Evolution of type 2 diabetes mellitus treatment approaches
2型糖尿病治疗方法的演变
A recent analysis of 271 174 patients with type 2 diabetes (T2D) registered in the Swedish National Diabetes Register showed that HbA1c >7% tracks much more strongly with mortality and cardiovascular disease (CVD) outcome among people with T2D than do blood pressure or low-density lipoprotein cholesterol.1 The Kaiser Permanente Northern California Diabetes Registry of 34 737 people followed for at least a decade (mean 13 years; mean age at diagnosis 57 years) found those with mean HbA1c ranging from 7.0% to <8.0% and those with HbA1c ≥9.0% had approximately 40% and 120% higher subsequent microvascular event rates, respectively, and approximately 30% and 50% higher macrovascular event rates, than those with mean HbA1c <6.5% during the first year after diagnosis.2 Both high HbA1c groups had approximately 30% higher mortality than those with the lower HbA1c levels.2 The implication of this and many other studies that better glycemic control is beneficial for people with T2D is inescapable.
The question then must be asked, can we make recommendations for “good” treatment approaches to improve the outcomes for people with T2D? Past approaches simply suggested hierarchies of treatments: first, use metformin, wait a period of time to observe response, then add a medication such as a sulfonylurea (SU), basal insulin, dipeptidyl peptidase-4 inhibitor (DPP-4i), glucagon-like peptide-1 receptor agonist (GLP-1RA), sodium-glucose cotransporter 2 inhibitor (SGLT2i), or thiazolidinedione (TZD), iteratively wait, and add further treatments.3 A refinement of this approach recommended treatments based on specific patient characteristics, baseline HbA1c (a greater number of agents would be used initially for those with worse glycemic control)4, obesity (avoiding agents that exacerbated weight gain, choosing those associated with weight loss), risk of and risk from hypoglycemia5 (avoiding insulin and SU because these are associated with a greater likelihood of these two side effects), and cost.3 The notion that recognition of adverse effects of each class of glucose-lowering agent ought to be used in avoiding their use in people prone to that side effect is included prominently in the algorithm of the American Association of Clinical Endocrinologists.4
New and evolving approaches for T2D now make concrete use of complications in suggesting treatments. Suggestions along these lines take the viewpoint that the presence of CVD should itself lead to treatment with agents that have been shown to reduce CVD outcomes,6 separately considering atherosclerotic CVD (ASCVD) and heart failure (HF), because TZDs, GLP-1RA, and SGLT2i all appear to improve ASCVD, but TZDs exacerbate HF, whereas HF is improved by SGLT2i. We may be able to similarly consider renal disease in determining the appropriate agent for a given person, given the evidence of benefit in reducing albuminuria with TZDs, GLP-1RA, and DPP-4i, and the evidence of reduction in albuminuria and preservation of glomerular filtration rate with SGLT2i.7
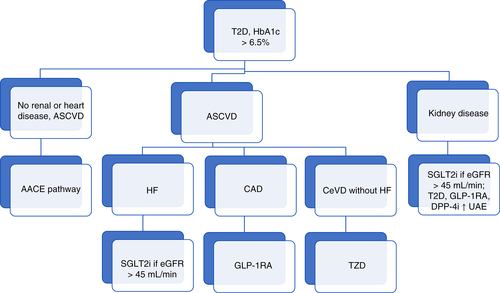
These considerations may lead us to novel algorithmic approaches, based on both glycemia and disease characteristics (e.g. see Figure 1). But such an approach may be overly reductionistic. Over time, people with diabetes have increasing CVD risk. When considered in terms of mortality, a recent meta-analysis of 128 843 deaths among 689 300 participants in randomized control trials found that the presence of diabetes led to the same risk as did that of stroke or myocardial infarction, with these conditions being additive in increasing cardiovascular mortality.8 Does it then make sense to consider one person with T2D at sufficiently high CVD risk to use an agent with CVD outcome benefit while not offering such an agent to another? Rather than being able to offer a decision tree in determining appropriate treatments, it may be more appropriate to consider people with T2D to be affected by a network of factors, to greater or lesser degrees in each. If, further, there are additive benefits of specific combinations, such as TZDs with SGLT2i,9, 10 the development of such a decision-guidance network may become the ideal tool for initiating and optimizing a given individual's treatment.
In an analysis of >300 000 propensity-score matched participants in the CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors) addressing the effect of SGLT2i on death or HF, approximately 13% had CVD at the time of initiation of glucose-lowering treatment,11 somewhat lower than the CVD prevalence of nearly one-third of people with T2D in a recent analysis.12 Although SGLT2i led to similar 40% to 50% relative reductions in HF plus mortality in the CVD-REAL study in those having and not having CVD, the absolute benefit of SGLT2i was 2.7 per 100 patient-years for those with established CVD, but 0.5 per 100 patient-years for those not having established CVD.11 Given the many-fold greater cost of newer glucose-lowering treatments compared with older agents, and the difficulties that many people with T2D have in lack of availability even of older treatments,13 we need to recall Shakespeare's caveat, “Striving to better, oft we mar what's well” (King Lear, Act 1, Scene 4). Careful ongoing attention to the true outcome benefit of various approaches will be crucial in determining appropriate treatments.
