Optimization of fluoride adsorption from aqueous solution over mesoporous titania-alumina composites using Taguchi method
Mohammed K. Al Mesfer
Department of Chemical Engineering, College of Engineering, King Khalid University, Abha, Saudi Arabia
Contribution: Conceptualization (equal), Funding acquisition (lead), Methodology (equal), Project administration (lead), Resources (lead), Validation (equal)
Search for more papers by this authorMohd Danish
Department of Chemical Engineering, College of Engineering, King Khalid University, Abha, Saudi Arabia
Contribution: Data curation (lead), Investigation (equal), Resources (supporting), Software (supporting), Validation (equal)
Search for more papers by this authorCorresponding Author
Mumtaj Shah
Chemical Engineering Department, Indian Institute of Technology Roorkee, Roorkee, India
Correspondence
Mumtaj Shah, Chemical Engineering Department, Indian Institute of Technology Roorkee, Roorkee 247667, India.
Email: [email protected]
Search for more papers by this authorMohammed K. Al Mesfer
Department of Chemical Engineering, College of Engineering, King Khalid University, Abha, Saudi Arabia
Contribution: Conceptualization (equal), Funding acquisition (lead), Methodology (equal), Project administration (lead), Resources (lead), Validation (equal)
Search for more papers by this authorMohd Danish
Department of Chemical Engineering, College of Engineering, King Khalid University, Abha, Saudi Arabia
Contribution: Data curation (lead), Investigation (equal), Resources (supporting), Software (supporting), Validation (equal)
Search for more papers by this authorCorresponding Author
Mumtaj Shah
Chemical Engineering Department, Indian Institute of Technology Roorkee, Roorkee, India
Correspondence
Mumtaj Shah, Chemical Engineering Department, Indian Institute of Technology Roorkee, Roorkee 247667, India.
Email: [email protected]
Search for more papers by this authorAbstract
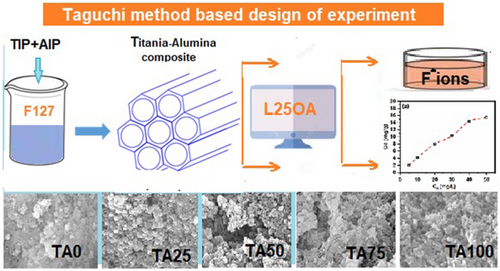
The optimization of fluoride removal from aqueous media was studied over the mesoporous titania-alumina composites using Taguchi method-based L25 orthogonal array experimental design. The chemical structure, surface chemistry, and morphology of as-prepared composite adsorbents were studied utilizing various analytical methods. The findings of the characterization demonstrated that the produced composites have high textural qualities, which are conducive to enhanced fluoride adsorption. The optimum conditions for maximum percentage removal of fluoride from aqueous solution were found as adsorbent type as TA75, adsorbent dose 4 g L−1, initial concentration of fluoride 40 ppm, solution pH 3 with a treatment time of 60 min. Under the optimum conditions, 98% of fluoride adsorption was achieved. Analysis of variance revealed that the solution pH followed by the adsorbent dose was the most significant for fluoride adsorption. The Langmuir model and pseudo-second-order kinetic model fit the adsorption data well, and the TA75 adsorbent had a maximum Langmuir fluoride adsorption capacity of 34.48 mg g−1 at pH = 3. The thermodynamic information suggests that the adsorption was spontaneous and endothermic under the given operating conditions. The synergic combination of Ti–Al nanoparticles demonstrated a high percentage removal of fluoride under the optimized operating conditions.
Practitioner Points
- The Taguchi method-based design of the experimental approach was implemented in the fluoride adsorption process.
- Mesoporous titania-alumina composites with 0 to 100 wt.% of alumina in titania were prepared and applied to remove fluoride from an aqueous solution.
- Solution pH was the most influential parameter for the fluoride adsorption process, while the synergistic combination of 75 wt.% alumina in titania showed the maximum adsorption capacity.
Graphical Abstract
The synthesis of mesoporous Ti-Al mixed oxide from TIP and AIP and its application in flouride removal from waste water wherein the optimization is done using a L25 Taguchi design. Due to the mopholoical variation in Ti-Al mixed oxide depending on the composition of oxide, a variation in adsorption capacity is expected.
