Improving outcomes in patients with type 2 diabetes mellitus and chronic heart failure: New hope
改善2型糖尿病合并慢性心力衰竭患者的预后:新的希望
Type 2 diabetes mellitus worsens heart failure mortality and morbidity
Type 2 diabetes mellitus (T2DM) is increasing in prevalence, and changes in lifestyles and the world we live in mean that the current estimated 400 million adults living with T2DM worldwide is likely to increase substantially as the 21st century unfolds. Patients with T2DM are at high risk of developing chronic heart failure (CHF) secondary to left ventricular (LV) systolic dysfunction.
Mortality in patients with CHF secondary to LV systolic dysfunction who do not suffer from T2DM has declined over the past 3 decades. A decline in annual mortality rates from 12.5% to 7.8% has been demonstrated in ambulant outpatients with CHF free of T2DM between 1995 and 2010.1 However, if a patient with CHF also suffers from T2DM, the outlook has been bleak. In addition, patients with CHF and T2DM have a risk of all-cause mortality double that of similar patients without diabetes; the risk of progressive heart failure death is also doubled, whereas the risk of sudden cardiac death (due to cardiac arrhythmia) is threefold greater in patients with CHF suffering from T2DM.2 To put this in context, if a patient with CHF suffers from T2DM, their risk of death at 3 years is approaching 40%. Studying approximately 2000 patients over a 10-year period has demonstrated that at least 25% of patients with CHF secondary to LV systolic dysfunction have coexisting T2DM.2 In a further study, it was shown that diabetes imparts a risk of hospitalization due to decompensated heart failure threefold that of a patient without diabetes.3 In addition, a study examining the characteristics of patients with CHF and T2DM demonstrated that diabetes has deleterious effects on renal function, hemoglobin, and symptoms, as assessed by New York Heart Association functional class.4
Improved understandings of disease mechanisms: New hope
Over the past 20 years, our understanding of the pathophysiology of CHF has advanced substantially5 (Fig. 1). An initial insult to LV function leading to reduced cardiac output, such as a myocardial infarction, is thought to be followed by compensatory changes in LV size and shape (LV remodeling), and activation of the renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system (SNS). This “neurohumoral activation” may initially be beneficial, but in the longer term becomes detrimental. The use of angiotensin-converting enzyme (ACE) inhibitors, β-adrenoceptor antagonists, aldosterone antagonists and, more recently, complex device therapies that address these maladaptions (Fig. 1) has led to a significant improvement in life expectancy in patients with CHF. Moreover, previous doubt about use of β-adrenoceptor antagonists in patients with CHF and T2DM was recently dispelled by our paper demonstrating that each 1-mg increment in bisoprolol (or its equivalent) led to an associated 9% reduction in mortality in patients with diabetes, compared with 3% in patients with CHF and no diabetes.6
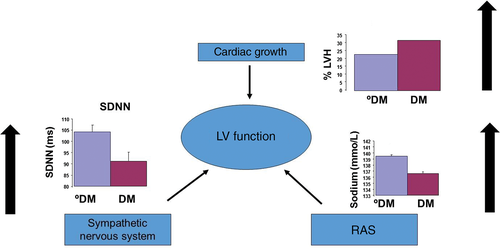
Using contemporary therapies to improve outcomes in patients with CHF and T2DM
We advocate a rationalized approach to treating patients with CHF and T2DM. At first assessment, loop diuretics should be used to control symptoms, carefully titrating the dose up and down according to fluid status. This should be accompanied by careful but aggressive use of disease-modifying agents (β-adrenoceptor antagonists and ACE inhibitors). If the patient has evidence of electrical dyssynchrony on their 12-lead electrocardiograph (QRS >120 ms) one should consider resynchronization therapy and, if at high risk of sudden death, an implantable defibrillator. On top of this, metformin7 and possibly sodium–glucose cotransporter 2 (SGLT2) inhibitors8 should be used to control glycemia as part of a holistic cardiometabolic treatment strategy.
Conclusions
An understanding of disease mechanisms is pivotal to effective treatment of T2DM and CHF. The combination of the two requires an integrated rational approach. Delivery of optimal current therapeutic options offers hope while we wait for new therapies and ways of thinking to emerge.
Disclosure
MK has received speaker fees from Merck and unrestricted research grants from Medtronic.
