Surgical vs percutaneous coronary revascularization in patients with diabetes following an acute coronary syndrome
糖尿病患者出现急性冠状动脉综合征后选择外科手术治疗与选择经皮冠状动脉血运重建术治疗的对照研究
Cardiovascular disease remains the leading cause of death in patients with diabetes mellitus (DM), and outcomes following an acute coronary syndrome (ACS) are worse in patients with than without DM.1, 2 One of the reasons for the poor prognosis is that patients with DM have a more complex coronary anatomy, with a higher prevalence of multivessel coronary disease (MVD).3 Patients with DM also have more comorbidities (eg, hypertension, peripheral artery disease, renal disease, and heart failure) and higher rates of local complications after stent placement (stent thrombosis, neoatherosclerosis, and restenosis).4 All these elements make the choice of the revascularization strategy following an ACS very challenging. The current evidence for coronary revascularization in patients with DM in the stable ischemic heart disease (SIHD) scenario supports coronary artery bypass grafting (CABG) as the optimal strategy rather than percutaneous coronary intervention (PCI).5 In the ACS setting, randomized evidence is lacking and although it is reasonable to think that the benefit derived from CABG would also be found after a non-ST elevation (NSTE) ACS, the most common standard of practice today still favors PCI6 (Figure 1).
In patients with SIHD, a subgroup analysis of the Bypass Angioplasty Revascularization Investigation (BARI) trial, published in 1996, first generated the hypothesis that CABG would lead to better long-term survival in the DM population than PCI with balloon angioplasty.7 Subsequently, the BARI 2 Diabetes (BARI 2D) trial randomized only patients with DM and suggested that CABG could result in lower rates of major cardiovascular events (MACE; ie, all-cause mortality, non-fatal myocardial infarction [MI] and stroke) than optimal medical therapy alone (5-year event rates 22.4% vs 30.5% respectively; P = 0.01).8 These results were further corroborated by other smaller trials9 and a subanalysis of large studies.10 The Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial was the first randomized study powered to compare CABG vs PCI with drug-eluting stents in patients with DM and MVD.5 After a median follow-up of 3.8 years, CABG resulted in a lower rate of MACE (18.7% vs 26.6%; P = 0.005) and MI (6.0% vs 13.9%; P < 0.001) than PCI.5 After an extended follow-up (8 years), CABG was also associated with lower all-cause mortality in this population (18.3% vs 24.3%; P = 0.01).11 In FREEDOM, 30.7% of patients had a history of recent ACS, and CABG patients experienced a numerically lower rate of MACE endpoints than PCI patients (5-year event rates 20.9% vs 30.7%; P = 0.09).5 Newer-generation stents do not seem to alter the superiority of CABG in patients with DM, especially when complete anatomical revascularization cannot be achieved.12 In the Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery Disease (BEST) trial comparing CABG to PCI with second-generation everolimus-eluting stents, CABG was associated with a lower