CONFLICT OF INTEREST
There is no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data will be available at the reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| wer1663-sup-0001-Supporting Information_CLEAN.docxWord 2007 document , 1 MB | Table S1. L25 OA for the adsorption experiment Table S2. Physical properties of the catalysts Figure S1. Representative TEM images of TA adsorbents (a) TA0, (b) TA75, (c) TA100. Table S3. Mean signal-to-noise ratio Table Figure S2. pHpzc of various TA adsorbents. Table S4. Regression data for Langmuir and Freundlich adsorption isotherms |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- Abdelaziz, M., & Abdelrazek, E. M. (2007). Effect of dopant mixture on structural, optical and electron spin resonance properties of polyvinyl alcohol. Physica B: Condensed Matter, 390(1–2), 1–9. https://doi.org/10.1016/j.physb.2006.07.067
- Akbari, H., Jorfi, S., Mahvi, A. H., Yousefi, M., & Balarak, D. (2018). Adsorption of fluoride on chitosan in aqueous solutions: Determination of adsorption kinetics. Fluoride, 51(4), 319–327. https://www.fluorideresearch.org/514/files/FJ2018_v51_n4_p319-327_sfs.pdf
- Asgari, G., Dayari, A., Ghasemi, M., Seid-mohammadi, A., Gupta, V. K., & Agarwal, S. (2019). Efficient fluoride removal by preparation, characterization of pyrolysis bone: Mixed level design experiment and Taguchi L8 orthogonal array optimization. Journal of Molecular Liquids, 275, 251–264. https://doi.org/10.1016/j.molliq.2018.10.137
- Asghar, A., Aziz, A., Raman, A., Mohd, W., & Wan, A. (2014). A comparison of central composite design and taguchi method for optimizing fenton process. The Scientific World Journal, 2014. 1–14. https://doi.org/10.1155/2014/869120
- Babaeivelni, K., & Khodadoust, A. P. (2013). Adsorption of fluoride onto crystalline titanium dioxide: Effect of pH, ionic strength, and co-existing ions. Journal of Colloid and Interface Science, 394(1), 419–427. https://doi.org/10.1016/j.jcis.2012.11.063
- Balarak, D., Mostafapour, F. K., Edris Bazrafshan, A., & Mahvi, A. H. (2017). The equilibrium, kinetic, and thermodynamic parameters of the adsorption of the fluoride ion on to synthetic nano sodalite zeolite. Flouride, 50(2), 223–234. https://www.cabdirect.org/cabdirect/abstract/20183123830
- Bazrafshan, E., Balarak, D., Panahi, A. H., Kamani, H., & Mahvi, A. H. (2016). Fluoride removal from aqueous solutions by cupricoxide nanoparticles. Fluoride, 49(3), 233. https://www.researchgate.net/profile/Ayat-Hossein-Panahi/publication/329572199_FLUORIDE_REMOVAL_FROM_AQUEOUS_SOLUTIONS_BY_CUPRICOXIDE_NANOPARTICLES/links/5c10087a299bf139c7521b2c/FLUORIDE-REMOVAL-FROM-AQUEOUS-SOLUTIONS-BY-CUPRICOXIDE-NANOPARTICLES.pdf
- Biswas, K., Gupta, K., & Ghosh, U. C. (2009). Adsorption of fluoride by hydrous iron (III)-tin (IV) bimetal mixed oxide from the aqueous solutions. Chemical Engineering Journal, 149(1–3), 196–206. https://doi.org/10.1016/j.cej.2008.09.047
- Chen, L., He, B. Y., He, S., Wang, T. J., Su, C. L., & Jin, Y. (2012). Fe-Ti oxide nano-adsorbent synthesized by co-precipitation for fluoride removal from drinking water and its adsorption mechanism. Powder Technology, 227, 3–8. https://doi.org/10.1016/j.powtec.2011.11.030
- Chen, L., Wu, H. X., Wang, T. J., Jin, Y., Zhang, Y., & Dou, X. M. (2009). Granulation of Fe–Al–Ce nano-adsorbent for fluoride removal from drinking water by spray coating on sand in a fluidized bed. Powder Technology, 193(1), 59–64. https://doi.org/10.1016/J.POWTEC.2009.02.007
