Effect of basal insulin supplement therapy on diabetic retinopathy in short-duration type 2 diabetes: A one-year randomized parallel-group trial†在短病程2型糖尿病患者的治疗方案中增加基础胰岛素对糖尿病视网膜病变的影响:一项1年随机平行对照临床研究
Funding information: National Key R&D Program of China, Grant/Award Number: 2017YFA0105803; General Program of National Natural Science Foundation of China, Grant/Award Number: 81770826; Clinical research projects of Sun Yat–sen University, Grant/Award Number: 2015015; science and technology plan projects of Guangdong province, Grant/Award Number: 2016A050502010; key special projects of medical and health collaborative innovation of Guangzhou city, Grant/Award Number: 201604020016; special scientific research project of Guangzhou city, Grant/Award Number: 2060404
Abstract
enBackground
In this study, we compared the effect on diabetic retinopathy (DR) between oral antidiabetic drugs (OADs) alone and in combination with basal insulin-supported OADs therapy (BOT). [Correction added on 11 November 2019, after first online publication: In Abstract under Background section, “DR” has been corrected into “diabetic retinopathy (DR)”.]
Methods
Between January 2015 and January 2018, this study enrolled 290 patients (age 18-65 years) with diabetes duration between 0 and 5 years. Patients were randomly assigned to receive OADs or BOT after 14 days intensive insulin treatment. Examinations were performed at the beginning and end of the study.
Results
Fewer patients developed DR in the BOT than OADs group (8 [6.06%] vs 12 [8.3%], respectively), and all cases of DR were non-proliferative. Blood glucose concentrations were higher in the BOT than OADs group at the 3rd month, but lower in the former at the 6th and 12th month. The rate of reaching target HbA1c ≤7% was lower in the BOT than OADs group at the 3rd month (63.6% vs 72.2%, respectively), similar between the two groups at the 6th month (60.6% vs 66.6%, respectively) and higher in the BOT group at the 12th month (75.0% vs 61.1%, respectively). The SD of fasting blood glucose (FBG), coefficient of variation of FBG, SD of blood glucose (SDBG), and mean amplitude of glycemic excursions were lower in the BOT than OADs group. Changes in the levels of three cytokines (interleukin [IL]-1β, IL-6, and IL-17α) were significantly less in the BOT than OADs group.
Conclusions
Twelve months of BOT decreased the incidence of DR in short-duration type 2 diabetes by reducing glycemia more effectively, stably, and completely than OADs alone.
摘要
zh背景
在本研究中, 我们比较单纯口服抗糖尿病药物(oral antidiabetic drugs, OADs)和基础胰岛素联合口服抗糖尿病药物(basal insulin-supported OADs therapy, BOT)两种治疗方案对糖尿病视网膜病变(diabetic retinopathy, DR)的影响。
方法
本研究在2015年1月到2018年1月共入组290例糖尿病患者, 他们的年龄介于18到65岁之间, 糖尿病病程在0到5年之间。入组的患者先接受14天的胰岛素强化治疗, 然后被随机分入OAD组或者BOT组。在研究开始时和研究结束时进行相应的检查。
结果
与OADs组相比, BOT组更少患者出现DR [BOT组8例 (6.60%) vs. OADs组12例 (8.3%)], 并且所有出现DR的患者, 其DR都属于非增殖性病变。BOT组第3个月的血糖水平较OADs组高, 但其第6个月和第12个月的血糖水平则较OADs组低。HbA1c(≤7%)的达标率, 第3个月BOT组低于OADs组(分别为63.6% 和72.2%), 第6个月两组近似(分别为60.6% 和66.6%), 第12个月BOT组高于OADs组(分别为75.0% 和61.1%)。BOT组的空腹血糖标准差、空腹血糖变异系数、血糖标准差和血糖波动平均幅度均低于OADs组。BOT组中三个细胞因子(IL-1β、IL-6和IL-17α)的变化水平显著低于OADs组。
结论
对于病程较短的2型糖尿病患者, 12个月的BOT方案能通过更有效、平稳、全面地降低血糖而降低DR的发生率。
1 INTRODUCTION
Diabetic retinopathy (DR) is a common and serious microvascular complication of type 2 diabetes (T2D) and a leading cause of blindness among the working-age population in developed countries.1 As an initial treatment, intensive glycemic treatment has been shown to delay the onset and progression of DR. As subsequent treatments, both oral antidiabetic drugs (OADs) alone and basal insulin-supported OADs therapy (BOT) are frequently administered to T2D patients. However, it remains unclear which subsequent treatment has the best effect on DR. Furthermore, it is debatable whether insulin treatment is beneficial or harmful with regard to DR. Traditionally, proliferative DR is an indication for insulin therapy, indicating that insulin is superior to OADs in treating diabetic patients with DR. However, some recent reports have claimed that insulin therapy is harmful to DR.2-6 Thus, the primary aim of this randomized controlled trial was to compare the effects of OADs and BOT as subsequent therapies on DR.
