when combining TBM thickness with GBM thickness. Moreover, a traditional clinical parameter like eGFR independently predicted progression to ESRD in the multivariable Cox hazard models (HR, 0.60; 95% CI, 0.45-0.81).
| Characteristics | Univariate Model | P value | Multivariable Modela | P value |
|---|---|---|---|---|
| HR (95% CI) | HR (95%CI) | |||
| Clinical characteristics | ||||
| Age (per 10 years) | 1.32 (0.87-1.98) | 0.19 | 1.61 (0.86-2.99) | 0.14 |
| Sex, (female) | 0.66 (0.22-1.97) | 0.46 | 0.95 (0.18-5.11) | 0.95 |
| eGFR (per 10 mL/min/1.73 m2) | 0.69 (0.57-0.83) | <0.001 | 0.60 (0.45–0.81) | <0.001 |
| 24-hour proteinuria (per 1 g/d) | 1.15 (1.07-1.24) | <0.001 | 1.06 (0.95-1.18) | 0.30 |
| HbA1c (per 1 mmol/mol) | 1.19 (0.97-1.45) | 0.11 | 1.81 (0.87-2.57) | 0.08 |
| Pathology characteristics | ||||
| lnGBM (per 1 SD) | 1.08 (1.02–1.84) | 0.04 | 1.21 (1.17-2.38) | 0.03 |
| lnTBM (per 1 SD) | 1.89 (1.18–3.04) | <0.01 | 1.90 (1.18–2.87) | 0.02 |
| GBM/TBM group | ||||
| GBMloTBMlo group | 1 (reference) | 1 (reference) | ||
| GBMhiTBMlo/GBMloTBMhi group | 1.26 (1.03–8.55) | 0.03 | 1.49 (1.21-9.75) | 0.02 |
| GBMhiTBMhi group | 1.98 (1.52–11.65) | 0.01 | 3.07 (2.87–12.78) | <0.01 |
| RPS classification | ||||
| Class I + IIa | 1 (reference) | 1 (reference) | ||
| Class IIb | 0.57 (0.13-2.59) | 0.47 | 0.17 (0.02-1.45) | 0.11 |
| Class III | 1.69 (1.00-4.28) | 0.05 | 1.60 (1.16-2.29) | 0.04 |
| IFTA | ||||
| Score 0 + 1 | 1 (reference) | 1 (reference) | ||
| Score 2 + 3 | 1.44 (0.58-3.56) | 0.43 | 1.37 (0.10-1.28) | 0.12 |
| Interstitial inflammation | ||||
| Score 0 + 1 | 1 (reference) | 1 (reference) | ||
| Score 2 | 1.96 (0.65-5.88) | 0.23 | 1.52 (0.38-6.02) | 0.55 |
| Arteriolar hyalinosis | ||||
| Score 0 | 1 (reference) | 1 (reference) | ||
| Score 1 | 1.05 (0.23-4.71) | 0.95 | 0.23 (0.01-3.49) | 0.29 |
| Score 2 | 1.59 (0.46-5.53) | 0.47 | 0.18 (0.02-2.03) | 0.16 |
| Arteriosclerosis | ||||
| Score 0 | 1 (reference) | 1 (reference) | ||
| Score 1 | 1.89 (0.52-6.9) | 0.33 | 1.33 (0.11-16.45) | 0.83 |
| Score 2 | 3.41 (0.91-12.84) | 0.07 | 3.39 (0.24-47.73) | 0.37 |
- a Multivariable model was adjusted for age, sex, eGFR, proteinuria, HbA1c, and all pathological parameters at the time of biopsy.
- Note: For Cox analyses, classes I and IIa were combined as the reference group for the RPS glomerular class. Scores of 0 and 1 were combined as 1, and scores of 2 and 3 were combined as 2 for IFTA. Scores of 0 and 1 were combined for interstitial inflammation.
- Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; GBM, glomerular basement membrane; HbA1c, hemoglobin A1c; HR, hazard ratio; IFTA, interstitial fibrosis and tubular atrophy; IQR, interquartile range; RPS, Renal Pathology Society; TBM, tubular basement membrane.