rate of the primary composite endpoint of all-cause mortality, MI, and repeat revascularization in the subgroup of patients with DM (9.1% vs 19.2%; P = 0.007 for the primary composite outcome at 4.6 years; Pinteraction = 0.06).13 No interaction was found for patients with unstable angina at baseline in that trial (43% of patients; Pinteraction = 0.35 for the composite primary outcome). Finally, in a recently published patient-level meta-analysis, PCI was associated with a higher risk of 5-year death in patients with DM and MVD (hazard ratio 1.48; 95% confidence interval 1.19-1.84; P = 0.0004; n = 3266).14
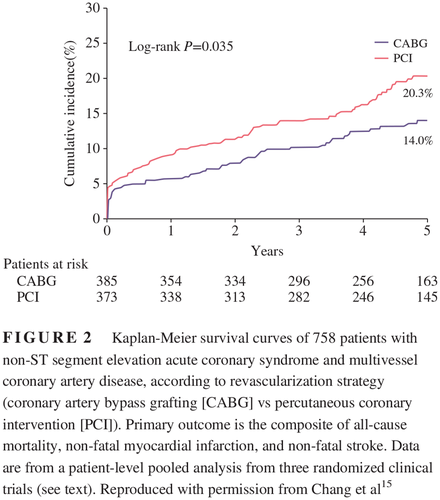
In the ACS setting, a meta-analysis of 1246 NSTE-ACS patients from three randomized trials (BEST trial, Premier of Randomized Comparison of Bypass Surgery vs Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease [PRECOMBAT] trial, and the Synergy between PCI with Taxus and Cardiac Surgery [SYNTAX] trial) showed that CABG was associated with a reduction in MACE, especially in patients with MVD (14.0% vs 20.3%; P = 0.035; Figure 2) compared with PCI with a drug-eluting stent.15 No interaction was observed between DM status (34.7% of all patients) and clinical outcomes (P = 0.885). A post hoc subanalysis of the Acute Catheterization and Early Intervention Triage Strategy (ACUITY) trial, including only patients with DM, MVD, and NSTE-ACS, also found lower rates of all-cause mortality, MI and repeat revascularization in the CABG vs PCI arm (17.3% vs 30.1%, respectively; P = 0.01).16 Perhaps the most impactful study to address the question of optimal revascularization for patients with diabetes presenting with an ACS was a registry-based analysis that included all revascularization procedures performed in the province of British Columbia, Canada, between 2007 and 2014, including 3017 patients with DM and MVD in the early phase of an ACS.17 In that study, CABG was associated with lower rates of MACE (20.8% vs 33.4%; P < 0.01), as well as lower individual rates of death (12.4% vs 22.3%; P < 0.01), MI (9.9% vs 17.6%; P < 0.01), and repeat revascularization (8.2% vs 22.6%; P < 0.01) compared with PCI during a median follow-up of 3.3 years.17 Of note, even in the first 30 days after the revascularization procedure, CABG was already associated with a reduction in MACE (4.3% vs 8.2%; P < 0.01), which is not usually observed in the SIHD population.17

The management of ACS and revascularization in patients with DM will continue to evolve in the coming decades, and CABG may be a viable strategy, leading to improved outcomes, especially following NSTE-ACS. Patients with DM and MVD who survive an ACS have been underrepresented in clinical trials, and further prospective studies are needed to define the optimum revascularization strategies in this population. In the meantime, in the absence of randomized evidence, cardiologists should guide their revascularization decisions using the best available evidence from the SIHD population and discussing these patients with an ACS together with an experienced heart team.