Favorable overall glycemic control is necessary to prevent the onset and progression of DR.7 Currently, glycemia comprises not only general glycemia, which is represented by HbA1c, but also glycemic stability, which is also called glycemic variability (GV). Because inflammation contributes to mechanisms underlying the occurrence and deterioration of DR, the secondary objective of this study was to compare the effects of these OADs alone and BOT on glycemia and inflammation.
2 METHODS
2.1 Study design and patients
This study was a single-center prospective randomized open-label trial conducted at the Third Affiliated Hospital of Sun Yat-sen University between January 2015 and January 2018. The study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University and the study is registered with ClinicalTrials.gov (ID NCT02587741). Written informed consent was obtained from all participants.
The inclusion criteria were as follows: T2D diagnosis according to the 1999 World Health Organization criteria,8 age 18 to 65 years, body mass index (BMI) 20 to 35 kg/m2, duration of diabetes 0 to 5 years, and HbA1c >7.0%. Patients with a history of macrovascular disease (including ischemic heart disease, heart failure, and cerebrovascular disease), acute diabetic complications in the previous 6 months, diabetic microvascular complications, hepatic or renal impairment, malignant tumors, autoimmune diseases, and acute or chronic infections were excluded from the study, as were pregnant and lactating women.
2.2 Study protocol
The study process is shown in Figure 1. Data for all eligible patients were collected via paper-based case report forms and included information on baseline characteristics (sex, age, duration of diabetes, previous medical history, medications etc.) and anthropometric data (body weight, height, BMI, heart rate, and blood pressure). Urinary albumin was measured in three consecutive 24-hour urine collections using a turbidimetric immunoassay and is expressed as the urine albumin excretion rate (UAER). For biochemical analysis, blood samples were drawn after an 8-hour overnight fast. Laboratory assessments consisted of fasting blood glucose (FBG) concentrations, 2-hour postprandial blood glucose (2hPBG) concentrations, liver function, renal function, lipid profiles, and electrolytes, which were measured using a HITACHI (Tokyo, Japan) 7180 Automatic Analyzer. C-peptide and insulin were determined by radioimmunoassay (Beijing Bio-Ekon Biotechnology, Beijing, China), whereas HbA1c was measured using the D-10 Hemoglobin Testing System (Bio-Rad Laboratories, Hercules, California). Serum concentrations of interleukin (IL)-1β, IL-6, and IL-17α were determined using the Human Cytokine/Chemokine Magnetic Bead Panel Kit (Asbio Technology, Guangzhou, China). Eye examinations comprised visual acuity testing, tonometry, and retinal exploration. Evaluation of the retina was made by SLM-JER slit-lamp biomicroscopy (Chongqing Kanghua, Chongqing, China) of the posterior pole using contact lenses after pupil dilation with tropicamide 0.5% and phenylephrine HCl 10% eye drops. Retinal angiography was tested by APS-GER digital fundus photography (Chongqing Kanghua, Chongqing, China) after intravenous injection of 10% fluorescein. Diabetic retinopathy was graded according to the International Classification of Diabetic Retinopathy.9 Diabetic retinopathy was diagnosed in the Department of Ophthalmology of the 3rd affiliated hospital of Sun Yat-sen university by a single specialist, who was blinded to the treatments.