4 DISCUSSION
GBM thickening has been well studied and is considered a characteristic early change in DN. However, changes in TBM structure in T2DM and its added value to GBM thickness in predicting renal outcome has not been previously investigated. The present study demonstrated that GBM and TBM thickness increased significantly in patients with biopsy-proven DN than the controls. TBM thickness was strongly correlated with GBM thickness, baseline eGFR, and proteinuria. The most interesting and potentially impactful conclusion is that the degree of TBM thickness provided additional value to GBM thickness for predicting ESRD progression in patients with type 2 diabetes and biopsy-proven DN.
Studies have shown that injured podocytes are likely to play a fundamental role in disturbing the balance between the GBM's synthetic and degradative pathways.7, 22 GBM thickening increases with the duration of diabetes.23 Previous studies reported that GBM thickness was an independent predictor for progression to proteinuria and/or ESRD in type 1 and type 2 diabetes.9, 24 Consistent with these studies, our results also demonstrated that greater GBM thickness was an independent risk factor for predicting the progression to ESRD in biopsy-proven DN.
Both the TBM and GBM undergo thickening in the early and advanced phases of diabetes, indicated that the glomerulotubular imbalance and tubuloglomerular feedback were involved in the progression of DN.25 Filtered proteins or high glucose content resulting from an impairment of the glomerular filtration apparatus driving the tubular injury in disease progression.26, 27 Increased proximal sodium reabsorption because of overactivity of sodium glucose cotransporter (SGLT)-2 and SGLT1 under hyperglycemia leads to decreased sodium delivery to and transport in the cells of the macula densa, with consequent reduction in ATP breakdown and adenosine production. Adenosine is a strong vasoconstrictor, and its reduction caused vasodilation of the afferent arteriole and thus glomerular hyperfiltration.28, 29 SGLT2 inhibition should increase sodium delivery to the macula densa activates the tubuloglomerulular feedback that leads to afferent arteriole vasoconstriction and a reduction in intraglomerular pressure.29 Besides, microRNAs in extracellular vesicles released by injured podocytes promote apoptosis of renal tubular epithelial cells, which may contribute to the development of tubular injury in glomerular disease.30 Podocytes share many elements of the innate and adaptive immune system as well. Several recent lines of evidence implicate that renal cells such as podocytes ectopically express costimulatory molecules during hyperglycemia, thus triggering immune response and maintaining inflammation.27, 31 The crosstalk between podocytes and immune response might play important role in both glomerular and tubular injury. The good correlations between the clinical renal functional parameters and GBM/TBM thickness in the present study further confirmed the structural-functional relationship in patients with T2DM.23 However, the relationship between duration of diabetes and GBM thickness was controversial.32 Younger patients with type 1 diabetes showed a linear relationship between GBM thickness and diabetes duration,32 whereas Zhang et al33 showed no strong relationship between them in patients with type 2 diabetes. Several studies showed that the duration of diabetes differed similarly between progressors and nonprogressors in patients with DN.9, 34, 35 The renal histopathological changes were associated with blood glucose control rather than the duration of diabetes.36 Thus, the absent relation between renal structural alterations and diabetic duration may be explained by the highly heterogeneous of type 2 diabetes and the onset of type 2 diabetes was insidious. A large number of people have undiagnosed diabetes or impaired glucose tolerance for a long time also lead to the inaccurate diabetes duration.37
The median GBM and TBM thickness in the nondiabetic controls with MCD was 380 nm and 574 nm, respectively. This result was consistent with previous studies.18, 38-40 Phillips et al41 indicated that TBM thickening represented a better indicator of DN than GBM thickening. However, whether TBM thickening can predict progression to ESRD remains unclear. In the present study, greater TBM thickness was not only independently associated with accelerated disease progression but also displayed an added value to GBM thickness for renal prognosis, which suggests that during DN progression, both GBM and TBM thickness might increase simultaneously. The thickened GBM in DN comprised primarily collagen types IV and VI and laminin.22 Similar to the GBM, the thickened TBM was characterized by a broad accumulation of basement membrane ECM material,18 which comprised mainly collagen types IV and V, laminin, and fibronectin.10, 42 In vitro, hyperglycemia stimulated proximal tubular epithelial cells to secrete approximately 2-fold more collagen type IV and decreased the degradation of ECM proteins, both of which played essential roles during TBM thickening.10, 43-45 The successful inhibition of sodium-glucose cotransporter 2 of the tubule in DN therapy reemphasized the important role of proximal tubular epithelial cells in the reversal of DN.46