2型糖尿病可导致心力衰竭的死亡率与发病率上升
2型糖尿病(T2DM)的患病率在日益上升, 而我们的生活方式以及我们所生活的这个世界在不断地变化, 由此带来的后果是, 目前估计全世界有4亿的成年人患T2DM,并且随着21世纪的展开这个数字还有可能会大幅增加。T2DM患者出现继发于左心室(LV)收缩期功能障碍的慢性心力衰竭(chronic heart failure,CHF)风险较高。
在过去的30年中, 没有合并T2DM的CHF患者继发于LV收缩功能障碍的死亡率已经有所下降。在1995至2010年之间, 没有合并T2DM的CHF门诊可自由活动的患者的年死亡率已经从12.5%下降到了7.8%1。然而, 如果一名患者同时合并CHF与T2DM,前景就很黯淡了。另外, 同时合并CHF与T2DM患者的全因死亡风险率是没有糖尿病的相似患者的2倍;进展性心力衰竭的死亡风险也增加了1倍, 而心源性猝死(由于心律失常)的风险在同时合并CHF与T2DM的患者中增加到了3倍2。把它放到上下文中, 如果CHF患者同时合并有T2DM,那么他们在3年内的死亡风险将接近40%。既往有一项研究在10年内大约调查了2000名患者, 结果发现至少有25%继发于LV收缩功能不全的CHF患者同时存在T2DM2。在一项进一步的研究中发现, 糖尿病导致的失代偿性心力衰竭住院风险是无糖尿病患者的3倍3。另外, 在一项调查合并CHF与T2DM患者特征的研究中, 使用纽约心脏协会心功能分类进行评估, 结果发现糖尿病对肾功能、血红蛋白以及症状都有不良的影响4。
对疾病机制的新认识:新的希望
在过去的20年中, 我们对CHF病理生理学的认识有了实质性的进展(图1)5。目前认为对LV功能的最初损害可导致心输出量减少, 例如心肌梗死之后,LV大小与形状(LV重塑)发生了代偿性改变, 并且还激活了肾素-血管紧张素-醛固酮系统(RAAS)以及交感神经系统(SNS)。这种“神经体液激活”最初可能是有益的, 但从长远来看是有害的。为了解决这些不良适应问题(图1),要使用血管紧张素转换酶(ACE)抑制剂、β-肾上腺素受体拮抗剂、醛固酮拮抗剂以及最近上市的复杂设备来治疗, 最终可使CHF患者的预期寿命得到显著改善。此外, 既往还有人怀疑CHF合并T2DM的患者使用β-肾上腺素受体拮抗剂治疗的益处, 最近我们的论文驱散了这个疑虑, 研究结果证实每增加1 mg的比索洛尔(或其等效药物)可导致糖尿病患者的相关死亡率降低9%,而对照的没有合并糖尿病的CHF患者只下降了3%6。
使用当代的治疗方法来改善CHF合并T2DM患者的预后
我们主张使用合理化的方法来治疗合并CHF与T2DM的患者。首先要评估的是使用袢利尿剂来控制症状, 并且根据液体出入状态仔细地上下滴定剂量。同时应该小心但积极地使用缓解病情药物(β-肾上腺素受体拮抗剂与ACE抑制剂)。如果患者在12导联心电图机上有电不同步(QRS> 120 ms)的证据, 则应该考虑再同步治疗, 并且, 如果有猝死的高风险, 则应该植入除颤器。最重要的是, 作为整体心脏代谢治疗策略的一部分, 应该使用二甲双胍7并且尽可能使用钠-葡萄糖共转运体2(SGLT2)抑制剂8来控制血糖。
结论
有效治疗T2DM与CHF的关键在于要了解疾病的发病机制。合并这两种疾病的患者需要一种综合理性的治疗方法。在我们等待新的治疗方法以及新思维方式出现的时候, 当前已经获得的最佳治疗方法为我们带来了希望。
图1 糖尿病可导致左心室收缩功能障碍的不良反应加重。图中显示的数值是平均数±SEM。DM,2型糖尿病;○DM,没有2型糖尿病;LV,左心室;LVH,LV肥厚;RAS,肾素-血管紧张素系统;SDNN,正常RR间期标准差(一种自主神经平衡的标志物)。