All eligible patients entered a 14-day intensive insulin treatment lead-in period. During the lead-in period, continuous subcutaneous insulin infusion was performed to achieve the glycemic target, which was defined as both FBG 4.4 to 5.6 mM and 2hPBG <7.8 mM. Then, participants were randomized in a ratio of 1: 1 to either BOT or OADs as the subsequent treatment. Sealed opaque envelopes, which were arranged in a computer-generated random order prepared by a statistician prior to the study, were opened to determine the patients' treatment assignment.
2.3 Treatments
2.3.1 Oral antidiabetic drugs
The OADs that patients received were determined by the physicians as part of routine clinical care. The algorithm for OAD selection was one OAD from the minimum dose to the maximum dose, sequentially supplemented with additional OADs until the glucose target was achieved. The order of selection of OADs was: metformin (Glucophage; Bristol-Myers Squibb, Shanghai, China), gliclazide (Diamicron; Servier, Tianjin, China), α-glucosidase inhibitor (acarbose; Bayer, Shanghai, China), and a dipeptidyl peptidase (DPP)-4 inhibitor (Januvia; Merck Sharp & Dohme, Shanghai, China).
2.3.2 Basal insulin-supported OADs therapy
Insulin glargine (Lantus; Sanofi-Aventis, Shanghai, China) was administered initially at a dose of 0.2 IU/kg. The dose was then adjusted according to Table 1. The OADs were supplemented if the glycemic target was not achieved or maintained. The OADs were prescribed in accordance with the algorithm used for the OAD group.
| Fasting blood glucose (mM) | Insulin dose adjustment |
|---|---|
| <4.4 | Decrease dose by 2 IU |
| 4.4-5.6 | No adjustment required |
| 5.6-8.0 | Increase dose by 2 IU |
| 8.0-10.0 | Increase dose by 4 IU |
| >10.0 | Increase dose by 6 IU |
2.4 Outpatient follow-up and outcome assessment
Fasting blood glucose, 2hPBG, and HbA1c values were collected every 3 months. All subjects were monitored using a continuous glucose monitoring system (MiniMed Paradigm 722; Medtronic, Northridge, California, USA) for three consecutive days at the time of randomization and at the12-month follow-up. At the same time, eye examinations were performed and UAER was measured. Blood samples were also collected to measure markers of inflammation. Anthropometric information, medications, self-monitored blood glucose, hypoglycemia events, and adverse events were recorded at every follow-up visit.
2.5 Glycemic control
The glycemic control in this study comprised both HbA1c to target and GV. The target HbA1c was ≤7%, and achieving this target was defined as HbA1c remission. In the present study, long-term GV was evaluated by the standard deviation (SD) and coefficient of variation (CV) of FBG and HbA1c. The short-term intraday GV was evaluated by the SD of blood glucose (SDBG) and the mean amplitude of glycemic excursions (MAGE). The short-term interday GV was evaluated by the mean of daily differences (MODD), which was calculated from the mean absolute value of differences between glucose values on two consecutive days at the same time point.
2.6 Statistical analysis
All statistical analyses were performed using PASW statistics 19.0 (IBM Corp., Armonk, New York, USA). Continuous variables are presented as the mean ± SD, whereas categorical variables are expressed as percentages. Differences in continuous variables between groups were evaluated using Student's t tests, whereas differences in categorical variables were evaluated by Pearson's χ2 tests. Two-sided P < 0.05 was considered significant.
3 RESULTS
3.1 Characteristics of study subjects
In all, 290 subjects were recruited in this study after the initial screening. Of these, 148 were randomly assigned to the OAD group and the remaining 142 were assigned to the BOT group. Four subjects in the OAD group and 10 in the BOT group were lost to follow-up, all of which were in euglycemic remission during their last documented study visit (Figure 2). At baseline, no characteristics differed significantly between the OAD and BOT groups (P > 0.05). At the 12-month follow-up, UAER was significantly higher in the OAD than BOT group (P < 0.05); there were no significant differences in any other parameters between the two groups (P > 0.05; Table 2).