Of note are the underlying changes that occur during the natural course of DN. An increasing prevalence of normoalbuminuric diabetic kidney disease (NADKD), characterized by renal insufficiency with normoalbuminuria, re-emphasized the important role of nonglomerular lesions. Ekinci et al47 reported that in patients with NADKD, interstitial changes rather than typical glomerular lesions played a predominant role. This result suggested a hypothesis that TBM thickening might be a possible contributory factor to the reduced eGFR of these patients. Nevertheless, whether TBM thickening was related to renal outcomes in NADKD needs to be elucidated in the future. Furthermore, recent studies suggested that tubulopathy may also contribute to glomerulopathy. This notion was supported by site-selective injury to the proximal tubule, which induced extensive glomerular injury, including glomerulosclerosis and elevated serum creatinine.48 The triggering of glomerular pathology by the proximal tubule was also confirmed by the nicotinamide mononucleotide released by proximal tubular epithelial cells diffusing back to the glomerulus and inducing podocyte foot process effacement.49 The effect of sodium-glucose cotransporter 2 blockade on the renal outcome further provides a potential therapy strategy aimed at tubulopathy in DN. The traditional clinical parameter the baseline eGFR was among independent clinical factors for ESRD in the present study.
The present study had several limitations. First, it was a retrospective cohort study performed in patients with type 2 diabetes and associated DN, and, therefore, its findings may not be extended to patients with T1DM and other types of diabetes such as maturity-onset diabetes of the young. Further studies in other types of DM is useful for our understanding of diabetic kidney injury. Second, a limited sample sized of diabetic patients that investigate the association between TBM and renal outcome in T2DM was another limitation in this study. The results require validation from multicenter studies with prospective cohorts in the future. Lastly, bcause of the retrospective design, the baseline 24-hour urinary albumin excretion was missing in the study. However, as ACR from a spot urine sample correlates well with 24-hour albuminuria,50 baseline ACR was obtained and analyzed in the current study. Proteinuria is a good biomarker for predicting clinical end points in diabetic patients too.50 Decrease in proteinuria has been recommended as a separate therapeutic target in patients with DN.51 Thus, proteinuria was measured and adjusted in the multivariable Cox proportional hazard models in this study.
In major contrast to the extensive studies on the GBM, few detailed ultrastructural studies have addressed TBM in diabetes. To the best of our knowledge, our study is the first to demonstrate that TBM thickness is not only an independent risk factor for ESRD progression in patients with type 2 diabetes, but when combined with GBM thickness assessment, it improved ESRD prognosis assessment. Further studies are needed to assess the TBM thickness changes in the disease progression, and to determine whether TBM thickness alters the overall outcome in T2DM.
ACKNOWLEDGEMENTS
We thank Robert Blakytny, DPhil, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. This study was supported by the National Natural Science Foundation of China (Grant numbers 81970626 and 81670662); Key Research and Development Project of Sichuan Science and Technology Department (Grant number 19ZDYF1273); Applied Basic Research Project of Sichuan Science and Technology Department (Grant number 2020YJ0063); Postdoctoral Research Foundation in West China Hospital (Grant number 2019HXBH101); Popularization Project of the Science and Technology Project of the Sichuan Health Planning Committee (Grant number 19PJ250). The funding source played no role in study design, data analysis, and manuscript writing or submission.
DISCLOSURE
The authors declare no conflict of interest.