- Daifullah, A. A. M., Yakout, S. M., & Elreefy, S. A. (2007). Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. Journal of Hazardous Materials, 147(1–2), 633–643. https://doi.org/10.1016/j.jhazmat.2007.01.062
- Dehghani, M. H., Gholami, S., Karri, R. R., Lima, E. C., Mahvi, A. H., Nazmara, S., & Fazlzadeh, M. (2021). Process modeling, characterization, optimization, and mechanisms of fluoride adsorption using magnetic agro-based adsorbent. Journal of Environmental Management, 286, 112173. https://doi.org/10.1016/j.jenvman.2021.112173
- Delgadillo-Velasco, L., Hernández-Montoya, V., Cervantes, F. J., Montes-Morán, M. A., & Lira-Berlanga, D. (2017). Bone char with antibacterial properties for fluoride removal: Preparation, characterization and water treatment. Journal of Environmental Management, 201, 277–285. https://doi.org/10.1016/j.jenvman.2017.06.038
- Deng, S., Liu, H., Zhou, W., Huang, J., & Yu, G. (2011). Mn–Ce oxide as a high-capacity adsorbent for fluoride removal from water. Journal of Hazardous Materials, 186(2–3), 1360–1366. https://doi.org/10.1016/J.JHAZMAT.2010.12.024
- Devi, R. R., Umlong, I. M., Raul, P. K., Das, B., Banerjee, S., & Singh, L. (2014). Defluoridation of water using nano-magnesium oxide. 9(5), 512–524. https://doi.org/10.1080/17458080.2012.675522
10.1080/17458080.2012.675522 Google Scholar
- Fröschl, T., Hörmann, U., Kubiak, P., Kucerová, G., Pfanzelt, M., Weiss, C. K., … Wohlfahrt-Mehrens, M. (2012). High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chemical Society Reviews, 41(15), 5313–5360. https://doi.org/10.1039/c2cs35013k
- Gonza, M., Trombetta, M., Busca, G., & Ramı, J. (1998). Characterization of alumina—Titania mixed oxide supports Part II: Al2O3-based supports. Microporous and Mesoporous Materials, 23, 265–275.
- Gutiérrez-Alejandre, A., Trombetta, M., Busca, G., & Ramirez, J. (1997). Characterization of alumina-titania mixed oxide supports I. TiO2-based supports. Microporous Materials, 12(1-3), 79–91. https://doi.org/10.1016/S0927-6513(97)00062-X
- He, Y., Zhang, L., An, X., Wan, G., Zhu, W., & Luo, Y. (2019). Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: Adsorption isotherms, kinetics, thermodynamics and mechanism. Science of the Total Environment, 688, 184–198. https://doi.org/10.1016/J.SCITOTENV.2019.06.175
- Ishihara, T., Shuto, Y., Ueshima, S., Ngee, H. L., Nishiguchi, H., & Takita, Y. (2002). Titanium hydroxide as a new inorganic fluoride ion exchanger. Journal of the Ceramic Society of Japan, 110(1285), 801–803. https://doi.org/10.2109/jcersj.110.801
- Jadhav, S. V., Bringas, E., Yadav, G. D., Rathod, V. K., Ortiz, I., & Marathe, K. V. (2015). Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. Journal of Environmental Management, 162, 306–325. https://doi.org/10.1016/j.jenvman.2015.07.020
- Jiang, G., Jin, L., Pan, Q., Peng, N., Meng, Y., Huang, L., & Wang, H. (2021). Structural modification of aluminum oxides for removing fluoride in water: Crystal forms and metal ion doping. Environmental Technology, 2021, 1–14. https://doi.org/10.1080/09593330.2021.1921044
- Kameda, T., Oba, J., & Yoshioka, T. (2015). Kinetics and equilibrium studies on Mg-Al oxide for removal of fluoride in aqueous solution and its use in recycling. Journal of Environmental Management, 156, 252–256. https://doi.org/10.1016/j.jenvman.2015.03.043