最近有一项研究对瑞典全国糖尿病登记册中的271174例2型糖尿病患者(T2D)进行了分析, 发现在T2D患者中HbA1c > 7%与血压或者低密度脂蛋白胆固醇相比, 前者与死亡率以及心血管疾病(CVD)预后之间的相关性更强1。对北加利福尼亚Kaiser Permanente糖尿病登记册中的34737例患者随访至少不少于10年(平均13年;诊断时的平均年龄为57岁)发现, 与那些在诊断后第一年期间平均HbA1c < 6.5%的患者相比, 平均HbA1c处于7.0%至< 8.0%以及平均HbA1c ≥ 9.0%的患者随后的微血管事件发生率大约分别增加了40%与120%,并且大血管事件发生率大约分别增加了30%与50%2。这两个高HbA1c组患者与HbA1c水平较低的患者相比死亡率大约都增加了30%2。这项研究与许多其他研究一样都明确证实了更好的血糖控制对于T2D患者来说是有益的。
接着必然要被问到的问题是, 我们能否推荐一些“好”的治疗方法以改善T2D患者的预后?过去的方法只是简单地提出了治疗的层次:首先, 使用二甲双胍, 等待一段时间观察治疗反应, 接着加用一种药物例如磺脲(SU)、基础胰岛素、二肽基肽酶-4抑制剂(DPP-4i)、胰高血糖素样肽-1受体激动剂(GLP-1 RA)、钠-葡萄糖共转运体2抑制剂(SGLT2i)或者噻唑烷二酮(TZD),反复等待, 然后进一步增加治疗药物3。对这种方法的进一步改进是建议采用基于特定患者特征的治疗方法, 需考虑患者的基线HbA1c(对于血糖控制较差的患者一开始就要使用更多的治疗药物)4、肥胖(避免使用增加体重的药物, 选择可以减重的药物)、低血糖风险以及低血糖导致的风险5(避免使用胰岛素与SU,因为它们导致这两种副作用的可能性更高)、以及费用问题3。在美国临床内分泌学家协会推荐的治疗策略中包括了这样的一个重要概念, 亦即要认识到每类降糖药物的副作用以免在易发生那些副作用的患者中使用这些药物4。
目前新的、不断发展的T2D治疗方法在用药时还会具体考虑到并发症。根据这些治疗策略建议采用以下这样的观点, 亦即存在CVD本身就要求采用那些已经被证实可减少CVD结果的药物来治疗6,需要分别衡量动脉粥样硬化CVD(ASCVD)与心脏衰竭(HF),因为TZDs、GLP-1RA与SGLT2i似乎都可以改善ASCVD,但是TZDs可以加重HF,然而SGLT2i却可以改善HF。同样, 对于一名既定的患者, 在选择合适治疗药物的同时也要考虑到肾脏疾病, 既往有证据表明TZDs、GLP-1RA与DPP-4i有益于减少蛋白尿, 并且也有证据表明SGLT2i可以减少蛋白尿和防止肾小球滤过率下降7。
考虑到以上所提的意见 在基于血糖与疾病特征下, 这可能会引导我们采用新的治疗策略。但是这种方法可能过于简化。随着时间的推移, 糖尿病患者的CVD风险在不断增加。对于死亡率的问题, 最近一项研究对689300名随机对照试验参与者中的128843例死亡进行了meta分析, 发现存在糖尿病所导致的死亡风险与中风或者心肌梗死一样, 这些因素都可以额外增加心血管的死亡率8。如果考虑到某个T2D患者存在较高的CVD风险对其使用具有CVD结果获益的药物, 但是却不对另外一名患者处方这种药物, 那么这样做有意义吗?与其用一个决策树来确定合适的治疗方法, 更为合适的治疗方法是要考虑到T2D患者可能会受到一系列因素的影响, 而每一种因素的影响程度多少不一。此外, 如果还有某种特定组合能给患者带来额外益处, 例如联用TZDs与SGLT2i9,10,那就应该将此作为在特定患者中决定起始治疗和优化方案的一种理想手段。
为了评估SGLT2i对死亡率或者HF的影响, 在CVD-REAL研究(Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors,新使用钠-葡萄糖共转运体-2抑制剂治疗的患者心血管预后疗效的对照研究)中纳入了>300000例倾向性评分相匹配的参与者, 在开始降糖治疗时大约有13%的参与者合并CVD11,CVD患病率与最近一项分析中将近三分之一的T2D患者合并CVD相比稍低一些12。在CVD-REAL研究中虽然那些患者无论是否合并CVD,SGLT2i导致的HF加上全因死亡的相对减少率都相似(40%-50%),对于既往已经有明确CVD的患者来说,SGLT2i导致的每100例患者-年的绝对获益有2.7例, 但是对于既往没有明确CVD的患者, 每100例患者-年的绝对获益只有0.5例11。鉴于与原有药物相比, 使用新型降糖药物治疗的成本要高出许多倍, 并且许多T2D患者困难到即使是原有的治疗药物也不能够获得13,我们需要牢记莎士比亚的告诫, “为了争取更好, 但是我们却常常毁掉现有的美好”(李尔王, 第1幕, 第4场)。为了选择更合适的治疗方法, 至关重要的就是我们需要密切地持续关注各种治疗方法真实结果有何益处。