心血管疾病仍然是糖尿病(DM)患者死亡的主要原因,并且与非DM患者相比,DM患者急性冠脉综合征(acute coronary syndrome,ACS)后的结果更差1,2。导致预后不良的原因之一就是DM患者的冠状动脉解剖结构更为复杂,并且多支冠状动脉病变(multivessel coronary disease,MVD)的患病率更高3。DM患者还存在更多的合并症(如高血压、外周动脉疾病、肾病与心力衰竭)并且支架置入术后局部并发症(支架血栓形成、新的动脉粥样硬化与再狭窄)的发生率更高4。所有的这些因素使得DM患者在ACS后选择何种血运重建策略成为非常具有挑战性的任务。目前的证据认为合并稳定缺血性心脏病(stable ischemic heart disease,SIHD)情况下的DM患者在选择冠状动脉血运重建方案时,应该采用冠状动脉旁路移植术(coronary artery bypass grafting,CABG)为最佳, 而不是经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)5。目前缺乏ACS患者相关的随机对照试验证据,虽然在发生非ST段抬高(non-ST elevation,NSTE)ACS后采用CABG也能够带来获益是合理的,但是目前最常见的实践标准仍然倾向于选择PCI6。
在合并SIHD的患者中,发表于1996年的旁路血管成形术血运重建研究(Bypass Angioplasty Revascularization Investigation,BARI)试验的亚组分析第一次提出了一个假说,亦即DM患者采用CABG后的长期生存率要高于采用经皮冠状动脉球囊成形术7。随后,在BARI 2糖尿病(BARI 2 Diabetes,BARI 2D)试验中只对DM患者进行随机化,结果表明采用CABG治疗与单纯采用最佳药物治疗相比,前者的主要心血管事件(MACE;亦即全因死亡率、非致命性心肌梗死[MI]、以及中风)发生率更低(5年事件发生率分别为22.4%与30.5%;P = 0.01)8。这些结果进一步得到了其他较小型试验9以及一项大型研究亚组分析的证实10。糖尿病患者未来血运重建评估:多支血管病变的最佳治疗(The Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease,FREEDOM)试验是第一个在合并MVD的DM患者中进行的旨在比较CABG术与药物洗脱支架PCI术疗效的随机研究5。经过中位数为3.8年的随访后,发现CABG组的MACE(分别为18.7%与26.6%;P = 0.005)以及MI(分别为6.0%与13.9%;P < 0.001)的发生率与PCI组相比都更低5。经过延长期随访(8年)后,发现CABG组患者的全因死亡率也更低(分别为18.3%与24.3%;P = 0.01)11。在FREEDOM试验中,30.7%的患者最近都有ACS病史,CABG组患者的MACE终点发生率与PCI组患者相比在数值上更低(5年事件发生率分别为20.9%与30.7%;P = 0.09)5。DM患者使用新一代支架似乎并不能够改变CABG术优于PCI术的情况,尤其是在不能够完全实现解剖学上血运重建的情况下12。在多支冠状动脉病变患者中比较冠状动脉搭桥手术与依维莫司-洗脱支架置入手术治疗效果的随机试验(Randomized Comparison of Coronary Artery Bypass Surgery and Everolimus-Eluting Stent Implantation in the Treatment of Patients with Multivessel Coronary Artery,BEST)中,对比了CABG组与第二代依维莫司-洗脱支架PCI组的疗效,在DM亚组患者中发现CABG治疗后的主要复合终点(包括全因死亡率、MI以及再次血运重建术)发生率更低(第4.6年时主要复合结果发生率分别为9.1%与19.2%;P = 0.007;P相互作用 = 0.06)13。在该试验中发现基线时不稳定型心绞痛患者没有相互作用(43%的患者;复合主要结果P相互作用 = 0.35)。最后,在最近发表的一篇患者水平meta分析中,合并MVD的DM患者PCI手术后的5年死亡率风险更高(风险比为1.48;95%置信区间为1.19-1.84;P = 0.0004;n = 3266)14。
针对ACS患者,有一项meta分析纳入了1246名来自3项随机试验(BEST试验、在左冠状动脉主干病变患者中第一次比较旁路手术与应用西罗莫司洗脱支架血管成形术的随机试验[Premier of Randomized Comparison of Bypass Surgery vs Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease,PRECOMBAT],以及使用紫杉醇洗脱支架与心脏手术协同作用[SYNTAX]试验)的NSTE-ACS患者,结果发现CABG组的MACE与药物洗脱支架PCI组相比明显更少,特别是在合并MVD的患者中(发生率分别为14.0%与20.3%;P = 0.035)15。在DM状态(在全部患者中占34.7%)与临床结果之间没有观察到相互作用(P = 0.885)。在紧急导管插入术与早期干预分诊策略(Acute Catheterization and Early Intervention Triage Strategy,ACUITY)试验的一项事后亚组分析中,只纳入了合并DM、MVD与NSTE-ACS的患者,结果也发现CABG组的全因死亡率、MI以及再次血运重建发生率与PCI组相比都有下降(分别为17.3%与30.1%;P = 0.01)16。对于出现ACS的糖尿病患者来说最佳血运重建方法是什么?也许能够回答这个问题的最有力的研究是一项基于登记表的分析研究,它纳入了2007至2014年间在加拿大不列颠哥伦比亚省进行的所有血运重建手术,共纳入了3017名合并MVD并且处于ACS早期的DM患者17。那项研究里,在中位数为3.3年的随访期间,CABG组的MACE发生率与PCI组相比显著更低(20.8%与33.4%;P < 0.01),除此之外患者的死亡率(12.4%与22.3%;P < 0.01)、MI(9.9%与17.6%;P < 0.01)以及再次血运重建(8.2%与22.6%;P < 0.01)的发生率也更低17。值得注意的是,即使在血运重建术后的前30天,CABG组的MACE也显著更少(4.3%与8.2%;P < 0.01),而这种情况在SIHD人群中却并不常见17。
在未来的几十年里DM患者的ACS以及血运重建的管理将会不断完善,CABG可能是一种切实可行的治疗策略,这将会改善患者的预后,特别是在发生了NSTE-ACS之后。在临床试验中发生ACS之后能幸存下来的合并MVD的DM患者数量没有足够的代表性,需要进一步的前瞻性研究来明确这个人群的最佳血运重建策略。与此同时,在缺乏随机试验证据的情况下,心脏病学家应该利用现有来自于SIHD人群的最佳证据,指导他们的血运重建策略,并且要与经验丰富的心脏病团队一起讨论这些ACS患者的治疗策略。
DISCLOSURE
LCG has no conflicts of interest to disclose. MEF has received research support from Amgen and Novo Nordisk.