- Li, Y., Song, X., Chen, G., Sun, Z., Xu, Y., & Yu, J. (2015). Preparation of calcium carbonate and hydrogen chloride from distiller waste based on reactive extraction–crystallization process. Chemical Engineering Journal, 278, 55–61. https://doi.org/10.1016/j.cej.2014.12.058
- Li, Z., Deng, S., Zhang, X., Zhou, W., Huang, J., & Yu, G. (2010). Removal of fluoride from water using titanium-based adsorbents. Frontiers of Environmental Science & Engineering in China, 4(4), 414–420. https://doi.org/10.1007/s11783-010-0241-y
- Lv, L., He, J., Wei, M., Evans, D. G., & Duan, X. (2006). Factors influencing the removal of fluoride from aqueous solution by calcined Mg-Al-CO3 layered double hydroxides. Journal of Hazardous Materials, 133(1–3), 119–128. https://doi.org/10.1016/j.jhazmat.2005.10.012
- Maliyekkal, S. M., Shukla, S., Philip, L., & Nambi, I. M. (2008). Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules. Chemical Engineering Journal, 140(1–3), 183–192. https://doi.org/10.1016/j.cej.2007.09.049
- Mohapatra, M., Anand, S., Mishra, B. K., Giles, D. E., & Singh, P. (2009). Review of fluoride removal from drinking water. Journal of Environmental Management, 91, 67–77. https://doi.org/10.1016/j.jenvman.2009.08.015
- Mohapatra, M., Hariprasad, D., Mohapatra, L., Anand, S., & Mishra, B. K. (2012). Mg-doped nano ferrihydrite—A new adsorbent for fluoride removal from aqueous solutions. Applied Surface Science, 258(10), 4228–4236. https://doi.org/10.1016/j.apsusc.2011.12.047
- Nazari, M., & Halladj, R. (2015). Optimization of fluoride adsorption onto a sonochemically synthesized nano-MgO/γ-Al2O3 composite adsorbent through applying the L 16 Taguchi orthogonal design. Desalination and Water Treatment, 56(9), 2464–2476. https://doi.org/10.1080/19443994.2014.961558
- Phadke, M. S. (1989). Quality engineering using robust design. Prentice Hall.
- Pholosi, A., Naidoo, E. B., & Ofomaja, A. E. (2020). Intraparticle diffusion of Cr (VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. South African Journal of Chemical Engineering, 32, 39–55. https://doi.org/10.1016/J.SAJCE.2020.01.005
10.1016/J.SAJCE.2020.01.005 Google Scholar
- Pillai, P., Dharaskar, S., Pandian, S., & Panchal, H. (2021). Overview of fluoride removal from water using separation techniques. Environmental Technology and Innovation, 21, 101246. https://doi.org/10.1016/j.eti.2020.101246
- Pillai, P., Dharaskar, S., Sinha, M. K., Sillanpää, M., & Khalid, M. (2020). Iron oxide nanoparticles modified with ionic liquid as an efficient adsorbent for fluoride removal from groundwater. Environmental Technology and Innovation, 19, 100842. https://doi.org/10.1016/j.eti.2020.100842
- Pudukudy, M., Yaakob, Z., & Akmal, Z. S. (2015). Direct decomposition of methane over Pd promoted Ni/SBA-15 catalysts. Applied Surface Science, 353, 127–136. https://doi.org/10.1016/j.apsusc.2015.06.073
- Rouquerol, J., Baron, G., Giesche, H., Groen, J., Klobes, P., Levitz, P., Neimark, A. V., Rigby, S., Sing, K., Thommes, M., & Unger, Klaus (2011). Liquid intrusion and alternative methods for the characterization of macroporous materials (IUPAC Technical Report). Pure and Applied Chemistry, Skudas, 84(1), 603. https://doi.org/10.1351/PAC-REP-10-11-19
- Roy, R. K. (2001). A primer on the Taguchi method. John Wiley & Sons, Ltd.