| Baseline | 12-month follow-up | |||||
|---|---|---|---|---|---|---|
| OAD group (n = 144) | BOT group (n = 132) | P-value | OAD group (n = 144) | BOT group (n = 132) | P-value | |
| Age (y) | 50.1 ± 6.7 | 51.7 ± 7.1 | NS | - | - | - |
| No. males/females | 83/61 | 78/54 | NS | - | - | - |
| Diabetes duration (y) | 2.3 ± 1.5 | 2.4 ± 2.0 | NS | - | - | - |
| SBP (mm Hg) | 134.1 ± 15.4 | 137.0 ± 20.2 | NS | 130.2 ± 18.6 | 122.4 ± 32.3 | NS |
| DBP (mm Hg) | 85.2 ± 11.9 | 84.4 ± 11.7 | NS | 79.6 ± 8.6 | 80.5 ± 9.4 | NS |
| Weight (kg) | 66.08 ± 10.86 | 67.59 ± 10.59 | NS | 65.66 ± 11.00 | 67.30 ± 9.95 | NS |
| BMI (kg/m2) | 25.32 ± 2.97 | 25.31 ± 2.91 | NS | 24.47 ± 4.26 | 24.53 ± 2.66 | NS |
| WC (cm) | 85.97 ± 7.72 | 88.00 ± 9.38 | NS | 84.58 ± 9.82 | 87.81 ± 8.86 | NS |
| FCP (pM) | 1.68 ± 0.63 | 1.94 ± 1.88 | NS | 1.86 ± 0.62 | 1.56 ± 1.22 | NS |
| 2 h-PCP (pM) | 5.02 ± 1.97 | 4.19 ± 2.68 | NS | 5.84 ± 2.29 | 4.98 ± 3.71 | NS |
| Cr (μM) | 57.90 ± 20.33 | 58.12 ± 20.09 | NS | 64.16 ± 17.58 | 65.83 ± 20.47 | NS |
| UA (μM) | 303.58 ± 71.26 | 313.95 ± 84.90 | NS | 357.31 ± 69.42 | 335.62 ± 74.11 | NS |
| TC (mM) | 6.09 ± 1.58 | 5.59 ± 1.55 | NS | 5.38 ± 1.12 | 5.29 ± 1.21 | NS |
| Median [25th to 75th percentile] TG (mM) | 2.72 [1.31-4.44] | 2.84 [1.34-3.77] | NS | 1.88 [0.83-2.32] | 1.58 [0.86-1.79] | NS |
| HDL-C (mM) | 1.48 ± 0.53 | 1.38 ± 0.21 | NS | 1.71 ± 0.71 | 1.64 ± 0.57 | NS |
| LDL-C (mM) | 3.48 ± 0.77 | 3.27 ± 0.96 | NS | 2.86 ± 0.74 | 2.88 ± 0.93 | NS |
| Cystatin C (μM) | 0.80 ± 0.27 | 0.85 ± 0.39 | NS | 0.65 ± 0.07 | 0.66 ± 0.40 | NS |
| UAER (μg/min) | 5.22 ± 4.78 | 6.77 ± 5.34 | NS | 11.03 ± 8.63 | 5.45 ± 4.15* | 0.012 |
- Unless indicated otherwise, data are given as the mean ± SD unless.
- OAD, oral anti-diabetic drug; BOT, basal insulin-supported OADs therapy; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; WC, Waist circumference; FCP, Fasting C-peptide; 2 h-PCP, 2 hour -postprandial C-peptide; Cr, plasma creatinine; UA, uric acid; TC, total cholesterol; TG, triglycerides; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; UAER, urine albumin excretion rate.