- Salifu, A., Petrusevski, B., Mwampashi, E. S., Pazi, I. A., Ghebremichael, K., Buamah, R., Aubry, C., Amy, G. L., & Kenedy, M. D. (2016). Defluoridation of groundwater using aluminum-coated bauxite: Optimization of synthesis process conditions and equilibrium study. Journal of Environmental Management, 181, 108–117. https://doi.org/10.1016/j.jenvman.2016.06.011
- Seoudi, R., Abd El Mongy, S., & Shabaka, A. A. (2008). Effect of polyvinyl alcohol matrices on the structural and spectroscopic studies of CdSe nanoparticles. Physica B: Condensed Matter, 403(10–11), 1781–1786. https://doi.org/10.1016/j.physb.2007.10.108
- Sepehr, M. N., Amrane, A., Karimaian, K. A., Zarrabi, M., & Ghaffari, H. R. (2014). Potential of waste pumice and surface modified pumice for hexavalent chromium removal: Characterization, equilibrium, thermodynamic and kinetic study. Journal of the Taiwan Institute of Chemical Engineers, 45(2), 635–647. https://doi.org/10.1016/J.JTICE.2013.07.005
- Shah, M., Bordoloi, A., Nayak, A. K., & Mondal, P. (2019). Effect of Ti/Al ratio on the performance of Ni/TiO2-Al2O3 catalyst for methane reforming with CO2. Fuel Processing Technology, 192, 21–35. https://doi.org/10.1016/j.fuproc.2019.04.010
- Shah, M., Das, S., Nayak, A. K. A. K., Mondal, P., & Bordoloi, A. (2018). Smart designing of metal-support interface for imperishable dry reforming catalyst. Applied Catalysis A: General, 556, 137–154. https://doi.org/10.1016/j.apcata.2018.01.007
- Shi, Q., Huang, Y., & Jing, C. (2013). Synthesis, characterization and application of lanthanum-impregnated activated alumina for F removal. Journal of Materials Chemistry A, 1(41), 12797–12803. https://doi.org/10.1039/C3TA12548C
- Singh, N. B., Nagpal, G., Agrawal, S., & Rachna. (2018). Water purification by using adsorbents: A review. Environmental Technology and Innovation, 11, 187–240. https://doi.org/10.1016/j.eti.2018.05.006
- Sinha, V., & Chakma, S. (2020). Synthesis and evaluation of CMC-g-AMPS/Fe/Al/AC composite hydrogel and their use in fluoride removal from aqueous solution. Environmental Technology and Innovation, 17, 100620. https://doi.org/10.1016/j.eti.2020.100620
- Suriyaraj, S. P., Pillai, M. M., Bhattacharyya, A., & Selvakumar, R. (2015). Scavenging of nitrate ions from water using hybrid Al2O3/bio-TiO2 nanocomposite impregnated thermoplastic polyurethane nanofibrous membrane. RSC Advances, 5(84), 68420–68429. https://doi.org/10.1039/C5RA09469K
- Suriyaraj, S. P., & Selvakumar, R. (2016). Advances in nanomaterial based approaches for enhanced fluoride and nitrate removal from contaminated water. RSC Advances, 6(13), 10565–10583. https://doi.org/10.1039/C5RA24789F
- Tang, Y., Guan, X., Su, T., Gao, N., & Wang, J. (2009). Fluoride adsorption onto activated alumina: Modeling the effects of pH and some competing ions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 337(1–3), 33–38. https://doi.org/10.1016/j.colsurfa.2008.11.027
- Thakre, D., Jagtap, S., Sakhare, N., Labhsetwar, N., Meshram, S., & Rayalu, S. (2010). Chitosan based mesoporous Ti–Al binary metal oxide supported beads for defluoridation of water. Chemical Engineering Journal, 158(2), 315–324. https://doi.org/10.1016/J.CEJ.2010.01.008
- Thathsara, S. K. T., Cooray, P. L. A. T., Mudiyanselage, T. K., Kottegoda, N., & Ratnaweera, D. R. (2018). A novel Fe-La-Ce tri-metallic composite for the removal of fluoride ions from aqueous media. Journal of Environmental Management, 207, 387–395. https://doi.org/10.1016/j.jenvman.2017.11.041
- Tor, A. (2006). Removal of fluoride from an aqueous solution by using montmorillonite. Desalination, 201(1–3), 267–276. https://doi.org/10.1016/j.desal.2006.06.003
- Wang, Y., & Reardon, E. J. (2001). Activation and regeneration of a soil sorbent for defluoridation of drinking water. Applied Geochemistry, 16(5), 531–539. https://doi.org/10.1016/S0883-2927(00)00050-0
- Yang, C., Gao, L., Wang, Y., Tian, X., & Komarneni, S. (2014). Fluoride removal by ordered and disordered mesoporous aluminas. Microporous and Mesoporous Materials, 197, 156–163. https://doi.org/10.1016/J.MICROMESO.2014.06.010
- Yu, Y., Yu, L., & Paul Chen, J. (2015). Adsorption of fluoride by Fe-Mg-La triple-metal composite: Adsorbent preparation, illustration of performance and study of mechanisms. Chemical Engineering Journal, 262, 839–846. https://doi.org/10.1016/j.cej.2014.09.006
- Zazouli, M. A., Belarak, D., Karimnezhad, F., & Khosravi, F. (2014). Removal of fluoride from aqueous solution by using of adsorption onto modified Lemna minor: Adsorption isotherm and kinetics study. Journal of Mazandaran University of Medical Sciences, 23(109), 195–204. https://jmums.mazums.ac.ir/article-1-3246-en.html
- Zazouli, M. A., Mahvi, A. H., Dobaradaran, S., Barafrashtehpour, M., Mahdavi, Y., & Balarak, D. (2014). Adsorption of fluoride from aqueous solution by modified Azolla filiculoides. Fluoride, 47(4), 349–358. https://www.researchgate.net/publication/286021886_Adsorption_of_fluoride_from_aqueous_solution_by_modified_Azolla_filiculoides
- Zeng, Y., Xue, Y., Liang, S., & Zhang, J. (2016). Removal of fluoride from aqueous solution by TiO2 and TiO2–SiO2 nanocomposite. 29(1), 25–32. https://doi.org/10.1080/09542299.2016.1269617
10.1080/09542299.2016.1269617 Google Scholar
- Zhang, Y., Dou, X. M., Yang, M., He, H., Jing, C. Y., & Wu, Z. Y. (2010). Removal of arsenate from water by using an Fe–Ce oxide adsorbent: Effects of coexistent fluoride and phosphate. Journal of Hazardous Materials, 179(1–3), 208–214. https://doi.org/10.1016/J.JHAZMAT.2010.02.081
- Zhang, Z., Wang, S., Chen, H., Liu, Q., Wang, J., & Wang, T. (2013). Preparation of polyamide membranes with improved chlorine resistance by bis-2,6-N,N-(2-hydroxyethyl) diaminotoluene and trimesoyl chloride. Desalination, 331, 16–25. https://doi.org/10.1016/J.DESAL.2013.10.006
- Zhao, X., Shi, Y., Wang, T., Cai, Y., & Jiang, G. (2008). Preparation of silica-magnetite nanoparticle mixed hemimicelle sorbents for extraction of several typical phenolic compounds from environmental water samples. Journal of Chromatography A, 1188(2), 140–147. https://doi.org/10.1016/j.chroma.2008.02.069
- Zhao, X., Wang, J., Wu, F., Wang, T., Cai, Y., Shi, Y., & Jiang, G. (2010). Removal of fluoride from aqueous media by Fe3O4@Al (OH)3 magnetic nanoparticles. Journal of Hazardous Materials, 173(1–3), 102–109. https://doi.org/10.1016/j.jhazmat.2009.08.054
- Zhao, X., Zhang, L., Xiong, P., Ma, W., Qian, N., & Lu, W. (2015). A novel method for synthesis of Co-Al layered double hydroxides and their conversions to mesoporous CoAl2O4 nanostructures for applications in adsorption removal of fluoride ions. Microporous and Mesoporous Materials, 201(C), 91–98. https://doi.org/10.1016/j.micromeso.2014.09.030
- Zheng, Y., Lv, K., Wang, Z., Deng, K., & Li, M. (2012). Microwave-assisted rapid synthesis of anatase TiO2 nanocrystals with exposed {0 0 1} facets. Journal of Molecular Catalysis A: Chemical, 356, 137–143. https://doi.org/10.1016/j.molcata.2012.01.006