3.2 Incidence of DR
At the 12-month follow-up, fewer patients had developed DR in the BOT than OAD group (8 [6.06%] vs 12 [8.3%]; P = 0.034]. The DR that developed during the follow-up period was non-proliferative. No diabetic macular edema was detected in any participant.
3.3 Glycemic control and HbA1c remission during follow-up
The results of FBG, 2hPBG, and HbA1c monitoring during the 12-month follow-up period are shown in Figure 3A–C. At baseline, there were no significant differences in FBG, 2hPBG, or HbA1c between the two groups (P > 0.05). At the 3rd month, FBG, 2hPBG, and HbA1c were higher in the BOT than OAD group (P < 0.05). However, at both the 6th and 12th month, FBG, 2hPBG, and HbA1c were all lower in the BOT than OAD group (P < 0.05).
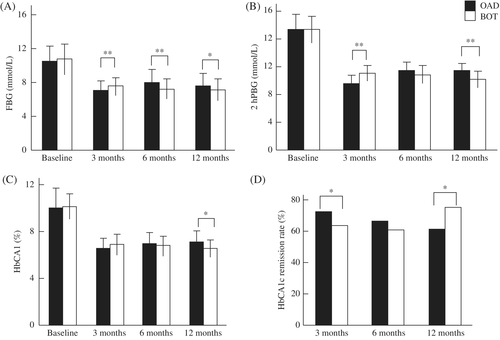
After 3 months of treatment, fewer patients had achieved the HbA1c target (≤7.0%) in the BOT than OAD group (63.6% [84/132] vs 72.2% [104/144], respectively; P < 0.05). However, among those who achieved the HbA1c target at the 3rd month, fewer patients in the BOT than OADs group failed to maintain the target at the 6th month (n = 4 vs 8). This led to a similar HbA1c remission rate between the two groups at the 6th month (60.6% and 66.6%, respectively; P > 0.05). At the 12th month, the HbA1c remission rate increased to 75.0% (99/132) in the BOT group, but declined further to 61.1% (88/144) in the OAD group. This difference was statistically significant (P < 0.05; Figure 3D).
3.4 Glycemic variability between the two groups
With regard to long-term GV, neither SD-HbA1c nor CV-HbA1c differed between the two groups (P > 0.05), whereas both SD-FBG and CV-FBG were significantly lower in the BOT than OAD group (P < 0.001). With regard to intraday GV, SDBG and MAGE were both significantly lower in the BOT than OAD group (P < 0.05). With regard to interday GV, neither FGE nor MODD differed significantly between the two groups (P > 0.05), as presented in Table 3.
| Baseline | 12-month follow-up | |||||
|---|---|---|---|---|---|---|
| OAD group (n = 144) | BOT group (n = 132) | P-value | OAD group (n = 144) | BOT group (n = 132) | P-value | |
| Long-term glycemic variability | ||||||
| SD-HbA1c | - | - | - | 0.404 ± 0.284 | 0.435 ± 0.264 | NS |
| CV-HbA1c | - | - | - | 0.061 ± 0.044 | 0.064 ± 0.038 | NS |
| SD-FBG | - | - | - | 1.183 ± 0.664 | 0.812 ± 0.587 | <0.001 |
| CV-FBG | - | - | - | 0.161 ± 0.079 | 0.110 ± 0.071 | <0.001 |
| Intraday glycemic variability | ||||||
| 24-hours mean glucose levels | 7.77 ± 1.51 | 7.25 ± 1.00 | 0.003 | 7.64 ± 1.26 | 7.33 ± 2.15 | NS |
| SDBG | 1.76 ± 0.50 | 1.60 ± 0.78 | NS | 2.00 ± 0.96 | 1.62 ± 0.97 | 0.006 |
| % CV of 24-hours glucose levels | 0.23 ± 0.05 | 0.22 ± 0.10 | NS | 0.25 ± 0.09 | 0.23 ± 0.12 | NS |
| MAGE | 3.04 ± 1.07 | 3.22 ± 1.72 | NS | 3.60 ± 1.47 | 3.18 ± 1.51 | 0.049 |
| FGE | 3.70 ± 1.55 | 3.45 ± 1.37 | NS | 3.86 ± 1.64 | 3.50 ± 1.56 | NS |
| Interday glycemic variability | ||||||
| MODD | 2.20 ± 1.24 | 2.33 ± 1.21 | NS | 2.32 ± 1.45 | 2.47 ± 1.43 | NS |
- Data are given as the mean ± SD.
- BOT, basal insulin-supported oral antidiabetic drug (OAD) therapy; CV-FBG, coefficient of variation of fasting blood glucose; CV-HbA1c, coefficient of variation of HbA1c; FGE, frequency of glucose excursion; MAGE, mean amplitude of glycemic excursions; MODD, mean of daily differences; SD-FBG, standard deviation of fasting blood glucose; SD-HbA1c, standard deviation of HbA1c; SDBG, standard deviation of blood glucose.
3.5 Inflammatory biomarkers
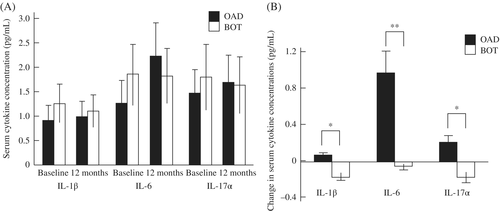
In the present study, IL-1β, IL-6, and IL-17α were selected as inflammatory biomarkers. There were no significant differences in any of these cytokines at baseline or at the 12-month follow-up between the two groups (Figure 4A). However, all changes in IL-1β, IL-6, and IL-17α were significantly smaller in the BOT than OAD group (Figure 4B).
3.6 Adverse events
There were no serious adverse events in either group. No significant changes in body weight were observed in either group (Table 2). Similar rates of hypoglycemia were observed in the OAD and BOT groups (9.87% vs 10.71%, respectively; P > 0.05).
4 DISCUSSION
The results of the present study indicate that, as a subsequent treatment, the BOT regimen was associated with a reduced occurrence of DR than OADs alone at the 12-month follow-up. The BOT regimen maintained the HbA1c target longer than OADs alone, with similar rates of hypoglycemia. In addition, the BOT regimen reduced long-term and intraday GV compared with OADs alone.
Our results regarding the reduction in the incidence of DR with basal insulin-supplemented therapy agree with those of previous studies. A few reports have indicated that insulin treatment prevents or delays DR in type 1 diabetes (T1D).10-14 A systematic review revealed that insulin treatment can also reduce DR in T2D, although the reduction was not as marked as that in T1D.15 The findings of an experimental animal study also suggested that insulin treatment benefited DR.16 However, some studies have suggested that insulin therapy could be harmful with regard to DR.2-6 These inconsistent results imply that the relationship between insulin treatment and DR is complicated. Indeed, numerous factors contribute to this relationship, such as the duration of diabetes, HbA1c, background treatment, complications, accompanying diseases, β-cell function, the timing and duration of insulin therapy, and the stage of DR. To the best of our knowledge, no investigation has compared the effects on DR of different regimens as subsequent therapies after intensive glycemic treatment. The findings of the present study suggest that BOT is superior to OADs alone as a subsequent therapy to prevent or delay DR, which implies that basal insulin supplementation benefited DR. Given that both BOT and OADs alone are widely used in clinical practice, further targeted studies are required.
Favorable overall glycemic control is necessary to prevent the onset of DR and to delay its progression.7 Currently, HbA1c is considered the gold standard for general glycemic control and it has been shown to be closely related to diabetic complications, such as DR.10-15 The results of the present study showed that HbA1c levels were lower in the BOT than OAD group at both the 6th and 12th month, although they were higher in the BOT than OAD group at the 3rd month. This result suggests that basal insulin supplementation provides a longer and better-maintained glycemic target, resulting in a lower incidence of DR. Notably, glycemia includes not only HbA1c but also GV. Recently, GV has emerged as one of the components of glycemia.17 The Diabetes Control and Complications Trial (DCCT) was the first study that suggested that GV should be considered. In the DCCT study, the DR risk was substantially lower in the intensive treatment group than in the conventional treatment group (6% vs 14%), although the HbA1c values were approximately 9% in both groups.18 This difference was attributed to GV. Numerous later studies have further indicated that GV plays an important role in the development and progression of DM complications.19-24 Hence, to prevent the occurrence of DR, optimized antidiabetic therapy should focus on not only HbA1c, but also GV. Glycemic variability is more complex than general glycemia. Unfortunately, there has been no gold standard to measure GV until now. Glycemic variability at a minimum includes long-term GV, short-term intraday GV, short-term interday GV, and the incidence of hypoglycemia. In the present study, we used SD-FBG, SD-HbA1c, CV-FBG, CV-HbA1c, SDBG, MAGE, and MODD to represent GV. As shown in Table 3, most of the indices of GV were lower in the BOT than OAD group. We therefore suggest that BOT can decrease glycemia more completely than OADs. At the same time, the incidence of hypoglycemia in the BOT group was similar to that in the OAD group. Overall, the BOT regimen decreased glycemia more effectively, stably, and completely than OADs without increasing hypoglycemia, which may contribute to its superiority in the prevention of DR.
Inflammation is a major pathogenic factor associated with DR.25, 26 Both general hyperglycemia and GV can induce and enhance inflammation.27-30 Therefore, we compared the levels of selected cytokines between the two treatment regimens. Among the cytokines, IL-1β, IL-6, and IL-17α have been reported to contribute greatly to DR,31-36 although there is some controversy regarding this issue.37, 38 Thus, we measured these three cytokines in the present study. Unfortunately, none of the cytokines showed any differences between the two groups at the 12-month follow-up. Notably, the changes in serum concentrations of all cytokines between the 12-month follow-up and baseline differed significantly between the two treatment regimens. The only difference between the BOT and OADs regimens was basal insulin supplementation. Consequently, the differences in the changes in cytokines may be due to a direct anti-inflammatory effect of insulin, which is independent of its hypoglycemic effect.39
Interestingly, UAER, which is usually used to screen for diabetic nephropathy (DN), decreased in the BOT group and increased in the OAD group at the 12-month follow-up. These results suggest that basal insulin supplementation also benefits DN. Given that both DN and DR are characteristic microvascular complications of T2D, the results indicate that basal insulin supplementation benefits the microvascular complications of diabetes.
This study had some limitations, the most important of which is that the follow-up time was relatively short. Diabetic retinopathy is a chronic complication of diabetes that usually appears in patients with poorly controlled glycemia of a long duration. A 12-month follow-up may not be sufficient, which may have contributed to the fact that few DR cases occurred in either group (8/132 in the BOT group vs 12/144 in the OAD group) and that all cases of DR were non-proliferative. Prospective studies with longer follow-up periods are required to validate this 12-month result. Because there were few cases of DR in the present study, the sample size appears to be relatively small, and hence studies with a larger sample size are needed. In this study, there were no differences in cytokine concentrations between the two groups at the 12-month follow-up. Consequently, the significance of differences in the changes in IL-1β, IL-6, and IL-17α between the two regimens was relatively slight. Therefore, we cannot determine whether these three cytokines were responsible for the superiority of basal insulin supplementation. More in-depth studies with expanded cytokine measurements are required to further investigate the mechanisms underlying the effects of BOT.
4.1 Conclusion
In conclusion, the findings of this study suggest that 12-month basal insulin supplementation as a subsequent therapy decreases the incidence of DR in short-duration T2D by reducing glycemia more effectively, stably, and completely.
ACKNOWLEDGEMENTS
The authors thank all the doctors, nurses, technicians, and patients involved in this study their dedication. The authors also thank Lian-xiong Yuan (department of science, the third affiliated hospital of Sun Yat-sen university, Guangzhou PRC) for help with statistical analyses.
DISCLOSURE
None of the authors has any conflicts of interest to declare.




